Abstract
When tetracycline was present, tetA(C) reduced acid tolerance, suppressed rpoS expression, and increased the concentration of total soluble proteins in stationary-phase Escherichia coli. The suppression of acid tolerance was reversed by 85 mM sodium, potassium, magnesium, and calcium ions but not by 85 mM sucrose. Implications for using TetA(C) are discussed.
Of the different modes of tetracycline resistance, the tetracycline-specific efflux system, such as the tetA(C) from pBR322 and tetA(B) from Tn10, is the most widely used in molecular biology (4, 12). In previous studies on acid tolerance in Escherichia coli, a decrease in acid tolerance was observed when plasmids carrying the tetA(C) gene were used as controls (2). Results from experiments reported here link tetracycline-specific efflux systems with a decrease in acid tolerance when tetracycline is present. This decrease was rescued by the presence of various cations. Tetracycline in the presence of TetA(C) was also correlated with other physiological changes, including a lowering of the steady-state mRNA level for rpoS, the dominant sigma transcription factor during the stationary phase (6), as well as an elevation in total soluble protein. The implications of the pleiotropic effects caused by tetracycline-specific efflux proteins in the presence of tetracycline are discussed.
Results and discussion.
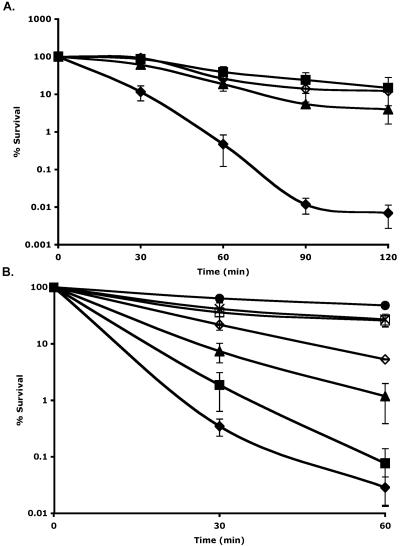
Many E. coli strains, such as DH1 and K-12, show tolerance to acidic conditions when stationary-phase (16 h of growth) cells are examined (1, 3). Acid challenges were performed by diluting stationary-phase cells (16 h, 37°C, 110-rpm shaking) at 1:1,000 into acid challenge medium (25 ml; medium was adjusted to pH 3.0 using HCl and then filter sterilized prior to use) followed by incubation (37°C, 110 rpm shaking). Samples were taken at specified time points, and viable cells were enumerated on tryptic soy agar plates (Difco, Detroit, MI). However, a DH1 strain transformed with the plasmid pJB3TA, which is a pBR322 derivative with the bla gene removed (SacI digestion followed by self-ligation), showed reduced acid tolerance when cultured in the presence of 36 μg tetracycline ml−1. Comparisons to the untransformed DH1 strain or to the DH1/pJB3TA strain grown without tetracycline selection (Fig. 1A) showed that this decrease in acid tolerance was a combined effect of both the tetracycline efflux system and tetracycline. This observation differs from several previous reports in which the tetracycline efflux system alone caused physiological changes (5, 7, 10, 13, 14). Similar results were observed when tetA(B) was assayed under these conditions (data not shown), suggesting that this suppression of acid tolerance is a result of the efflux mechanism instead of unique functions of the proteins. The absence of tetracycline selection during growth did not lead to a significant loss of the plasmid (P > 0.48, t test [Excel software; Microsoft, Redmond, WA]). This tetracycline-induced reduction in acid tolerance of E. coli cells expressing tetA(C) was ameliorated when Luria-Bertani broth (LB; 10% Bacto tryptone, 5% yeast extract, 5% NaCl), minimal medium (MM), or tryptic soy broth (TSB; Difco, Detroit, MI) was used as the acid challenge medium instead of nutrient broth (NB; Difco, Detroit, MI) (Table 1). Analyses of the compositions of the media showed that the addition of various salts to NB rescued the loss of acid tolerance. Specifically, when NaCl or MgCl2 was added at 85 mM or when Na2SO4 was added at 42.5 mM to NB, K-12/pJB3TA cells grown under selection with 36 μg tetracycline ml−1 exhibited acid tolerance that was comparable to that of untransformed K-12 cells, demonstrating that the presence of some cations can restore this loss of acid tolerance (Table 1). When calcium (CaCl2) and potassium (KCl) salts were tested under identical conditions, similar results were obtained (Table 1). Furthermore, the rescue of acid tolerance by KCl addition occurred in a dose-dependent manner (Fig. 1B). Sodium, magnesium, and calcium salts elicited similar dose-dependent rescue of acid tolerance (data not shown).
FIG. 1.
Percent survival of stationary-phase (16 h) E. coli during acid challenge. (A) ▪, DH1; ⋄, DH1/pJB3TA; ▴, DH1/pJB3TA grown with 12 μg tetracycline ml−1; ⧫, DH1/pJB3TA grown with 36 μg tetracycline ml−1. (B) Strain K-12/pJB3TA was used. Except for the control experiment (⋄), where no antibiotic selection was used, all experiments used cells that were cultured with 36 μg tetracycline ml−1 during overnight growth. The acid challenge medium had been supplemented with the following compounds: ⋄ and ⧫, none; ▴, 5 mM KCl; ×, 50 mM KCl; •, 75 mM KCl; ▪, 85 mM sucrose; □, 400 mM sucrose.
TABLE 1.
Percent survival of acid-challenged E. coli in different acid challenge media
| Strain | Challenge medium | % Survival with medium exposure time ofa:
|
|||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | ||
| DH1/pJB3TA | NB | 5.94 ± 1.34 | 0.78 ± 0.65 | 0.21 ± 0.24 | 0.09 ± 0.13 |
| LBb | 92.60 ± 7.94 | 78.43 ± 0.73 | 92.59 ± 0.50 | 57.15 ± 8.07 | |
| TSBb | 70.94 ± 30.76 | 75.02 ± 30.00 | 66.76 ± 30.45 | 66.74 ± 34.24 | |
| MMb | 91.30 ± 21.76 | 78.26 ± 18.07 | 65.52 ± 9.65 | 65.71 ± 8.25 | |
| K-12/pJB3TA | NB | 3.31 ± 0.25 | 0.35 ± 0.18 | 0.11 ± 0.08 | 0.02 ± 0.01 |
| NB + 85 mM NaClb | 33.89 ± 13.95 | 27.40 ± 9.76 | 17.84 ± 4.93 | 12.62 ± 2.59 | |
| NB + 42.5 mM Na2SO4b | 40.84 ± 8.50 | 29.24 ± 0.66 | 22.41 ± 3.13 | 14.46 ± 4.06 | |
| NB + 85 mM KClb | 47.19 ± 9.42 | 35.85 ± 8.84 | 27.97 ± 10.84 | 21.85 ± 6.53 | |
| NB + 85 mM MgCl2b | 58.55 ± 24.46 | 25.02 ± 15.32 | 24.97 ± 8.10 | 17.84 ± 4.27 | |
| NB + 80 mM CaCl2b | 93.10 ± 16.19 | 60.91 ± 16.95 | ND | ND | |
Acid challenge experiments were conducted with stationary-phase cells (16 h) grown with 36 μg tetracycline ml−1. All results are reported as the averages of three independent trials ± standard deviations. The percent survival was calculated by normalizing the CFU ml−1 results for each condition to its respective value at 0 min. ND, not determined.
Percent survival under these conditions is statistically different from percent survival under the NB condition (P < 0.05) for the corresponding host strain (DH1 or K-12).
To determine if changes in osmotic pressure resulting from salt addition were responsible for the rescue of acid tolerance, 85 mM sucrose was added to the acid challenge medium (NB) as a control. In addition, 400 mM sucrose, which has previously been shown to induce an osmotic stress response in E. coli (9), was tested. The results showed that 85 mM sucrose had no effect on acid tolerance, whereas 400 mM sucrose did (Fig. 1B). These data suggested that the addition of cations, rather than an osmotically triggered response, was responsible for the reversal of tetracycline-induced acid sensitivity.
The ability of tetracycline to affect acid tolerance in cells containing a tetracycline efflux system and the amelioration by the addition of some cations can both be explained by the transport of tetracycline in and out of the cell. The equilibrium between the noncharged form (Tc) and charged form ([M-Tc]+) of tetracycline depends on the abundance of both cations and protons (Tc + M2+ ⇆ [M-Tc]+ + H+, where M is usually a divalent cation) (4). In the cytoplasm, due to the higher pH and abundance of cations, Tc is coupled with a divalent cation (usually Mg2+) to form [M-Tc]+, which the tetracycline efflux system transports into the periplasm in exchange for one proton (15). This equilibrium indicates that when the periplasmic space is acidified, as in the case of exposing the cells to acidic medium, the balance between the Tc and [M-Tc]+ will shift towards the Tc form. We speculate that this shift will create two effects. First, since diffusion of Tc into the medium is a low-efficiency process, there will be an accumulation of tetracycline in the periplasmic space. Second, since Tc is freely permeable across the plasma membrane, the accumulation of Tc in the periplasmic space will likely increase tetracycline influx into the cytoplasm. We hypothesize that these two effects, together with a tetracycline-proton antiport system, will set up a futile loop of tetracycline transport under acidic conditions. Since one proton is imported with the expulsion of one [M-Tc]+ molecule, the net result is likely to be the acidification of the cytoplasm, which is likely the cause for lowered acid tolerance in E. coli cells expressing the tetracycline-specific efflux proteins in the presence of tetracycline.
In addition to suppressing acid tolerance, the tetracycline efflux system in the presence of tetracycline can affect global cell physiology. For instance, rpoS promoter activity, as revealed by the lacZ reporter gene fused to the rpoS promoter (6), in tetA(C)-expressing cells grown with tetracycline was lower (Table 2). Further, when the levels of endogenous RpoS protein, assayed using anti-RpoS antibody (11) in a Western blot, were examined, a consistent trend of tetracycline lowering the levels of RpoS was observed (data not shown). Moreover, results of experiments aimed at determining whether the reduction in rpoS transcription correlates with reduction in activity of genes under its regulation showed a reduction in endogenous β-galactosidase activity when stationary-phase DH1/pJB3TA was grown in the presence of 36 μg tetracycline ml−1 was compared to control conditions (Table 2). This assay was performed using o-nitrophenyl-β-d-galactopyranoside (ONPG; Sigma-Aldrich, St. Louis, MO) as a substrate (8). Furthermore, in three different strains of E. coli (DH1, K-12, and MC4100), the combined presence of tetA(C) and tetracycline in stationary-phase (16 h) cultures led to an increase in the total soluble protein (BPER bacterial protein extraction reagents and BCA protein quantification kit by Pierce, Rockford, IL). These data indicated that the overall physiological state of the cells was altered by the combined presence of tetracycline efflux system and tetracycline.
TABLE 2.
Effects of tetracycline on rpoS transcription, endogenous β-galactosidase activity, and protein levels in stationary-phase E. coli
| Measurement | Strain | Result with tetracycline concn (μg ml−1) showna
|
|||
|---|---|---|---|---|---|
| 0 (no plasmid) | pJB3TA
|
||||
| 0 | 12 | 36 | |||
| rpoS expression (U cell−1)b | RO91 | 199.9 ± 6.2 | 201.8 ± 10.8 | 174 ± 6.2 | 62.2 ± 5.1 |
| β-Gal (U cell−1)c | DH1 | 998.4 ± 216.4 (222.6 ± 72.2d) | 877.5 ± 271.6 | 195.1 ± 18.5 | 103.1 ± 20.2 |
| Soluble protein (μg/108 cells) | K-12 | 1.76 ± 0.24 | 2.51 ± 0.07 | 2.44 ± 0.42 | 3.46 ± 0.10 |
| DH1 | 6.99 ± 0.75 | 4.85 ± 0.22 | 5.36 ± 0.20 | 7.43 ± 0.76 | |
| MC4100 | 26.08 ± 6.70 | 5.96 ± 0.34 | 9.19 ± 2.62 | 13.40 ± 4.30 | |
Results are reported as average of three independent trials ± standard deviation.
Units of β-galactosidase activity per cell under the λRZ5 [rpoS742::lacZ(Hyb)] construct (6) in an MC4100 Δ(argF-lac)U169 background.
Activity of endogenous β-galactosidase following induction with IPTG (isopropyl-β-thiogalactopyranoside) for 1 h.
No IPTG induction.
Tetracycline efflux systems have been reported to have pleiotropic effects on cell physiology, although this is the first report showing effects caused by the combined presence of tetracycline and tetracycline efflux systems. Given the reported effects on protein levels and on the activity level of a major transcription factor, experimental data using tetracycline efflux proteins as selection markers should be interpreted with care, with due consideration for proper control experiments and strains.
Acknowledgments
We thank David Baumler for technical assistance and helpful discussions of this work.
Financial support was provided in part by the National Renewable Energy Laboratory, Boulder, CO; the USDA; NRICGP grant 96-35201-3430; and the College of Agricultural and Life Sciences, University of Wisconsin—Madison. J.J.B. was supported in part by a development grant from St. Mary's College of Maryland.
REFERENCES
- 1.Arnold, K. W., and C. W. Kaspar. 1995. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:2037-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumler, D. J., K. F. Hung, J. L. Bose, B. M. Vykhodets, C. Cheng, K.-C. Jeong, and C. W. Kaspar. Enhancement of acid tolerance in Zymomonas mobilis by a proton-buffering peptide. Appl. Biochem. Biotechnol., in press. [DOI] [PubMed]
- 3.Choi, S. H., D. J. Baumler, and C. W. Kaspar. 2000. Contribution of dps to acid stress tolerance and oxidative stress tolerance in Escherichia coli O157:H7. Appl. Environ. Microbiol. 66:3911-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert, B., and C. F. Beck. 1989. Overproduction of transposon Tn10-encoded tetracycline resistance protein results in cell death and loss of membrane potential. J. Bacteriol. 171:3557-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange, R., and R. Hengge-Aronis. 1994. The cellular concentration of the σs subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 8:1600-1612. [DOI] [PubMed] [Google Scholar]
- 7.Lee, S. W., and G. Edlin. 1985. Expression of tetracycline resistance in pBR322 derivatives reduces the reproductive fitness of plasmid-containing Escherichia coli. Gene 39:173-180. [DOI] [PubMed] [Google Scholar]
- 8.Miller, J. H. 1992. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 9.Muffler, A., D. D. Traulsen, R. Lange, and R. Hengge-Aronis. 1996. Posttranscriptional osmotic regulation of the σs subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 178:1607-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen, T. N., Q. G. Phan, L. P. Duong, K. P. Bertrand, and R. E. Lenski. 1989. Effects of carriage and expression of the Tn10 tetracycline-resistance operon on the fitness of Escherichia coli K12. Mol. Biol. Evol. 6:213-225. [DOI] [PubMed] [Google Scholar]
- 11.Rockabrand, D., K. Livers, T. Austin, R. Kaiser, D. Jensen, R. Burgess, and P. Blum. 1998. Roles of DnaK and RpoS in starvation-induced thermotolerance of Escherichia coli. J. Bacteriol. 180:846-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnappinger, D., and W. Hillen. 1996. Tetracyclines: antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 165:359-369. [DOI] [PubMed] [Google Scholar]
- 13.Stavropoulos, T. A., and C. A. Strathdee. 2000. Expression of the tetA(C) tetracycline efflux pump in Escherichia coli confers osmotic sensitivity. FEMS Microbiol. Lett. 190:147-150. [DOI] [PubMed] [Google Scholar]
- 14.Valenzuela, M. S., K. A. Siddiqui, and B. L. Sarkar. 1996. High expression of plasmid-encoded tetracycline resistance gene in E. coli causes a decrease in membrane-bound ATPase activity. Plasmid 36:19-25. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi, A., Y. Iwasaki-Ohba, N. Ono, M. Kaneko-Ohdera, and T. Sawai. 1991. Stoichiometry of metal-tetracycline/H+ antiport mediated by transposon Tn10-encoded tetracycline resistance protein in Escherichia coli. FEBS Lett. 282:415-418. [DOI] [PubMed] [Google Scholar]