Abstract
The effect of Mn2+/Mg2+ concentration on the activity of intact, homogeneous phosphoenolpyruvate carboxykinase (PEPCK) from leaves of the C4 grass, Guinea grass (Panicum maximum), have been investigated. Assay conditions were optimized so that PEPCK activity could be measured at concentrations of Mn2+/Mg2+ similar to those found in the cytosol (low micromolar Mn2+ and millimolar Mg2+). PEPCK activity was totally dependent on Mn2+ and was activated at low micromolar concentrations of Mn2+ by millimolar concentrations of Mg2+. Therefore, at physiological concentrations of Mn2+, PEPCK has a requirement for Mg2+. Assay at physiological concentrations of Mn2+/Mg2+ led to a marked decrease in its affinity for ATP and a 13-fold increase in its affinity for CO2. The Km (CO2) was further decreased by assay at physiological ATP to ADP ratios, reaching values as low as 20 μm CO2, comparable with the Km (CO2) of ribulose 1,5-bisphosphate carboxylase-oxygenase. This means that PEPCK will catalyze a reversible reaction and that it could operate as a carboxylase in vivo, a feature that could be particularly important in algal CO2-concentrating systems.
Phosphoenolpyruvate carboxykinase (PEPCK-ATP; EC 4.1.1.49) is a Mn2+-dependent enzyme that catalyzes the reversible reaction:
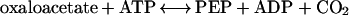 |
This reaction is important in plant metabolism because it lies at an interface between organic acid, amino acid, and sugar metabolism. In keeping with the importance of this reaction the presence of PEPCK in a wide range of plant tissues is now emerging, including structures involved in plant defense, such as trichomes and oil and resin ducts, in flowers, fruits, and developing seeds, and in the phloem of some plants. In at least some of these tissues, it plays a role in nitrogen metabolism and its abundance may change greatly and rapidly in response to changes in the nitrogen status of the tissue (Leegood and Walker, 1999; Walker et al., 1999, 2001). In addition, it is well established that PEPCK is involved gluconeogenesis, converting stored fats to sugars after germination in oil-storing seeds (Leegood and ap Rees, 1978) and in the photosynthetic CO2-concentrating mechanisms present in both PEPCK-type and some NADP-malic enzyme-type C4 plants and in plants with Crassulacean acid metabolism (Leegood et al., 1996; Walker and Leegood, 1996; Wingler et al., 1999). In higher plants it has always been thought that PEPCK acts as a decarboxylase in vivo because of its low affinity for CO2 (Ray and Black, 1976; Urbina and Avilan, 1989). In both gluconeogenesis and in C4 and Crassulacean acid metabolism photosynthesis, PEPCK certainly acts as a decarboxylase, but in some aquatic plants and algae it has also been proposed to act as a carboxylase (Reiskind and Bowes, 1991).
A difficulty with the suggestion that PEPCK acts as a carboxylase in some tissues has been the low affinity of the enzyme for CO2 when measured in in vitro assay. It is possible that this low affinity is a result of assay of the enzyme at non-physiological concentrations of metal ions (Walker et al., 1997). For example, all previous studies have assayed PEPCK at completely unphysiological concentrations of Mn2+ (>0.5 mm) and in the absence of Mg2+. Mg2+ has also been shown to be inhibitory (Burnell, 1986). However, the concentration of Mn2+ in the cytosol of plant and animal cells is submicromolar as, for example, in maize roots (Quiquampoix et al., 1993), and the concentration of Mg2+ in the cytosol of plant cells is millimolar as, for example, in mung bean (Vigna radiata) roots (Yazaki et al., 1988). In addition, previous studies of PEPCK have used the proteolytically cleaved form of the enzyme, the properties of which may differ from the intact enzyme (Walker et al., 1997, 2002).
In this paper we show how PEPCK activity can be measured in in vitro assay at physiological concentrations of Mn2+/Mg2+ and that under such conditions the affinity of PEPCK for CO2 is greatly increased.
RESULTS
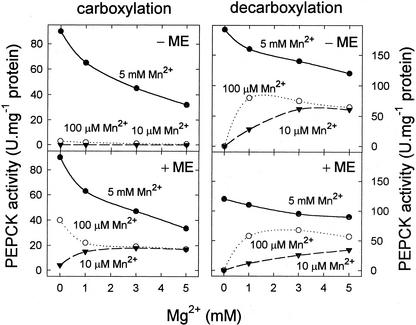
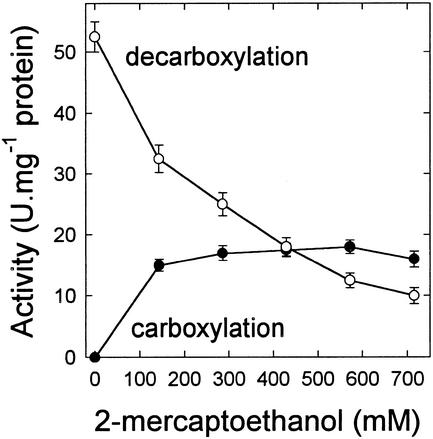
The effects of Mn2+ and Mg2+ concentration on the carboxylation and decarboxylation activities of pure, intact PEPCK from illuminated leaves of the C4 plant, Guinea grass (Panicum maximum) were characterized (Fig. 1). For the carboxylation reaction, in the absence of 2-mercaptoethanol (ME), there was no activity at 10 μm Mn2+, little activity at 100 μm Mn2+ and maximum activity at millimolar concentrations of Mn2+. Under these conditions, Mg2+ was inhibitory, with more than 50% inhibition at 5 mm Mg2+. In the presence of high concentrations of ME (500 mm), the characteristics of the carboxylation reaction were different. First, there was some activity at 10 μm Mn2+, and this was greatly increased by inclusion of Mg2+. Second, there was substantial activity at 100 μm Mn2+, but Mg2+ was inhibitory, as at 5 mm Mn2+. For the decarboxylation reaction, in the absence of ME, there was essentially no activity at 10 or 100 μm Mn2+, but activity was greatly stimulated by the presence of Mg2+. Maximum activity was still observed at 5 mm Mn2+ and Mg2+ was inhibitory. In the presence of ME, the pattern of response to Mn2+ and Mg2+ was much the same, except that there was an inhibition of PEPCK activity compared with the absence of ME. ME was therefore inhibitory to the decarboxylation reaction, but stimulated the carboxylation reaction dramatically at low micromolar concentrations of Mn2+ in the presence of millimolar concentrations of Mg2+ (Fig. 2).
Figure 1.
Effect of Mn2+ and Mg2+ concentration on the carboxylation and decarboxylation activities of Guinea grass PEPCK, measured in either the presence or absence of ME. Substrate concentrations for the carboxylation assay were 0.5 mm ADP/5 mm PEP and for the decarboxylation assay 0.5 mm ATP/200 μm OAA.
Figure 2.
Effect of ME concentration on both the carboxylation and decarboxylation activities of Guinea grass PEPCK, measured at 10 μm Mn2+/5 mm Mg2+. Substrate concentrations for the carboxylation assay were 0.5 mm ADP/5 mm PEP and for the decarboxylation assay 0.5 mm ATP/200 μm OAA.
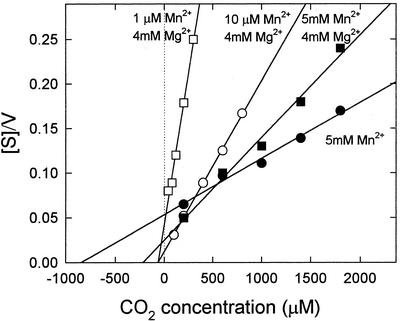
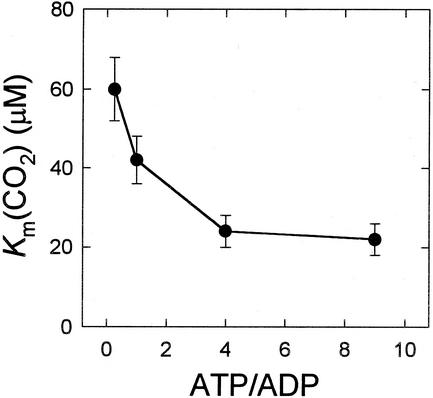
Kinetic constants were determined for PEPCK from illuminated leaves (light) at both 5 mm Mn2+ and 10 μm Mn2+/4 mm Mg2+. There were decreases in the affinities of PEPCK for phosphoenolpyruvate (PEP) and ADP in the carboxylation reaction and for oxaloacetate (OAA) and ATP in the decarboxylation reaction when assayed at micromolar concentrations of Mn2+ in the presence of 4 mm Mg2+. The increase in Km was substantial in the case of ATP. However, there was a large increase in the affinity of PEPCK for CO2 when Mn2+ was lowered from 5 mm to 10 μm. Figure 3 shows that at least part of the decrease in Km (CO2) was the result of the inclusion of Mg2+ (compare plots with 5 mm Mn2+ and 5 μm Mn2+/4 mm Mg2+). There was no further decrease in the Km (CO2) when PEPCK was assayed in the presence of 1 μm Mn2+ (Fig. 3). However, inclusion of ATP as well as ADP in the carboxylation reaction resulted in a further 3-fold reduction in the Km (CO2) as the ATP to ADP ratio was increased (Fig. 4).
Figure 3.
Hanes-Woolf plots, which show the effect of Mn2+ and Mg2+ concentration on the affinity of Guinea grass PEPCK for CO2. Substrate concentrations were 5 mm PEP/0.5 mm ADP.
Figure 4.
Effect of ATP to ADP ratio (total adenylate concentration1 mm) on the affinity of Guinea grass PEPCK for CO2. These assays used 10 μm Mn2+/4 mm Mg2+/5 mm PEP. Michaelis constants were determined from Hanes-Woolf plots and are the means ± se of three separate determinations.
When the properties of PEPCK purified from darkened leaves (dark) were compared with that purified from illuminated leaves (light) at 10 μm Mn2+/4 mm Mg2+ (Table I), there was no difference in the affinity of the light and dark enzymes for CO2 and there were no significant changes in affinities for PEP and ADP in the carboxylation reaction. There were substantial decreases in the affinities for OAA and ATP in the decarboxylation reaction with an approximate doubling of the Km. These are the result of light-dependent changes in the phosphorylation state of PEPCK, as discussed by Walker et al. (2002).
Table I.
Kinetic constants for PEPCK from illuminated leaves of Panicum maximum
| Metal Ions in Assay |
Km
|
||||
|---|---|---|---|---|---|
| CO2 | PEP | ADP | OAA | ATP | |
| μm | mm | mm | μm | μm | |
| 5 mm Mn2+ | 732 ± 90 | 1.38 ± 0.21 | 18 ± 2.0 | 85 ± 15 | 1.5 ± 0.2 |
| 5 mm Mn2++ 4 mm Mg2+ | 238 ± 55 | 1.67 ± 0.30 | 17 ± 2.5 | 100 ± 16 | 1.1 ± 0.3 |
| 10 μm Mn2++ 4 mm Mg2+ | |||||
| Light | 55 ± 18 | 2.10 ± 0.25 | 36 ± 3.3 | 156 ± 20 | 25.7 ± 3.5 |
| Dark | 55 ± 19 | 2.65 ± 0.31 | 44 ± 3.1 | 304 ± 25 | 43.5 ± 3.3 |
Carboxylase activity was measured using 10 mm CO2/0.5 mm ADP/5 mm PEP) and decarboxylase activity using 0.5 mm ATP/200 μm OAA. Michaelis constants were determined from Hanes-Woolf plots and are the means ± se of three separate determinations. Measurements are for the enzyme purified from illuminated leaves except for the row labelled dark, which are for the enzyme purified from darkened leaves.
DISCUSSION
Previous investigators of the assay conditions and kinetics of PEPCK from plants have assayed it at unphysiological concentrations of Mn2+ (> 0.5 mm) and have concluded that plant PEPCK is strongly inhibited by millimolar concentrations of Mg2+ (Burnell, 1986; Walker et al., 1997). This behavior is different to that reported for the enzyme from non-plant tissues, in which a synergistic activation by a combination of Mn2+ and Mg2+ occurs, e.g. the enzyme from yeast (Cannata and Stoppani, 1963) and Trypanosoma cruzii (Jurado et al., 1996), which show substantial sequence similarity to the plant enzyme, and the enzyme from rat liver, which shows no significant sequence similarity (Foster et al., 1967). It has been suggested that, in the rat liver enzyme, Mg2+ forms a MgITP2− complex that acts as a substrate for the reaction, whereas Mn2+ acts as an activator at a separate site (Foster et al., 1967). The present results resolve this discrepancy between PEPCK from plant and non-plant tissues and show that PEPCK from Guinea grass is (a) totally dependent on Mn2+, (b) that it can operate at physiological (μm) concentrations of Mn2+, and (c) that physiological (mm) concentrations of Mg2+ activate the enzyme at physiological concentrations of Mn2+ (presumably forming an MgATP2− substrate complex). PEPCK thus has a dual requirement for both Mn2+ and Mg2+. However, it is also clear that measurements of the maximum activity of PEPCK in plant extracts should still be made under conditions of saturating Mn2+ in the absence of Mg2+.
The improvements made to the assay of PEPCK involve the inclusion of high concentrations of ME. A notable property of both ATP-dependent (plant) and GTP-dependent PEPCKs is their inactivation by thiol-modifying reagents (Chang and Lane, 1966), affecting nucleotide binding at the active site (Lewis et al., 1993). Although the sequence of the ATP-dependent PEPCK from higher plants, yeast, and many bacteria shows little similarity to the GTP- or ITP-dependent enzyme found in animals and other bacteria, the active site has considerable homology in both ATP- and GTP- or ITP-dependent PEPCKs. Reactive thiol groups in ATP-dependent PEPCKs occur in yeast (Cardemil et al., 1990) and T. cruzii (Jurado et al., 1996). Cardemil et al. (1990) reported that the inactivation of yeast PEPCK by thiol reagents is caused by modification of both thiol and vicinal dithiol groups within the active site of each subunit and that loss of activity was effectively prevented by the combined presence of ATP plus Mn2+ (see also Walker et al., 1997). Whether or not this loss of activity, that can be more severe for the carboxylation than for the decarboxylation reaction (e.g. Ray and Black, 1976), is related to the changes in metal ion dependence with ME for the carboxylation reaction, as shown in Figure 1, has yet to be resolved. Another possibility is that ME is acting to chelate inhibitory trace metals or to stabilize the concentration of Mn2+. However, the fact that ME stimulated the carboxylation reaction but was inhibitory to the decarboxylation reaction suggests that this explanation is less likely.
An important consequence of assay of PEPCK at physiological concentrations of metal ions is the effect on its substrate affinities, in particular a marked decrease in its affinity for ATP and a 13-fold increase in its affinity for CO2. PEPCK from higher plant and algal sources has always been notable for its low affinity for CO2. For example, the Km (CO2) in Guinea grass has been estimated at 1.36 and 1.61 mm (calculated from Urbina and Avilan [1989] and Ray and Black [1976], respectively), 0.497 mm in pineapple (calculated from Daley et al. [1977]) and 0.175 to 1.21 mm in a range of brown and green algae (Table II in Johnston and Raven, 1983). This low affinity for CO2 has been particularly controversial in the algae in which it has been proposed that PEPCK may sometimes act as a carboxylase (Reiskind and Bowes, 1991; Johnston and Raven, 1983). The present results suggest that the Km(CO2) of PEPCK can be many-fold lower than previous estimates, reaching as low as 20 μm CO2 at physiological ATP to ADP ratios (an ATP to ADP ratio above 4:1 is typical in the cytosol of wheat leaf mesophyll protoplasts in the light; Stitt et al., 1982). It is not clear how the ATP to ADP ratio affects the Km(CO2). Although chelation of metal ions could be involved, ATP is known to be an effector of PEPCK (Walker et al., 2002). The Km(CO2) of PEPCK at high ATP to ADP ratios is comparable with the Km(CO2) of Rubisco from C3 and Crassulacean acid metabolism plants (12–26 μm) or from C4 plants (28–63 μm; Yeoh et al., 1980, 1981). This means that PEPCK will catalyze a reversible reaction and that it could operate as a carboxylase in vivo. Clearly the balance between carboxylation and decarboxylation will be strongly influenced by the ATP to ADP ratio, that will affect both the equilibrium of the reaction and the Km (CO2), and by the availability of reductant or amino donors that will determine the concentration of OAA. Further studies of algal PEPCK are now required.
MATERIALS AND METHODS
Plant Material
Seeds of Guinea grass (Panicum maximum) were obtained from the Kew Seed Bank (Royal Botanical Gardens, Kew, UK). Plants were grown in soil in a greenhouse during the summer with no supplementary light.
Purification of PEPCK
PEPCK was purified from both darkened and illuminated leaves of Guinea grass as described by Walker et al. (2002).
Assay of PEPCK
Carboxylase activity was measured as described by Walker et al. (1995), and decarboxylase activity was measured as described by Lee et al. (1981). Modifications to these procedures in individual experiments are described in the text. For determination of CO2 affinity, solutions were made up in boiled acidified water that had been purged with N2. The CO2 concentration was calculated using a pK of 6.365 at 25°C (Umbreit et al., 1972). One unit of PEPCK activity corresponds to the production of 1 μmol product/min at 25°C.
Footnotes
This research was supported by the Biotechnology and Biological Sciences Research Council, UK (research grant nos. CO5229 and RSP07804), by a David Phillips Research Fellowship to R.P.W., and by a research studentship to R.M.A.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010431.
LITERATURE CITED
- Burnell JN. Purification and properties of phosphoenolpyruvate carboxykinase from C4 plants. Aust J Plant Physiol. 1986;13:577–587. [Google Scholar]
- Cannata JJB, Stoppani AOM. Phosphoenolpyruvate carboxylase from baker's yeast: II. Properties of the enzyme. J Biol Chem. 1963;238:1208–1212. [PubMed] [Google Scholar]
- Cardemil E, Encinas MV, Jabalquinto AM. Reactive sulfhydryl groups in Saccharomyces cerevisiae phosphoenolpyruvate carboxykinase. Biochim Biophys Acta. 1990;1040:71–76. doi: 10.1016/0167-4838(90)90147-8. [DOI] [PubMed] [Google Scholar]
- Chang HC, Lane MD. The enzymatic carboxylation of phosphoenolpyruvate: II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966;241:2421–2430. [PubMed] [Google Scholar]
- Daley LS, Ray TB, Vines HM, Black CC., Jr Characterization of phosphoenolpyruvate carboxykinase from pineapple leaves Ananas comosus (L.) Merr. Plant Physiol. 1977;59:618–622. doi: 10.1104/pp.59.4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DO, Lardy HA, Ray PD, Johnston JB. Alteration of rat liver phosphoenolpyruvate carboxykinase activity by l-tryptophan in vivo and metals in vivo. Biochemistry. 1967;6:2120–2128. doi: 10.1021/bi00859a033. [DOI] [PubMed] [Google Scholar]
- Johnston AM, Raven JA. Extraction, partial purification and characterization of phosphoenolpyruvate carboxykinase from Ascophyllum nodosum (Phaeophyceae) J Phycol. 1983;25:568–576. [Google Scholar]
- Jurado LA, Machín I, Urbina JA. Trypanosoma cruzi phosphoenolpyruvate carboxykinase (ATP-dependent): transition metal ion requirement for activity and sulphydryl group reactivity. Biochim Biophys Acta. 1996;1292:188–196. doi: 10.1016/0167-4838(95)00201-4. [DOI] [PubMed] [Google Scholar]
- Lee MH, Hebda CA, Nowak T. The role of cations in avian liver phosphoenolpyruvate carboxykinase catalysis. J Biol Chem. 1981;256:12793–12801. [PubMed] [Google Scholar]
- Leegood RC, ap Rees T. Phosphoenolpyruvate carboxykinase and gluconeogenesis in cotyledons of Cucurbita pepo. Biochim Biophys Acta. 1978;524:207–218. doi: 10.1016/0005-2744(78)90119-5. [DOI] [PubMed] [Google Scholar]
- Leegood RC, von Caemmerer S, Osmond CB. Metabolite transport and photosynthetic regulation in C4 and CAM plants. In: Dennis DT, Turpin DH, Layzell DD, Lefebvre DK, editors. Plant Metabolism. London: Longman; 1996. pp. 341–369. [Google Scholar]
- Leegood RC, Walker RP. Phosphoenolpyruvate carboxykinase in plants: its role and regulation. In: Bryant JA, Burrell MM, Kruger NJ, editors. Plant Carbohydrate Biochemistry. Oxford: BIOS Scientific Publishers; 1999. pp. 201–213. [Google Scholar]
- Lewis CT, Seyer JM, Cassell RG, Carlson GM. Identification of vicinal thiols of phosphoenolpyruvate carboxykinase (GTP) J Biol Chem. 1993;268:1628–1636. [PubMed] [Google Scholar]
- Quiquampoix H, Loughman B, Ratcliffe RG. A 31P-NMR study of the uptake and compartmentation of manganese by maize roots. J Exp Bot. 1993;44:1819–1827. [Google Scholar]
- Ray TB, Black CC., Jr Characterization of phosphoenolpyruvate carboxykinase from Panicum maximum. Plant Physiol. 1976;58:603–607. doi: 10.1104/pp.58.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind JB, Bowes G. The role of phosphoenolpyruvate carboxykinase in a marine macroalga with C4-like photosynthetic characteristics. Proc Natl Acad Sci USA. 1991;88:2883–2887. doi: 10.1073/pnas.88.7.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RMC, Heldt HW. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982;70:971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit WW, Burris RH, Stauffer JF. Manometric techniques and related methods for the study of tissue metabolism. Minneapolis: Burgess Publishing Co.; 1972. [Google Scholar]
- Urbina JA, Avilan L. The kinetic mechanism of phosphoenolpyruvate carboxykinase from Panicum maximum. Phytochemistry. 1989;28:1349–1353. [Google Scholar]
- Walker RP, Acheson RM, Técsi LI, Leegood RC. Phosphoenolpyruvate carboxykinase in C4 plants: its role and regulation. Aust J Plant Physiol. 1997;24:459–468. [Google Scholar]
- Walker RP, Chen Z-H, Johnson KE, Famiani F, Tecsi LI, Leegood RC. Using immunohistochemistry to study plant metabolism: the examples of its use in the localization of amino acids in plant tissues, and of phosphoenolpyruvate carboxykinase and its possible role in pH regulation. J Exp Bot. 2001;52:565–576. [PubMed] [Google Scholar]
- Walker RP, Chen Z-H, Acheson RM, Leegood RC. Effects of phosphorylation on phosphoenolpyruvate carboxykinase from the C4 plant, Guinea grass. Plant Physiol. 2002;128:165–172. [PMC free article] [PubMed] [Google Scholar]
- Walker RP, Leegood RC. Phosphorylation of phosphoenolpyruvate carboxykinase in plants: studies in plants with C4 photosynthesis and Crassulacean acid metabolism and in germinating seeds. Biochem J. 1996;317:653–658. doi: 10.1042/bj3170653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RP, Trevanion SJ, Leegood RC. Phosphoenolpyruvate carboxykinase from higher plants: purification from cucumber and evidence of rapid proteolytic cleavage in extracts from a range of plant tissues. Planta. 1995;195:58–63. [Google Scholar]
- Wingler A, Walker RP, Chen Z-H, Leegood RC. Phosphoenolpyruvate carboxykinase is involved in the decarboxylation of aspartate in the bundle-sheath of maize. Plant Physiol. 1999;120:539–545. doi: 10.1104/pp.120.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki Y, Asukagawa N, Ishikawa Y, Ohta E, Sakata M. Estimation of cytoplasmic free Mg2+ levels and phosphorylation potentials in mung bean root tips by in vivo 31P NMR spectroscopy. Plant Cell Physiol. 1988;29:919–924. [Google Scholar]
- Yeoh H-H, Badger MR, Watson L. Variation in Km(CO2) of ribulose-1,5-bisphosphate carboxylase among grasses. Plant Physiol. 1980;66:1110–1112. doi: 10.1104/pp.66.6.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh H-H, Badger MR, Watson L. Variations in kinetic properties of ribulose-1,5-bisphosphate carboxylases among plants. Plant Physiol. 1981;67:1151–1155. doi: 10.1104/pp.67.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]