Production of farnesol by Candida albicans is the first quorum-sensing system discovered in a eukaryote (29). In C. albicans, accumulated farnesol affects both dimorphism (29, 50) and biofilm formation (62). Fungal dimorphism is defined (64) as an environmentally controlled reversible interconversion of morphology, particularly yeast and mycelial morphologies. Interest in this shift derives from the dimorphic character of many fungi that are pathogenic toward plants and animals (64). Numerous chemical and environmental parameters can shift the yeast-mycelium dimorphism, including temperature, pH, glucose levels, nitrogen source, carbon dioxide levels, transition metals, chelating agents, and inoculum size or initial cell density (64). Of these, the inoculum size effect is probably the least well studied. For fungi such as Ceratocystis ulmi (28, 42) and C. albicans (29), cells develop as budding yeasts when inoculated at ≥106 cells per ml and as mycelia when inoculated at <106 cells per ml. We believe the inoculum size effect is a general phenomenon in dimorphic fungi (Table 1). In keeping with the precedent established by homoserine lactone-based signaling in gram-negative bacteria (22), the inoculum size effect in fungi is also called quorum sensing (29) and the extracellular cell density-dependent signals are called quorum-sensing molecules (QSMs). Thus, the chemical identity of the respective QSMs is of interest. Apart from C. ulmi (28) and C. albicans (29), it is a “leap of faith” on our part that the other cell density phenomena listed in Table 1 are mediated by QSMs.
TABLE 1.
Fungi reported to exhibit inoculum size effects
| Fungus | Reference(s) | Physiological change | Comment |
|---|---|---|---|
| Ceratocystis ulmi | 28, 42 | Yeast-mycelium dimorphism | Dutch elm disease |
| Candida albicans | 29, 57 | Yeast-mycelium dimorphism | Candidiasis |
| Histoplasma capsulatum | 88 | Yeast-mycelium dimorphism | Histoplasmosis |
| Mucor rouxii | 2, 18 | Yeast-mycelium dimorphism | Zygomycete |
| Aureobasidium pullulans | 63 | Yeast-mycelium dimorphism | Black yeast |
| Cladosporium werneckii | 25 | Yeast-mycelium dimorphism | |
| Penicillium isariaeforme | 53 | Light-induced coremia | Photomorphogenic |
| Trigonopsis variabilis | 70 | Yeast-triangular transition | Triangular cells |
| Dictyostelium discoideum | 13 | Germination efficiency | Cellular slime mold |
| Neurospora crassa | 68 | Galactosamine in cell wall | Model system |
| Neurospora crassa | 23 | Suppression of cot-1 phenotype | |
| Histoplasma capsulatum | 41 | α(1,3)-Glucan in cell wall | |
| Schizophyllum commune | 37 | Light-induced growth inhibition in dikaryons | Basidiomycete |
The QSM for Candida albicans is E,E-farnesol.
Inclusion of spent medium from C. albicans as part of the fresh growth medium reduces the percentage of mycelial cells (29). The active principle is lipophilic and can be extracted with many organic solvents. The active molecule was identified by gas chromatography-mass spectrometry as farnesol (1-hydroxy-3,7,11-trimethyl-2,6,10-dodecatriene; C15H26O; molecular weight, 222.37). Farnesol is a component of many perfumes, including Chanel No. 5, and its distinctive aroma was used initially in its purification from C. albicans (29). Farnesol can exist as four isomers, but only the E,E isomer has QSM activity (72). Farnesol prevents mycelial development in both growth morphology and differentiation assays. The differentiation assay can be varied by using three common chemical triggers for germ tube formation: l-proline, N-acetylglucosamine, and serum. In all cases, farnesol prevented the yeast-to-mycelium conversion, resulting in actively budding yeasts without otherwise altering cellular growth rates, even at concentrations up to 300 μM (29, 62). Our early work was done with C. albicans strain A72, a clinical isolate, and the supernatant from that strain was active on cells from strains MEN, LGH1095, 10261, SG3314, and SC5314 (at that time, this strain was mistakenly listed as SG5314) and vice versa (29). Farnesol is produced at a level of ∼0.13 mg/g (dry weight) by two laboratory strains (A72 and CAI-4) and four recent clinical isolates of C. albicans; only strain 10231 did not produce detectable farnesol (31).
Several technical precautions need to be taken when evaluating the farnesol concentrations needed: (i) only E,E-farnesol is active; (ii) farnesol oils and stock solutions should be stored under argon or nitrogen with desiccation; (iii) despite the long-term stability of E,E-farnesol in water (29), farnesol solutions in methanol are not as stable; and (iv) the composition of the growth medium is important (50). For any of five defined media, 1 to 2 μM farnesol sufficed to reduce germ tube formation to 50%. Inclusion of serum increased the amount of farnesol needed to block yeast-to-mycelium conversion in a dose-dependent manner. The concentrations needed were ∼10, 50, 150, and 250 μM farnesol with 2, 5, 10, and 20% serum, respectively (50). These increases probably are due to the nonspecific lipid binding ability of serum albumin. We emphasize these experimental considerations because conflicting observations regarding farnesol and its role in quorum sensing can be attributed to differences in experimental design.
Commitment.
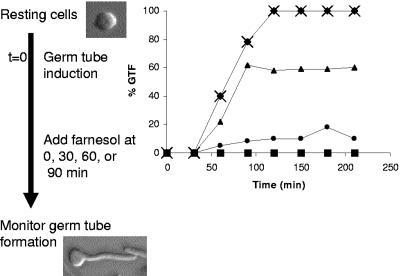
Although farnesol blocks the yeast-to-mycelium conversion (29, 72), it does not block the elongation of preexisting hyphae (50, 62). Thus, there is a limited time during which cells can respond to farnesol. Resting cells transferred to hypha-inducing conditions are sensitive to farnesol, and when the farnesol is added at time zero (Fig. 1), the cells do not differentiate into hyphae. However, the percentage of cells committed to hyphal growth increases with time under hypha-inducing conditions. By ∼60 min (Fig. 1), some cells are committed to hyphal growth and are therefore insensitive to farnesol, and by 90 min, almost all cells are committed to hyphal growth and thus are insensitive to farnesol (54). This insensitivity to farnesol is likely another manifestation of the commitment phenomenon (10, 49), in which, once visible germ tubes have appeared, a shift from hypha-inducing conditions to bud-inducing conditions no longer causes yeast formation, because the cells are no longer totipotent (10, 49). The onset of commitment in C. albicans is rather synchronous and easily studied (Fig. 1). However, commitment is not permanent (49, 54). Sooner or later, the cells return to being totipotent. In liquid culture this return usually happens during stationary phase (49), and at this point the cells should again become farnesol sensitive. The timing of this decommitment point remains poorly characterized, in part because it is probably asynchronous. The effect of commitment and decommitment on farnesol sensitivity will provide insight for interpreting the effects of farnesol on biofilm formation.
FIG. 1.
The commitment phenomenon. Resting cells transferred into hypha-inducing conditions are sensitive to farnesol at time zero; they do not differentiate into hyphae. As the time of farnesol addition is delayed, there is an increase in insensitivity to farnesol. Emergence of insensitivity corresponds with the appearance of germ tubes. Symbols in the graph stand for no farnesol added (×) or farnesol (30 μM) added at time zero (▪), 30 min (•), 60 min (▴), or 90 min (⧫). GTF, germ tube formation. The graph was originally published in reference 50.
Candida biofilms.
The formation of biofilms by C. albicans and their importance in infection are well documented (15). In particular, the formation of biofilms on medical equipment, e.g., catheters, is a serious concern in the progression of infections caused by C. albicans. This concern is due in part to the introduction of infective organisms and in part to the greater resistance to antifungal agents observed for Candida biofilms.
Biofilm formation has been examined by scanning electron microscopy and confocal laser scanning microscopy. The initial colonization by yeast cells is followed 3 to 6 h later by germ tube formation. The adhering yeast cells form a basal layer that firmly attaches the biofilm to the substrate, while subsequent germination generates the bulk of the biofilm. After 48 h, a mature biofilm typically contains yeasts, mycelia, and pseudomycelia (15). Mycelia-only mutants of C. albicans form a relatively loose attachment to the surface, whereas yeast-only mutants form only the basal layer, and therefore only a very thin biofilm (15). The presence of cells of multiple morphologies in biofilms suggests that farnesol has a role in regulating cellular morphology and therefore in the establishment of mature biofilms. Ramage et al. (62) found that the effect of farnesol on biofilm development was time dependent. Addition of 30 to 300 μM farnesol at time zero inhibited biofilm development, but addition 1 to 2 h later did not. That is, once hyphal formation had been initiated, it could no longer be inhibited by addition of farnesol (62). Interestingly, mature biofilms (24 h) once more became sensitive to farnesol (62). These results are consistent with the idea that developing biofilms go through commitment and decommitment just like planktonic cells in liquid culture (50). In this model, the release of yeast cells from mature biofilms would be triggered by the in situ accumulation of farnesol (62) some time after the decommitment point.
Another line of evidence for involvement of diffusible molecules, possibly farnesol, in Candida biofilms is that when medium flows across a developing biofilm, e.g., by gentle shaking, the overall size of the biofilm is significantly larger than if the liquid is static (15). Based on farnesol's effects on cellular morphology and biofilm development, we hypothesize that liquid flowing across a solid surface removes farnesol, leading to larger biofilms with more cells in the mycelial morphology. When the rate of flow decreases or when flow is absent, more farnesol accumulates and, after decommitment, causes new cells to develop with the yeast morphology. These yeast cells would diffuse away from the mature biofilm, with the capacity to start a new biofilm elsewhere.
Anaerobic Candida.
Very little research has been done on the anaerobic growth of C. albicans. This lack is surprising, since C. albicans infections can spread into the body from the anaerobic gastrointestinal tract (56). There is a defined liquid medium for the anaerobic growth of C. albicans (16) based on the Hungate technique for stringent anaerobes. A distinctive feature of anaerobic growth was that the cells neither produced nor responded to exogenous farnesol, even at concentrations as high as 1.2 mM (16). This difference in farnesol production between aerobically (31) and anaerobically (16) grown cells shows that farnesol synthesis is regulated. Additionally, anaerobic C. albicans cells are highly resistant to amphotericin B and four azole antifungals, and wild-type C. albicans cells grow exclusively as mycelia at all temperatures tested: 25, 30, and 37°C (16).
The distinctive physiology of anaerobically grown cells raises the question, “How anaerobic are the interiors of C. albicans biofilms?” (16). C. albicans cells are more resistant to fluconazole and other antifungal drugs when in biofilms than when they are planktonic (15, 61). Biofilm cells might be more resistant because they are growing anaerobically. The interiors of many bacterial biofilms are highly anaerobic, including those associated with tooth decay and periodontal disease (39). Anaerobically grown C. albicans does not produce biofilms on plastic or acrylic surfaces (4), but this observation does not preclude an aerobically formed biofilm from having an anaerobic interior. Finally, physiologically anaerobic cells would be excellent candidates for “persister” cells, which remain viable in biofilms following treatment with antimicrobial agents (15, 61).
Farnesol synthesis and the sterol pathway.
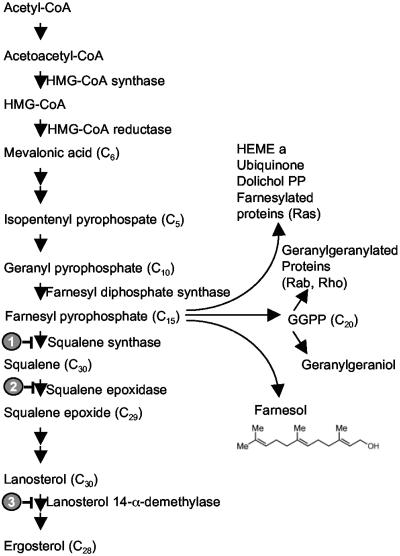
Farnesol is produced by an alternative pathway from the sterol biosynthetic intermediate farnesyl pyrophosphate (FPP). FPP is an important branch point in lipid metabolism (Fig. 2). Cell extracts of C. albicans contain an enzymatic activity that can convert [3H]FPP to [3H]farnesol (30). Saccharomyces cerevisiae contains two relevant pyrophosphate phosphatases, Lpp1p (81) and Dpp1p (80). Their C. albicans homologs are designated Dpp2 and Dpp3, respectively. Farnesol production is elevated in the presence of drugs that block sterol synthesis in fungi. For 0.5 to 1 μM zaragozic acid B, fluconazole, clotrimazole, ketoconazole, and miconazole, farnesol levels were increased 8-, 10-, 45-, 45-, and 44-fold, respectively (30, 31). For both zaragozic acid B (30) and fluconazole (31), there was a dose-dependent relationship between the drug concentration and the amount of farnesol produced. Zaragozic acid inhibits squalene synthase (3), while the azoles inhibit lanosterol 14α-demethylase, a key enzyme in ergosterol biosynthesis (Fig. 2). Production of ∼0.13 mg farnesol per g (dry weight) is equivalent to a concentration of 2 to 4 μM farnesol at a yeast cell density of 108/ml. We estimated the energetic cost of farnesol production through the respective carbon flows to farnesol and ergosterol. If the sterol content of C. albicans is 0.8% (dry weight) (56), then ∼1.6% of the FPP is directed to farnesol, with the remaining ≥98% going to sterol synthesis. This calculation ignores any additional pathways for carbon flow through the FPP branch point (Fig. 2).
FIG. 2.
Farnesyl pyrophosphate as a metabolic branch point in lipid metabolism. Circled and highlighted numbers 1, 2, and 3 indicate the enzyme targets for zaragozic acid, terbinafine, and fluconazole, respectively.
Industrial production of farnesol.
A series of patents have been issued for the microbial production of prenyl alcohols, e.g., farnesol and geranylgeraniol (52, 59, 82), for use in the chemical synthesis of vitamins and hormones. Two general procedures have been used to achieve farnesol overproduction by various microbes. In the first approach, genes in the early mevalonate pathway for FPP synthesis (Fig. 2) are overexpressed. For example, overexpression of HMG1 (which encodes HMG-coenzyme A [CoA] reductase) in S. cerevisiae increases farnesol production to 160 mg/liter. In a second approach, squalene synthase is repressed (48, 59) or inhibited (21, 52), allowing its substrate, FPP, to accumulate. One squalene synthase-deficient mutant produced 2.5 g/liter (11.3 mM) of farnesol in 10 days of fed-batch cultivation (48). With both approaches, prenyl alcohol production depends on the growth medium and fermentation conditions. Productive media included (i) high levels of carbohydrate (2 to 7%), (ii) 1 to 3% soybean oil, fish oil, olive oil, or almond oil, and (iii) 0.1 to 0.5% of a nonionic detergent such as NP-40 or Triton X-100 (52). The farnesol yield could be increased more than 1,000 times, to 250 mg/liter, by use of a squalene synthase inhibitor called SQAD (52) combined with an improved farnesol production medium.
The enzymology for farnesol production is common among fungi. One-third of the Ascomycetes tested had the potential to produce farnesol or geranylgeraniol (52). In the presence of SQAD (20 mg/liter), at least 47 fungal species, including 8 Candida species—Candida albicans, Candida utilis, Candida stellata, Candida solani, Candida intermedia, Candida krusei, Candida tenuis, and Candida zeylanoides—excrete farnesol (52). Having such a broad range of microorganisms with the potential for farnesol production suggests that farnesol excretion proceeds either via simple diffusion or by a very common transport system. In a rich medium (yeast-malt broth) supplemented with 5% glucose, 1% soybean oil, and SQAD (52), C. albicans excreted 500 μM farnesol. Soybean oil and nonionic detergents likely promote farnesol production by creating an external lipid sink. Farnesol has a maximum water solubility of only 1 to 1.2 mM (38), and an external lipid sink would shift the equilibrium toward production and excretion of farnesol. Why, then, do most wild-type strains of C. albicans excrete farnesol (29, 31), whereas most other fungi do not? Two testable explanations are that C. albicans might have a larger FPP pool size or that one of the pyrophosphatases in C. albicans (Dpp2 or Dpp3) might have a significantly lower Km for FPP. Resolution of this question awaits measurement of the FPP pool sizes (77) as well as the availability of purified FPP pyrophosphatases.
Farnesol and the fungal physiology of Candida albicans.
Farnesol was identified as a QSM by its ability to block the yeast-to-mycelium shift. But what else might it do? Two simple hypotheses have already been eliminated. The temperature dependence of polymorphism (64) is not correlated with discontinuities in farnesol production, because farnesol is produced aerobically from 15 to 42°C (29), although it remains possible that the subcellular localization of farnesol is altered at different growth temperatures. Also, farnesol production is not correlated with morphology. For two cases of strictly filamentous growth the farnesol production levels were quite opposite. During anaerobic growth the filamentous cells produced no detectable farnesol (16). In contrast, three obligately filamentous mutants (6-8) of C. albicans (tup1Δ/tup1Δ, nrg1Δ/nrg1Δ, and rbf1Δ/rbf1Δ mutants) produce 6 to 19 times more farnesol than do wild-type cells (D. Navarathna, A. L. Atkin, and K. W. Nickerson, unpublished data).
Three other effects of farnesol on C. albicans also have been reported. Each of these effects requires a much higher concentration of farnesol than that needed to block germ tube formation. First, Hog1, a stress-activated protein kinase, is rapidly phosphorylated after cells are treated with 100 μM farnesol (76). Second, there is a link between farnesol and the oxidative stress response in C. albicans (85). Conditioned medium from a stationary-phase culture protected yeast cells 8- to 10-fold from H2O2 and superoxide anion-generating agents. Extracellular farnesol is at least partially responsible for this protective effect (85). It is not known if or how farnesol is altered in the process of protecting the cells from oxidative stress. Finally, Jensen et al. (33) isolated 1,111 stable mutants of C. albicans with altered colony morphology, i.e., they had hairy or wrinkled colonies, usually associated with filamentous growth. Most (96%) of these mutants were farnesol remedial. That is, when grown on yeast-malt agar plates with 50 μM farnesol, the mutants partially or completely reverted to wild-type colony morphology (33). The observation that farnesol-remedial mutants are so common (96%) relative to mutants that fail to respond to farnesol (4%) suggests that farnesol activates/induces a pathway that can override many of the morphogenesis defects in these mutants (33).
Farnesol and other fungi.
Reports of farnesol production or response by other fungi are rare. The S. cerevisiae erg20 mutant is defective in FPP synthase (11) and excretes five prenyl alcohols (isopentenol, dimethylallyl alcohol, linalool, geraniol, and farnesol) at a combined concentration of 12.7 mg/liter of culture. Of these, farnesol was the least common (0.18 mg/liter). The degradation of HMG-CoA reductase, the rate-limiting enzyme of sterol synthesis (Fig. 3), also is regulated by farnesol in both S. cerevisiae (73, 74) and Chinese hamster ovary (47) cells. Low levels of farnesol are produced by many yeasts used for wine making as a volatile flavor compound (20).
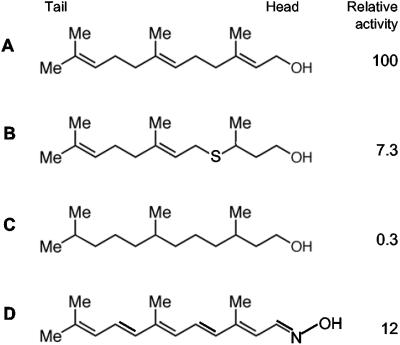
FIG. 3.
Structures of three farnesol analogs. (A) E,E-farnesol; (B) the 2,3-hydrogenated 4-thia analog (72); (C) the fully hydrogenated analog (72); (D) the fluorescent oxime (71). Relative activity refers to QSM activity, i.e., the ability to block N-acetylglucosamine-induced germ tube formation (29).
In Neurospora crassa, farnesol has a role in the circadian rhythm clock that governs conidiation (24). Mutations in three different genes led to the loss of the circadian rhythm, but the wild-type rhythms could be restored by 10 to 100 μM exogenous farnesol or geraniol. Also in N. crassa, 40 to 70 μM exogenous farnesol restored the wild-type phenotype to cot-1 mutants (O. Yarden, personal communication), which have abnormal polar extension and branching patterns when grown at restrictive temperatures (23). The cot-1 gene function is linked to environmental stress response signaling (23). This response also is cell density dependent, as cot-1 mutants have near-wild-type morphology even at restrictive temperatures when the growth medium is supplemented with spent medium from high-cell-density cultures. Although farnesol is effective in suppressing the cot-1 phenotype, there is no evidence that N. crassa actually makes farnesol or that farnesol is a natural QSM for N. crassa. The natural QSM for Neurospora remains to be identified. Also, the suggestion we made above with regard to our own data on farnesol-remedial mutants of C. albicans (33), i.e., that farnesol activates/induces a pathway that can override many of the morphogenesis defects in C. albicans altered-colony mutants, applies equally well to farnesol's ability to restore wild-type phenotypes to cot-1 (23) and circadian rhythm (24) mutants of N. crassa. The identity of such a pathway would be of considerable interest.
Schizophyllum commune is the only basidiomycete listed in Table 1. Klein et al. (37) described an asymmetric pattern of dikaryotic growth, which suggested the presence of a light-stimulated autoinhibitor of growth. This inhibitor may be schizostatin, a close relative of farnesol. Schizostatin, a C20 trans-1,3-dicarboxylic acid of geranylgeranioic acid, is a novel squalene synthase inhibitor produced by S. commune (79). As a squalene synthase inhibitor, schizostatin could cause the observed periodic growth inhibition itself or via the intracellular accumulation of farnesol.
Antagonism between fungi.
Fungal antagonisms have been documented for at least 65 years (84), and farnesol may play a role in some of these interactions. For example, in Aspergillus nidulans, 100 μM farnesol triggers apoptosis (69), even though A. nidulans does not produce detectable extracellular farnesol (69). The fungus is presumably responding to farnesol produced by other fungal species. In coculture of A. nidulans with C. albicans, the number of A. nidulans CFU/ml was reduced ≥180-fold within 24 h. This inhibitory interaction was eliminated if the coculture contained 1% bovine serum albumin (69). The dermatophyte Trichophyton rubrum also does not grow when it is cocultivated with C. albicans or if it is grown on filtered spent medium from C. albicans (34). Thus, C. albicans is secreting a metabolic product with fungistatic action. One hypothesis is that C. albicans uses farnesol to eliminate fungal competitors within a mammalian host environment. This hypothesis provides a potential explanation as to why pure cultures of C. albicans generally are isolated from clinical lesions (43). How does C. albicans tolerate relatively high levels of farnesol (≥300 μM [29, 62]) that are fungistatic (46) or fungicidal for other fungi (69)? This question becomes even more interesting in light of the fact that some S. cerevisiae strains can be genetically modified to produce high levels of farnesol (11, 48, 52).
The 3-oxo-C12 homoserine lactone QSM from the bacterium Pseudomonas aeruginosa has enough structural similarity to farnesol that at high concentrations (200 μM) it can mimic farnesol's action and prevent the yeast-to-mycelium shift by C. albicans (27). Interestingly, the signal cross talk between C. albicans and P. aeruginosa goes both ways, as evidenced by the fact that 10 to 25 μM farnesol lowers the production of four homoserine lactones (C6 to C12) by P. aeruginosa by ≥10-fold each, which in turn reduces pyocyanin production by those P. aeruginosa cells (J. Robinson, personal communication).
Farnesol analogs.
One strategy for studying farnesol receptors is to develop farnesol analogs. Shchepin et al. (72) examined 2 natural and 38 synthetic farnesol analogs (Fig. 3) and found that 22 of the 40 analogs had QSM activity as measured by their ability to reduce germ tube formation by 50%. Even the most active of the analogs tested (Fig. 3B), however, had only 7.3% of the activity of E,E-farnesol. Farnesoic acid, which also is reported to be a QSM for C. albicans (58), had only 3.2% of the activity of E,E-farnesol (72). Note, however that C. albicans strain 10231 was used in the study in which farnesoic acid was identified as a QSM. This strain of C. albicans is the only strain of this yeast that we know of that does not produce farnesol (31).
QSM activity is quite sensitive to structural changes. The 10,11-epoxide is 58-fold less active than E,E-farnesol, while the 2,3-epoxide is 260-fold less active. Air oxidation causes epoxide formation, so these dramatic decreases in QSM activity for the epoxides are consistent with the susceptibility of farnesol to air oxidation. Similarly, the 3-methyl side chain is important; in the 4-thia series, removal of the 3-methyl group reduced activity 5-fold, while replacement of the 3-methyl with the bulkier 3-ethyl reduced activity 16-fold (72). Based on these structure-activity relationships, a direct effect of farnesol on membrane fluidity can be ruled out and the existence of a highly specific farnesol receptor inferred.
Fluorescent farnesols.
The structural constraints identified in the analog studies means that simply attaching a fluorescent chromophore to farnesol is unlikely to be effective. Instead, we designed an analog with two additional double bonds in the farnesol backbone, making five conjugated double bonds while preserving the approximate length, cross section, and hydrophobicity of farnesol (71). This fluorescent farnesol had an excitation maximum of 360 nm and an emission maximum of 465 nm. The corresponding oxime (Fig. 3D) with six conjugated double bonds has an excitation maximum of 382 nm and an emission maximum of 530 nm (71). The oxime avoids the autofluorescence common in many fungi and extends the excitation range beyond 400 nm, an important feature for confocal microscopy. These designs parallel those for the study of parinaric acid, a naturally fluorescent fatty acid with four conjugated double bonds (75). Both the fluorescent farnesol and its oxime had stronger QSM activity (71) than any of the other analogs we examined (72). The existence of a farnesol binding protein/receptor (72) as well as its location can be studied with fluorescent farnesol analogs (71).
Pathogenicity and therapeutic potential.
Cell density regulation of morphology in the dimorphic fungi is important because many of these fungi are plant or animal pathogens (Table 1), and the ability to switch from one cellular morphology to another is associated with pathogenicity. This hypothesis is consistent with the roles of each morphological type during an infection. Yeast cells usually are involved in the initial infection and in dispersal or dissemination through the vascular system, whether in an animal or in a plant. On the other hand, mycelia are invasive forms that can penetrate host tissues. Morphology changes are accompanied by changes in cell surface antigens that enable the pathogen to effectively elude the host immune response (65). Dermatophytic fungi may be limited because they cannot switch morphologies under host physiological conditions and therefore cannot cause deep-seated infections (67). The correlation between dimorphism and pathogenicity suggests that interfering with the morphological switch could be an attractive method for controlling pathogenicity. Manipulating dimorphism could force an opportunistic pathogen to exist only in a form that should not damage the host. Many studies of farnesol production and localization have been done exclusively in vitro (29-31, 55, 62, 85). Since fungal membranes appear to serve as a sink for farnesol, we expect that host tissues would behave similarly and that farnesol dynamics in vivo would differ accordingly. The effect of host tissues on Candida's response to farnesol remains to be evaluated.
An early hypothesis was that in a mouse model of candidiasis, exogenous farnesol would block hyphal growth and thus prevent infection and mortality in the subjects. However, mice treated with farnesol (intraperitoneally, intravenously, or orally) died significantly faster than did untreated mice (D. Navarathna and K. W. Nickerson, unpublished data). Mice also were challenged with C. albicans that had been treated with subinhibitory doses of fluconazole to increase farnesol production (31). The extracellular, membrane bound, and intracellular farnesol concentrations of C. albicans cells pretreated with 1.0 μM fluconazole were 12, 2, and 6 times those of untreated cells, respectively (55). Mice administered C. albicans pretreated with 0.5 to 1.0 μM fluconazole died 2.5 days earlier and had four-times-higher mortality rates than did mice given untreated C. albicans. Thus, the fluconazole-pretreated cells were 4.2 to 8.5 times more lethal (P < 0.001) than untreated cells (55). One, but certainly not the only, explanation for this enhanced pathogenicity is that excreted farnesol acts as a QSM in vitro but as a virulence factor in vivo when a virulence factor is defined as something produced by the organism to enhance pathogenicity (1). Farnesol is not an innocuous molecule and may influence mammalian cells in several ways, including blocking calcium channels, triggering apoptosis, targeting HMG-CoA reductase (Fig. 2) for degradation, and stimulating cell differentiation (17).
Our studies have focused strictly on intravenously inoculated C. albicans cells. Maintaining these cells as yeasts after they enter the circulatory system should aid their dispersal, making treatment with farnesol counterproductive (55). However, gastrointestinal challenge with C. albicans accompanied by farnesol administration might be different, because this route of exposure requires the dimorphic lifestyle for entry. Use of farnesol as a preventative therapeutic in this context is still being analyzed.
Farnesol analogs might also be of interest as antagonists. Some synthetic farnesol analogs can competitively inhibit the QSM activity of farnesol (J. M. Hornby and K. W. Nickerson, unpublished data). For example, the QSM activity of farnesol was reduced threefold by the simultaneous presence of equimolar hexahydro (saturated) farnesol (Fig. 3C). Similar levels of QSM antagonism also were found for alternative stereoisomers of farnesol. The biologically active (E,E)-farnesol is inhibited to a small extent by (Z,Z)-farnesol. The next step is to analyze C. albicans pathogenesis in a mouse model with and without the addition of these competitive farnesol analogs.
The effect of farnesol on morphogenic signaling pathways.
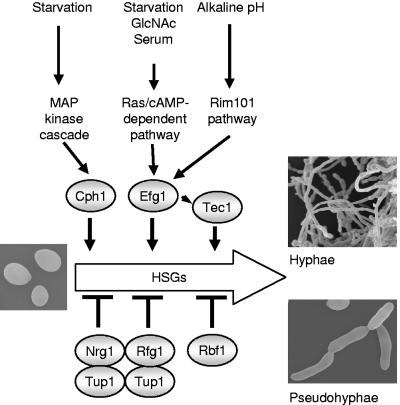
The ability of C. albicans to change morphological forms from unicellular budding yeasts to hyphae and pseudohyphae is controlled largely by changes in transcription (83) and can be induced by various environmental conditions. These environmental conditions are transduced into a change in morphology via a network of signal transduction pathways whose activity is ultimately coordinated by transcription regulators (Fig. 4) (reviewed in references 14, 45, 78, and 86). Components of the CEK1 mitogen-activated protein kinase pathway, the Ras/cyclic AMP-dependent pathway, the calcium signaling pathway, the Rim101-independent pathway, and two-component signal transduction pathways all have been implicated in filamentation. These pathways are to some degree specialized, since they respond to different environmental inducers. Farnesol blocks filament development induced by environmental signals for most, if not all, of the signaling pathways that activate filament development. Thus, farnesol could act to specifically block each of the morphogenic signaling pathways or could act at a common control point in morphogenesis.
FIG. 4.
Abbreviated scheme showing positive and negative regulators of hypha-specific gene (HSG) expression.
There are at least two negative regulators of the yeast-to-hypha and yeast-to-pseudohypha morphological changes in C. albicans: the Tup1-containing Tup1/Rfg1 and Tup1/Nrg1 complexes and Rbf1. The Tup1/Rfg1 and Tup1/Nrg1 complexes are transcription repressors. Activation of these complexes represses filament-specific gene expression (5, 7, 35, 51). In the absence of TUP1, RFG1, or NRG1, C. albicans CAI-4 cells are filamentous, without yeast-to-hypha induction (6, 8, 35, 51). The role of Rbf1 (RPG-box-binding factor) is less clear. This DNA-binding protein binds to the S. cerevisiae RPG box in vitro and the telomeric repeat sequence of C. albicans (32). Repression of filament-specific gene expression is a common control point in morphogenesis (78, 86).
Farnesol increases expression of TUP1 but not that of NRG1 or RFG1 (9). In the presence of farnesol, TUP1 mRNA levels doubled within 40 min of N-acetylglucosamine stimulation of germ tube formation (B. Kebaara, A. L. Atkin, and K. W. Nickerson, unpublished data). This increase coincides with the time by which cells normally become committed to filament formation and beyond which exogenous farnesol no longer blocks germ tube formation (50). Farnesol also prevents induction of the Tup1-regulated, filament-specific genes HWP1, RBT1, CPH1, and HST7 (19, 62, 66). Cph1 and Hst7 are essential components in the CEK1 mitogen-activated protein kinase pathway, one of the signal transduction pathways that regulate morphogenesis, and Cph1 is a transcription activator of filament-specific genes. Thus, the down-regulation of CPH1, HST7, and GAP1 (a gene regulated by Cph1) observed by Sato et al. (66) is consistent with a secondary effect of farnesol on TUP1.
A two-component signal transduction pathway also may be involved in response to farnesol. Candida has three histidine kinases. Mutants lacking these kinases either do not produce hyphae (chk1-null mutants) or produce only a limited number of hyphae at the peripheries of colonies (nik1- and sln1-null mutants) on serum agar (87). However, the same null mutants form hyphae on M199 (pH 7.5) at 37°C. The addition of farnesol blocks hyphal formation by wild-type cells and by nik1- and sln1-null mutants but not by chk1-null mutants (40). Thus, Chk1 is important for inhibition of hyphal development by farnesol. It is not known if Chk1 is directly involved in the response to farnesol or if it is a negative regulator of a hyphal developmental pathway that is unresponsive to farnesol. CHK1 also is regulated by Tup1 (36).
Effect of farnesol on global gene expression.
The effect of farnesol on global gene expression during resumption of growth following stationary phase (19) and in developing biofilms (9) has been examined. Consistent with the observation that farnesol affects expression of Tup1 and Cph1, the presence of farnesol influenced gene expression. Farnesol had a similar effect on only 26 genes, but in both studies there were changes in equivalent functional categories including iron transport, cell wall synthesis, drug resistance, and progression through the cell cycle. The differences in specific gene expression patterns reported from different laboratories probably reflect differences in growth conditions and in the time when cells were collected for RNA extraction.
Enjalbert and Whiteway (19) analyzed the transcriptional reprogramming caused by the resumption of growth in the presence and absence of farnesol. Their strategy allowed them to distinguish gene expression changes due to farnesol-induced morphological differences, e.g., differences in cell adhesion, cell wall formation, chromatin, DNA replication, and the cell cycle, from those due to the continued presence of farnesol, e.g., drug response and fatty acid oxidation. These data (19) must be analyzed with some caution, however, since the presence of farnesol did not completely suppress hyphal formation under the conditions used.
Cao et al. (9) allowed the cells to adhere, then added farnesol, and collected cells 24 h after farnesol addition for RNA and microarray analysis. Twenty-four hours is the time required to form a mature biofilm in the absence of farnesol. There are three caveats regarding the interpretation of the Cao et al. (9) data. First, mRNA was measured at only a single time point, 24 h after the addition of farnesol. Second, cells that have adhered are a mixed population of yeasts, hyphae, and pseudohyphae. Finally, the level of farnesol used was ∼40-fold higher than that required for a response by the responsive cells, and at high levels, lipophilic molecules such as farnesol can trigger nonspecific changes. These points are significant because Cao et al. (9) detected only stable long-term changes in gene expression from a mixed population of cells, some of which are insensitive to farnesol. Additional microarray studies are needed to examine the effect of farnesol on transcription at the time of morphological commitment, using minimum farnesol concentrations on cultures that are ∼100% responsive to farnesol.
Other QSMs from C. albicans.
The levels of E,E-farnesol made by C. albicans (31) can account for all of the QSM activity present in C. albicans cell-free supernatants (72). However, these corresponding numbers do not prove that farnesol is the only QSM made. More than 20 years ago, Hazen and Cutler (26) described a morphogenic autoregulatory substance (MARS) isolated from C. albicans. Both farnesol and MARS are extracellular molecules that suppress the yeast-to-hypha transition. However, MARS differs from farnesol in that it has a UV maximum at 270 nm, has a nitrogen-containing ring system, reacts with ninhydrin to form a yellow color, must be bioassayed within 2 days, is inactivated at pHs of >9.0 or <4.5, and has no aroma. The chemical structure of MARS has not yet been identified.
Oh et al. (58) identified E,E-farnesoic acid as the QSM for C. albicans, but we found that farnesoic acid has only 3.3% of the QSM activity of farnesol (72). Significantly, Oh et al. (58) used C. albicans strain 10231, which is the only strain of C. albicans known not to produce farnesol (31). Chen et al. (12) identified tyrosol as a QSM produced by C. albicans strain SC5314. They provided evidence that this molecule stimulated the yeast-to-hypha conversion rather than inhibiting it as farnesol does. However, the exact function of tyrosol remains unclear. When farnesol and tyrosol are present in direct competition, tyrosol does not alter the QSM activity of farnesol, even when the tyrosol is present at a 16-fold molar excess (S. Ghosh and K. W. Nickerson, unpublished data). Similarly, tyrosol did not contribute to the protection from oxidative stress provided by stationary-phase supernatants of C. albicans (85) or to the stimulation by C. albicans of homoserine lactone production by P. aeruginosa (J. Robinson, personal communication). C. albicans also has been reported to secrete phenethyl alcohol and tryptophol (44), and presumably the three aromatic alcohols have a common biosynthetic origin. A pathway whereby the respective aromatic amino acids undergo transamination followed by decarboxylation seems likely.
Final thoughts.
Farnesol's action as a QSM for C. albicans appears to be highly specific. Farnesol does not affect the morphology of either C. ulmi or Penicillium isariaeforme (28), and it is not the cell density modulator (41) of α(1,3)-glucan synthesis in Histoplasma capsulatum (W. E. Goldman, personal communication). However, the mechanism for this specificity is not yet clear. It could be resolved by identifying the farnesol binding proteins from C. albicans along with their cellular localization.
Are the many effects of farnesol on C. albicans manifestations of one or more underlying phenomena? We think that blockage of the yeast-to-mycelium shift at 1 to 2 μM farnesol (50, 72) is quite distinct from the reversible shift in colony morphology at 50 to 100 μM farnesol (33) and from other physiological changes requiring ≥100 μM farnesol (23, 24, 76, 85). Additionally, it is not clear how C. albicans balances using farnesol as a QSM to regulate its own morphology (29), as a potential virulence factor during pathogenesis (55), and as a trigger for apoptosis in other fungi (69). In particular, how does C. albicans tolerate high levels of farnesol and avoid apoptosis when it is itself susceptible to apoptosis triggered by environmental stresses (60)? Based on microarray analysis (9), C. albicans perceives farnesol as an environmental stress, but somehow that stress signal is either sidetracked or sidestepped. The numerous and complex effects of farnesol on C. albicans suggest that much remains to be done to understand the multitude of changes this molecule can induce in this very important yeast.
Acknowledgments
We thank Judy Berman, Raluca Dumitru, Steve Harris, Ellen Jensen, Bessie Kebaara, Masayoshi Muramatsu (Toyota Research), Dhammika Navarathna, Daniel Nickerson, Camile Semighini, and Malcolm Whiteway for helpful discussions and Bill Goldman, Jayne Robinson, and Oded Yarden for allowing us to cite their unpublished data.
REFERENCES
- 1.Alonso-Monge, R., F. Navarro-Garcia, E. Roman, B. Eisman, C. Nombela, and J. Pla. 2003. Strategies for the identification of virulence determinants in human pathogenic fungi. Curr. Genet. 42:301-312. [DOI] [PubMed] [Google Scholar]
- 2.Bartnicki-Garcia, S., and W. J. Nickerson. 1962. Nutrition, growth, and morphogenesis of Mucor rouxii. J. Bacteriol. 84:841-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergstrom, J. D., C. Dufresne, G. F. Bills, M. Nallin-Omstead, and K. Byrne. 1995. Discovery, biosynthesis, and mechanism of action of the zaragozic acids: potent inhibitors of squalene synthase. Annu. Rev. Microbiol. 49:607-639. [DOI] [PubMed] [Google Scholar]
- 4.Biswas, S. K., and W. L. Chaffin. 2005. Anaerobic growth of Candida albicans does not support biofilm under similar conditions used for aerobic biofilm. Curr. Microbiol. 51:100-104. [DOI] [PubMed] [Google Scholar]
- 5.Braun, B. R., W. S. Head, M. X. Wang, and A. D. Johnson. 2000. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 156:31-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, B. R., and A. D. Johnson. 1997. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science 277:105-109. [DOI] [PubMed] [Google Scholar]
- 7.Braun, B. R., and A. D. Johnson. 2000. TUP1, CPH1, and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 155:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, B. R., D. Kadosh, and A. D. Johnson. 2001. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J. 20:4753-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao, Y.-Y., Y.-B. Cao, Z. Xu, K. Ying, Y. Li, Y. Xie, Z.-Y. Zhu, W.-S. Chen, and Y. Y. Jiang. 2005. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol. Antimicrob. Agents Chemother. 49:584-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaffin, W. L., and D. E. Wheeler. 1981. Morphological commitment in Candida albicans. Can. J. Microbiol. 27:131-137. [DOI] [PubMed] [Google Scholar]
- 11.Chambon, C., V. Ladeveze, A. Oulmouden, M. Servouse, and F. Karst. 1990. Isolation and properties of yeast mutants affected in farnesol diphosphate synthetase. Curr. Genet. 18:41-46. [DOI] [PubMed] [Google Scholar]
- 12.Chen, H., M. Fujita, Q. Feng, J. Clardy, and G. R. Fink. 2004. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 101:5048-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlberg, K. B., and D. A. Cotter. 1978. Autoactivation of spore germination in mutant and wild type strains of Dictyostelium discoideum. Microbios 23:153-166. [PubMed] [Google Scholar]
- 14.Dhillon, N. K., S. Sharma, and G. K. Khuller. 2003. Signaling through protein kinases and transcriptional regulators in Candida albicans. Crit. Rev. Microbiol. 29:259-275. [DOI] [PubMed] [Google Scholar]
- 15.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 16.Dumitru, R., J. M. Hornby, and K. W. Nickerson. 2004. Defined anaerobic growth medium for studying Candida albicans: basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 48:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards, P. A., and J. Ericsson. 1999. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu. Rev. Biochem. 68:157-185. [DOI] [PubMed] [Google Scholar]
- 18.Elmer, G. W., and W. J. Nickerson. 1970. Filamentous growth of Mucor rouxii under nitrogen. J. Bacteriol. 101:592-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enjalbert, B., and M. Whiteway. 2005. Release from quorum-sensing molecules triggers hyphal formation during Candida albicans resumption of growth. Eukaryot. Cell 4:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fagan, G. L., R. E. Kepner, and A. D. Webb. 1981. Production of linalool, cis- and trans-nerolidol, and trans,trans-farnesol by Saccharomyces fermentati growing as a film on simulated wine. Vitis 20:36-42. [Google Scholar]
- 21.Flint, O. P., B. A. Masters, R. E. Gregg, and S. K. Durham. 1997. Inhibition of cholesterol synthesis by squalene synthase inhibitors does not induce myotoxicity in vitro. Toxicol. Appl. Pharmacol. 145:91-98. [DOI] [PubMed] [Google Scholar]
- 22.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the luxR-luxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorovits, R., and O. Yarden. 2003. Environmental suppression of Neurospora crassa cot-1 hyperbranching: a link between COT1 kinase and stress sensing. Eukaryot. Cell 2:699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granshaw, T., M. Tsukamoto, and S. Brody. 2003. Circadian rhythms in Neurospora crassa: farnesol or geraniol allow expression of rhythmicity in the otherwise arrhythmic strains Frq10, wc-1, and wc-2. J. Biol. Rhythms 18:287-296. [DOI] [PubMed] [Google Scholar]
- 25.Hardcastle, R. V., and P. J. Szaniszlo. 1974. Characterization of dimorphism in Cladosporium werneckii. J. Bacteriol. 119:294-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazen, K. C., and J. E. Cutler. 1983. Isolation and purification of morphogenic autoregulatory substance produced by Candida albicans. J. Biochem. 94:777-783. [DOI] [PubMed] [Google Scholar]
- 27.Hogan, D. A., A. Vik, and R. Kolter. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212-1223. [DOI] [PubMed] [Google Scholar]
- 28.Hornby, J. M., S. M. Jacobitz, D. J. McNeel, E. C. Jensen, D. S. Treves, and K. W. Nickerson. 2004. The inoculum size effect in dimorphic fungi: extracellular control of yeast mycelial dimorphism in Ceratocystis ulmi. Appl. Environ. Microbiol. 70:1356-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornby, J. M., B. W. Kebaara, and K. W. Nickerson. 2003. Farnesol biosynthesis in Candida albicans: cellular responses to sterol inhibition by zaragozic acid B. Antimicrob. Agents Chemother. 47:2366-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornby, J. M., and K. W. Nickerson. 2004. Enhanced production of farnesol by Candida albicans treated with four azole antibiotics. Antimicrob. Agents Chemother. 48:2305-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishii, N., M. Yamamoto, H.-W. Lahm, S. Iizumi, F. Yoshihara, N. Nakayama, M. Arisawa, and Y. Aoki. 1997. A DNA-binding protein from Candida albicans that binds to the RPG box of Saccharomyces cerevisiae and the telomeric repeat sequence of C. albicans. Microbiology 143:417-427. [DOI] [PubMed] [Google Scholar]
- 33.Jensen, E. C., J. M. Hornby, N. E. Pagliaccetti, C. E. Wolter, K. W. Nickerson, and A. L. Atkin. 2006. Farnesol restores wild-type colony morphology to 96% of Candida albicans colony morphology variants recovered following treatment with mutagens. Genome 49:346-353. [DOI] [PubMed]
- 34.Jillson, O. F., and W. J. Nickerson. 1948. Mutual antagonism between pathogenic fungi. Inhibition of dimorphism in Candida albicans. Mycologia 40:369-385. [PubMed] [Google Scholar]
- 35.Kadosh, D., and A. D. Johnson. 2001. Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans. Mol. Cell. Biol. 21:2496-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadosh, D., and A. D. Johnson. 2005. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: a genome-wide analysis. Mol. Biol. Cell 16:2903-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein, K. K., J. Landry, T. Friesen, and T. Larimer. 1997. Kinetics of asymmetric mycelial growth and control by dikaryosis and light in Schizophyllum commune. Mycologia 89:916-923. [Google Scholar]
- 38.Knobloch, K., A. Pauli, B. Iberl, N. Weis, and H. Weigand. 1988. Mode of action of essential oil components on whole cells of bacteria and fungi in plate tests, p. 287-299. In P. Schreier (ed.), Bioflavour '87. Walter de Gruyter, Berlin, Germany.
- 39.Kolenbrander, P. E., and J. London. 1993. Adhere today, here tomorrow: oral bacterial adherence. J. Bacteriol. 175:3247-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruppa, M., B. P. Krom, N. Chauhan, A. V. Bambach, R. L. Cihlar, and R. A. Calderone. 2004. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot. Cell 3:1062-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kügler, S., T. S. Sebghati, L. G. Eissenberg, and W. E. Goldman. 2000. Phenotypic variation and intracellular parasitism by Histoplasma capsulatum. Proc. Natl. Acad. Sci. USA 97:8794-8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulkarni, R. K., and K. W. Nickerson. 1981. Nutritional control of dimorphism in Ceratocystis ulmi. Exp. Mycol. 5:148-154. [Google Scholar]
- 43.Lewis, G. M., and M. E. Hooper. 1943. Concurrent, combined, and consecutive fungous infections of the skin. Arch. Dermat. Syph. 47:27-35. [Google Scholar]
- 44.Lingappa, B. T., M. Prasad, Y. Lingappa, D. F. Hunt, and K. Biemann. 1969. Phenethyl alcohol and tryptophol: autoantibiotics produced by the fungus Candida albicans. Science 163:192-194. [DOI] [PubMed] [Google Scholar]
- 45.Liu, H. 2001. Transcriptional control of dimorphism in Candida albicans. Curr. Opin. Microbiol. 4:728-735. [DOI] [PubMed] [Google Scholar]
- 46.Machida, K., T. Tanaka, K. Fujita, and M. Taniguchi. 1998. Farnesol-induced generation of reactive oxygen species via indirect inhibition of the mitochondrial electron transport chain in the yeast Saccharomyces cerevisiae. J. Bacteriol. 180:4460-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meigs, T. E., and R. D. Simoni. 1997. Farnesol as a regulator of HMG-CoA reductase degradation: characterization and role of farnesol pyrophosphatase. Arch. Biochem. Biophys. 345:1-9. [DOI] [PubMed] [Google Scholar]
- 48.Millis, J. R., G. G. Saucy, J. Maurina-Brunker, and T. W. McMullin. January2000. Vitamin production by fermentative biosynthesis of intermediates using genetically engineered microorganisms followed by chemical synthesis. Worldwide patent WO 2,000,001,650.
- 49.Mitchell, L. H., and D. R. Soll. 1979. Commitment to germ tube or bud formation during release from stationary phase in Candida albicans. Exp. Cell Res. 120:167-179. [DOI] [PubMed] [Google Scholar]
- 50.Mosel, D. D., R. Dumitru, J. M. Hornby, A. L. Atkin, and K. W. Nickerson. 2005. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl. Environ. Microbiol. 71:4938-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murad, A. M. A., P. Leng, M. Straffon, J. Wishart, S. Macaskill, D. MacCallum, N. Schnell, D. Talibi, D. Marechal, F. Tekaia, C. d'Enfert, C. Gaillardin, F. C. Odds, and A. J. P. Brown. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 2:4742-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muramatsu, M., S. Obata, and S. Shimizu. July2002. Microorganisms for production of geranylgeraniol and analogous compounds. European patent EP 1,219,714.
- 53.Muthukumar, G., E. C. Jensen, A. W. Nickerson, M. K. Eckles, and K. W. Nickerson. 1991. Photomorphogenesis in Penicillium isariaeforme: exogenous calcium substitutes for light. Photochem. Photobiol. 53:287-291. [Google Scholar]
- 54.Muthukumar, G., and K. W. Nickerson. 1985. Ca(II)-calmodulin regulation of morphological commitment in Ceratocystis ulmi. FEMS Microbiol. Lett. 27:199-202. [Google Scholar]
- 55.Navarathna, D. H. M. L. P., J. M. Hornby, N. Hoerrmann, A. M. Parkhurst, G. E. Duhamel, and K. W. Nickerson. 2005. Enhanced pathogenicity of Candida albicans pre-treated with subinhibitory concentrations of fluconazole in a mouse model of disseminated candidiasis. J. Antimicrob. Chemother. 56:1156-1159. [DOI] [PubMed] [Google Scholar]
- 56.Odds, F. C. 1988. Candida and candidosis. Baillière Tindall, London, United Kingdom.
- 57.Odds, F. C., C. A. Hall, and A. B. Abbott. 1978. Peptones and mycological reproducibility. Sabouraudia 16:237-246. [DOI] [PubMed] [Google Scholar]
- 58.Oh, K.-B., H. Miyazawa, T. Naito, and H. Matsuoka. 2001. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. Proc. Natl. Acad. Sci. USA 98:4664-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohto, C., and S. Obata. July2002. Repression of expression of squalene synthase in Saccharomyces cerevisiae to increase the efficiency of production of prenyl alcohol. Worldwide patent WO 2,002,053,747.
- 60.Phillips, A. J., I. Sudbery, and M. Ramsdale. 2003. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. USA 100:14327-14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramage, G., S. P. Saville, D. P. Thomas, and J. L. Lopez-Ribot. 2005. Candida biofilms: an update. Eukaryot. Cell. 4:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramage, G., S. P. Saville, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramos, S., and I. G. Acha. 1975. A vegetative cycle of Pullularia pullulans. Trans. Br. Mycol. Soc. 64:129-135. [Google Scholar]
- 64.Romano, A. 1966. Dimorphism, p. 181-209. In G. C. Ainsworth and A. S. Sussman (ed.), The Fungi, vol. 2. Academic Press, New York, N.Y. [Google Scholar]
- 65.Rooney, P. J., and B. S. Klein. 2002. Linking fungal morphogenesis with virulence. Cell. Microbiol. 4:127-137. [DOI] [PubMed] [Google Scholar]
- 66.Sato, T., T. Watanabe, T. Mikami, and T. Matsumoto. 2004. Farnesol, a morphogenetic autoregulatory substance in the dimorphic fungus Candida albicans, inhibits hyphae growth through suppression of a mitogen-activated protein kinase cascade. Biol. Pharm. Bull. 27:751-752. [DOI] [PubMed] [Google Scholar]
- 67.Scherr, G. H., and R. H. Weaver. 1953. The dimorphism phenomenon on yeasts. Bacteriol. Rev. 17:51-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmit, J. C., C. M. Edson, and S. Brody. 1975. Changes in glucosamine and galactosamine levels during conidial germination in Neurospora crassa. J. Bacteriol. 122:1062-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Semighini, C. P., J. M. Hornby, R. Dumitru, K. W. Nickerson, and S. D. Harris. 2006. Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol. Microbiol. 59:753-764. [DOI] [PubMed] [Google Scholar]
- 70.Sentheshanmuganathan, S., and W. J. Nickerson. 1962. Nutritional control of cellular form in Trigonopsis variabilis. J. Gen. Microbiol. 27:437-449. [DOI] [PubMed] [Google Scholar]
- 71.Shchepin, R., R. Dumitru, K. W. Nickerson, M. Lund, and P. H. Dussault. 2005. Biologically active fluorescent farnesol analogs. Chem. Biol. 12:639-641. [DOI] [PubMed] [Google Scholar]
- 72.Shchepin, R., J. M. Hornby, E. Burger, T. Niessen, P. Dussault, and K. W. Nickerson. 2003. Quorum sensing in Candida albicans: probing farnesol's mode of action with 40 natural and synthetic farnesol analogs. Chem. Biol. 10:743-750. [DOI] [PubMed] [Google Scholar]
- 73.Shearer, A. G., and R. Y. Hampton. 2004. Structural control of endoplasmic reticulum-associated degradation: effect of chemical chaperones on 3-hydroxy-3-methylglutaryl-CoA reductase. J. Biol. Chem. 279:188-196. [DOI] [PubMed] [Google Scholar]
- 74.Shearer, A. G., and R. Y. Hampton. 2005. Lipid-mediated, reversible misfolding of a sterol-sensing domain protein. EMBO J. 24:149-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sklar, L. A., B. S. Hudson, and R. D. Simoni. 1975. Conjugated polyene fatty acids as membrane probes: preliminary characterization. Proc. Natl. Acad. Sci. USA 72:1649-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith, D. A., S. Nicholls, B. A. Morgan, A. J. Brown, and J. Quinn. 2004. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol. Biol. Cell 15:4179-4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song, L. 2003. Detection of farnesyl diphosphate accumulation in yeast ERG9 mutants. Anal. Biochem. 317:180-185. [DOI] [PubMed] [Google Scholar]
- 78.Sudbery, P., N. Gow, and J. Berman. 2004. The distinct morphogenic states of Candida albicans. Trends Microbiol. 12:317-324. [DOI] [PubMed] [Google Scholar]
- 79.Tanimoto, T., K. Onodera, T. Hosoya, Y. Takamatsu, T. Kinoshita, K. Tago, H. Kogen, T. Fujioka, K. Hamano, and Y. Tsujita. 1996. Schizostatin, a novel squalene synthase inhibitor produced by the mushroom, Schizophyllum commune. I. Taxonomy, fermentation, isolation, physico-chemical properties, and biological activities. J. Antibiot. 49:617-623. [DOI] [PubMed] [Google Scholar]
- 80.Toke, D. A., W. L. Bennett, D. A. Dillon, W.-I. Wu, X. Chen, D. B. Ostrander, J. Oshiro, A. Cremesti, D. R. Voelker, A. S. Fischl, and G. M. Carman. 1998. Isolation and characterization of the Saccharomyces cerevisiae DPP1 gene encoding diacylglycerol pyrophosphate phosphatase. J. Biol. Chem. 273:3278-3284. [DOI] [PubMed] [Google Scholar]
- 81.Toke, D. A., W. L. Bennett, J. Oshiro, W.-I. Wu, D. R. Voelker, and G. M. Carman. 1998. Isolation and characterization of the Saccharomyces cerevisiae LPP1 gene encoding a Mg2+-independent phosphatidate phosphatase. J. Biol. Chem. 273:14331-14338. [DOI] [PubMed] [Google Scholar]
- 82.Tokuhiro, K., N. Muramoto, Y. Yamada, O. Asami, M. Hirai, C. Ohto, S. Obata, and M. Muramatsu. July2002. Transgenic yeast expressing phosphatases for increase the efficiency of producing prenyl alcohol. Worldwide patent WO 2,002,053,751.
- 83.Uhl, M. A., M. Biery, N. Craig, and A. D. Johnson. 2003. Haploinsufficiency-based large-scale forward genetic analysis of filamentous growth in the diploid human fungal pathogen C. albicans. EMBO J. 22:2668-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Waksman, S. A. 1941. Antagonistic relations of microorganisms. Bacteriol. Rev. 5:231-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Westwater, C., E. Balish, and D. A. Schofield. 2005. Candida albicans-conditioned medium protects yeast cells from oxidative stress: a possible link between quorum sensing and oxidative stress resistance. Eukaryot. Cell 4:1654-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whiteway, M., and U. Oberholzer. 2004. Candida morphogenesis and host-pathogen interactions. Curr. Opin. Microbiol. 7:350-357. [DOI] [PubMed] [Google Scholar]
- 87.Yamada-Okabe, T., T. Mio, N. Ono, Y. Kashima, M. Matsui, M. Arisawa, and H. Yamada-Okabe. 1999. Role of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J. Bacteriol. 181:7243-7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yen, C. M., and D. H. Howard. 1970. Germination of blastospores of Histoplasma capsulatum. Sabouraudia 8:242-252. [PubMed] [Google Scholar]