Abstract
Autophagy is a transport system mediated by vesicles, ubiquitous in eukaryotic cells, by which bulk cytoplasm is targeted to a lysosome or vacuole for degradation. In the yeast Saccharomyces cerevisiae, autophagy is triggered by nutritional stress conditions (e.g., carbon- or nitrogen-depleted medium). In this study we showed that there is induction of autophagy in second-fermentation yeasts during sparkling wine making. Two methods were employed to detect autophagy: a biochemical approach based on depletion of the protein acetaldehyde dehydrogenase Ald6p and a morphological strategy consisting of visualization of autophagic bodies and autophagosomes, which are intermediate vesicles in the autophagic process, by transmission electron microscopy. This study provides the first demonstration of autophagy in second-fermentation yeasts under enological conditions. The correlation between autophagy and yeast autolysis during sparkling wine production is discussed, and genetic engineering of autophagy-related genes in order to accelerate the aging steps in wine making is proposed.
The process of obtaining sparkling wines by the champenoise or traditional method involves two fermentation steps. As a consequence of the first fermentation, must is converted into base wine. For the second fermentation, sucrose, selected yeasts, and bentonite are added to the base wine, and the mixture is bottled and allowed to ferment and age in a cellar for extended periods (at least 9 months for “cava” in Spain and 12 months for “champagne” in France). During this time and after the second fermentation is complete, yeast autolysis takes place in the bottle. Yeast autolysis involves the release of different products, resulting from the degradation of yeast macromolecules, into the wine. The amino acids, peptides, proteins, polysaccharides, nucleic acid derivatives, and lipids released during autolysis have a positive effect on the quality of the aroma, flavor, and foam of the wine (9). Finally, the yeasts are removed from the bottle by disgorging, and the sparkling wine is marketed in the bottle that was used for aging (6).
A positive correlation between the autolytic capacity of yeast strains and the quality of the sparkling wines obtained has been shown by several groups of workers (2, 13, 20, 21). However, autolysis in enological conditions is a slow process that requires long aging periods in order to obtain the desired results, which contributes to the relatively high production costs of these wines. Some still wines also benefit from yeast autolysis. Indeed, “aging on lees” is becoming increasingly popular due to its positive effect on several wine properties, including the chemical stability of white wines and the color stability or mouth feel, among other properties, of red wines (14).
For the most part, two methods to accelerate the acquisition of aging-like properties during sparkling wine production have been tested: adding yeast autolysates to the wine and aging at higher temperatures (9). Both of these alternatives cause organoleptic defects in the final product, probably due to excess autolysis (25). Other alternatives to accelerate the process that have been tested in the laboratory or under real production conditions include the use of a combination of killer and killer-sensitive yeast strains (33) and the use of autolytic strains derived from industrial second-fermentation strains by meiosis (32) or by UV mutagenesis (15).
Autophagy is a ubiquitous process in eukaryotic cells that involves bulk degradation of the cytoplasm and organelles in the vacuole or lysosome (1, 4, 30). In Saccharomyces cerevisiae, autophagy is triggered by starvation conditions and is considered an adaptive response that allows survival of the cell due to recycling of the building blocks resulting from its own digestion (1, 30). Autophagic transport is carried out by autophagosomes, which are double-membrane vesicles containing fractions of the cytoplasm that are targeted to the vacuole (1, 3). The outer membrane of the autophagosome fuses with the vacuolar membrane, and an autophagic body, an internal single-membrane-bound vesicle, is released into the lumen of the vacuole (1, 3, 30). Finally, the membrane of the autophagic body is degraded, and the cytoplasm becomes accessible to the resident hydrolytic enzymes (12, 28, 31). The first evidence of autophagy in S. cerevisiae was obtained in a morphological study performed with vacuolar proteinase-deficient mutants, which accumulated autophagic bodies when they were shifted from a rich medium to a nitrogen-depleted medium (30). The same results were obtained for wild-type cells shifted to starvation medium in the presence of phenylmethylsulfonyl fluoride (PMSF), an inhibitor of serine threonine proteinases (30).
Although autophagy is considered a nonselective mechanism for degradation of cytoplasm in the vacuole, different proteins, including the vacuolar hydrolases aminopeptidase 1 (Ape1p) and α-mannosidase (Ams1p), have been shown to be selectively included in autophagosomes (16). Alternatively, Ape1p and Ams1p can be sorted to the vacuole by the cytosol-to-vacuole targeting (CVT) pathway, a synthetic constitutive pathway that has morphological and molecular homologies to autophagy (17, 26). Recently, Onodera and Ohsumi (24) showed that the cytosolic acetaldehyde dehydrogenase (Ald6p) is also specifically targeted to the vacuole by autophagosomes. Under nutrient starvation conditions, the Ald6p in cells was quickly depleted as a result of preferential degradation of this protein during autophagy (24).
Since autophagy is a highly regulated process that is essential for homeostasis and cell maintenance under stressful conditions, we hypothesized that wine yeasts exhibiting deregulated autophagy would die earlier and also undergo accelerated autolysis. One premise for this hypothesis is that autophagy precedes autolysis under wine-making conditions. However, autolysis under industrial conditions is usually defined as a self-degradation process that involves disturbance of internal membrane systems and release of vacuolar hydrolases into the cytoplasm (11, 14). Curiously, there are no references to autophagy in the previously published descriptions of yeast behavior during wine fermentation and aging. In recent work, we showed that S. cerevisiae laboratory strains undergo autophagy under experimental second-fermentation conditions, using a dealcoholized base wine (8). In the current study we used morphological and biochemical approaches in order to show that industrial second-fermentation yeasts undergo autophagy during sparkling wine elaboration. Detection of autophagic bodies and autophagosomes by transmission electron microscopy (TEM) and the disappearance of Ald6p as determined by Western blotting were used as markers to study the progression of the autophagic process in the commercial yeast strain EC1118 during second-fermentation experiments under industrial conditions.
MATERIALS AND METHODS
Strains.
The S. cerevisiae strains employed in this study were BY4741 (MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and the isogenic strain ΔATG1 (MATα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ygl180w::kanMX4) with the ATG1 gene deleted. ATG1 codes for a protein kinase essential for both the CVT pathway and autophagy (22, 27). Both strains were obtained from Euroscarf (European S. cerevisiae Archive for Functional Analysis). The commercial second-fermentation S. cerevisiae strain EC1118 was provided by Lallemand Inc. (Montreal, Canada).
Media.
YPD contains 1% yeast extract, 2% peptone, and 2% glucose; 2% agar was added to solid YPD. SD-N contains 0.17% yeast nitrogen base without amino acids and ammonium sulfate (Difco Laboratories Inc., Detroit, Mich.), as well as 2% glucose. Adaptation medium contains 0.08 g/liter diammonium phosphate, 2.4 g/liter tartaric acid, 20 g/liter sucrose, 482 ml/liter base wine (Cavas Castellblanch, Sant Sadurní D'Anoia, Spain). For second-fermentation experiments sucrose was added to the base wine to a final concentration of 20 g/liter.
Nitrogen starvation experiments.
Yeast cells, grown to the exponential phase in YPD, were washed with a sterile 0.9% NaCl solution, inoculated into SD-N with or without 1 mM PMSF to obtain a final density of 108 cells/ml, and incubated at 17°C. One-milliliter samples from cultures without PMSF were removed every hour and centrifuged for 5 min at 3,000 × g and 4°C. Pellets were stored at −20°C until they were used to detect Ald6p by Western blotting. Yeast cells from SD-N containing PMSF were pelleted after 8 h of incubation, and cells were prepared for analysis by TEM. Yeast cells incubated in YPD were used as a control.
Second-fermentation experiments.
One hundred milliliters of YPD was inoculated to obtain a concentration of 105 cells/ml using an overnight S. cerevisiae EC1118 culture. After 48 h of incubation at 30°C and 150 rpm, cells were inoculated into fresh adaptation medium to obtain a concentration of 106 cells/ml. The cells were incubated for 4 to 5 days at 30°C and 150 rpm, and base wine with sucrose (20 g/liter) was subsequently inoculated at a concentration of 106 cells/ml with cells adapted to ethanol. The base wine was either bottled or kept in an Erlenmeyer flask, and it was incubated in static conditions at 17°C for 16 to 19 days. At different times, samples containing 108 cells were centrifuged for 5 min at 3,000 × g and 4°C (in the case of bottled wine, one bottle was opened for each sampling time). The supernatants were used to monitor the fermentation, and the pellets were used to detect Ald6p by Western blotting. Cells were also prepared for TEM analysis. In the second-fermentation experiments using Erlenmeyer flasks, PMSF (1 mM) was added to the base wine 8 days after inoculation, and cells were incubated with the inhibitor for an additional 8 h before samples were taken for TEM analysis.
Protein extracts and Western blotting.
The proteins from total extracts were concentrated by precipitation with trichloroacetic acid. One hundred microliters of total protein extract was mixed with 37 μl of a trichloroacetic acid solution (100%, wt/vol). After 10 min for incubation at 4°C, a protein pellet was obtained by centrifugation at 16,000 × g and 4°C. The pellet was washed three times with cold acetone (−20°C) and suspended with 10 μl of sterile water. Western blotting was performed as described by Leber et al. (19), using a rabbit anti-acetaldehyde dehydrogenase monoclonal antibody (Rockland, Gilberstville, PA) and the ECL detection system (Amersham Biosciences Europe, Barcelona, Spain). This antibody simultaneously reacted with Ald6p and Ald4p, providing an internal control for extract concentration and gel loading as described below (24).
Monitoring of second-fermentation experiments.
Residual reducing sugars from cell-free base wine samples were quantified by using 3,5-dinitrosalicylic acid as described by Bernfeld (5). Before quantification, the samples were incubated overnight at 37°C with invertase (2.8 mg/liter; Sigma-Aldrich Química, Tres Cantos, Spain) to ensure that residual sucrose was completely hydrolyzed. Determinations were performed in duplicate.
Differential interference contrast microscopy.
Cell morphology was examined with a Leitz Aristoplan microscope (Ernst Leitz Weltzar GMBH) equipped for Nomarski optics for interference contrast.
Transmission electron microscopy.
Cells from the second-fermentation experiments were mixed with 1 ml of phosphate buffer (40 mM sodium phosphate [pH 7], 1 mM NaCl, 1 mM MgCl2) containing 2% glutaraldehyde and 4% paraformaldehyde. After fixation for 1.5 h at room temperature in an orbital shaker, cells were washed three times with phosphate buffer. Cells were subjected to rapid freezing and freeze substitution fixation and were examined as previously described (30).
RESULTS
Degradation of Ald6p and appearance of autophagic bodies are suitable markers for detecting autophagy at a low temperature.
We used depletion of Ald6p and visualization of autophagic bodies to monitor autophagy progression during sparkling wine making. Both of these criteria, especially the morphological criterion, have been successfully used to detect autophagy at 30°C (10, 24, 30). As a preliminary step for analysis of autophagy under second-fermentation conditions, we studied the suitability of the autophagic markers used to monitor autophagy at 17°C (the temperature of second fermentation).
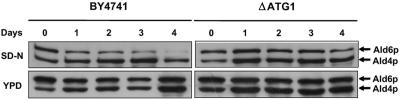
When S. cerevisiae strain BY4741 cells were starved in SD-N at 17°C (as described in Materials and Methods), a decrease in the Ald6p level with time was observed, until Ald6p almost completely disappeared after 4 days of incubation (Fig. 1). However, the amount of Ald6p remained constant in the S. cerevisiae ΔATG1 mutant under the same starvation conditions (Fig. 1). This result is consistent with the known phenotype of strains from which ATG1 is deleted, which are completely unable to form autophagosomes (22, 27). When the same experiment was carried out using the rich medium YPD, in which no autophagy was expected, the quantities of Ald6p in strains BY4741 and ΔATG1 remained the same for the time of incubation (Fig. 1). According to these results a reduction in the Ald6p level can be used as a biochemical marker to detect autophagy at 17°C.
FIG. 1.
Western blot analysis of protein extracts from strains BY4741 and ΔATG1 incubated in SD-N or YPD at 17°C. The membranes were probed with rabbit monoclonal anti-acetaldehyde dehydrogenase antibody.
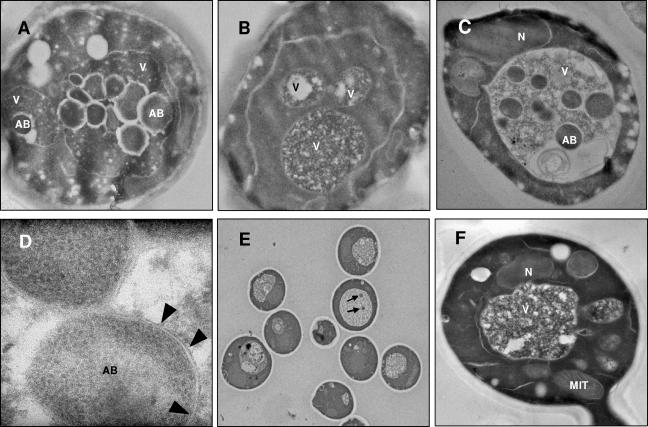
In the same way, S. cerevisiae BY4741 was incubated at 17°C in SD-N supplemented with 1 mM PMSF, a proteinase inhibitor that prevents the degradation of the autophagic bodies (30). As a consequence, the vacuoles of most cells (more than 50%) were filled with vesicles, which took up almost the whole lumen after 8 h of incubation under nitrogen starvation conditions (Fig. 2A). However, in the mutant defective in autophagy, ΔATG1, the vacuoles were free of vesicles under the same experimental conditions (Fig. 2B shows a representative example). No autophagic bodies were detected in BY4741 or ΔATG1 cells incubated in PMSF-containing YPD (data not shown).
FIG. 2.
Transmission electron micrographs of BY4741 (A), ΔATG1 (B), and EC1118 (C) cells after 8 h of incubation in SD-N containing 1 mM PMSF. In panel D arrowheads indicate the single membrane delimiting an autophagic body. Isolated autophagic bodies (arrows) were detected in EC1118 incubated for 8 h in SD-N without PMSF (E). No vesicles were detected in EC1118 vacuoles after 8 h of incubation in YPD containing 1 mM PMSF (F). AB, autophagic body; MIT, mitochondrion; N, nucleus; V, vacuole.
Similar results were obtained with the commercial yeast strain EC1118. After 8 h of incubation in PMSF-containing SD-N, single-membrane-bound vesicles accumulated in the vacuole. The lumen of each of these vesicles had a density for TEM which was indistinguishable from that of the cytoplasm (Fig. 2C and 2D). They also showed Brownian movement, as previously described for autophagic bodies (23), as determined by differential interference contrast microscopy (data not shown). In the absence of PMSF, intravacuolar vesicles were rarely observed. However, occasionally one or two isolated vesicles were identified in the vacuoles of some cells (one example is shown in Fig. 2E). No autophagic bodies were detected when S. cerevisiae EC1118 was incubated for 8 h in YPD with or without PMSF (Fig. 2F shows a representative example).
Our results show that depletion of Ald6p and detection of autophagic bodies can be used as markers of the progression of autophagy at 17°C, the temperature used for second fermentation of base wine. Hence, we considered these indicators appropriate for detection of autophagy during sparkling wine making.
Demonstration of autophagy with industrial yeasts under wine-making conditions.
For simplicity, second-fermentation experiments were first performed in Erlenmeyer flasks instead of closed bottles. This allowed PMSF to be added to the base wine for morphological detection of autophagy at any time after inoculation. The use of PMSF was precluded in closed bottles (to which it had to be added at the time of inoculation), both because of its inhibitory effect on the fermentation and because of its low stability in aqueous solutions.
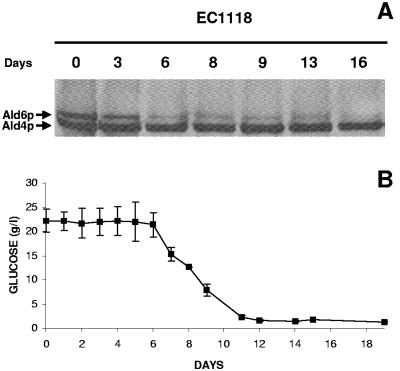
For these experiments S. cerevisiae EC1118 was previously adapted to ethanol as described above in order to fully reproduce industrial sparkling wine-making conditions. The Ald6p levels decreased throughout the second-fermentation process; after 6 days it was hardly detectable, and it completely disappeared after 16 days of incubation in the base wine (Fig. 3A). The Ald4p levels, used as an internal control for the assay, remained the same. In similar experiments but with PMSF added, the Ald6p levels remained high, as expected for inhibition of autophagy (data not shown). These results showed that industrial yeasts induce autophagy during the second fermentation of base wine.
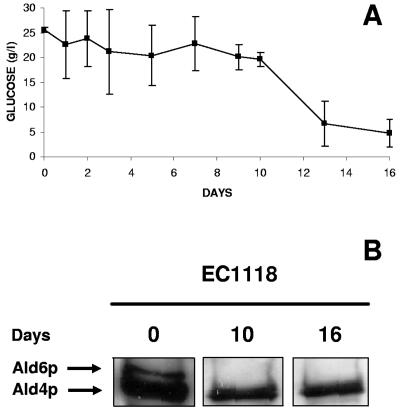
FIG. 3.
(A) Western blot of EC1118 extracts for 16 days of incubation at 17°C in Erlenmeyer flasks with base wine. The membranes were probed with rabbit monoclonal anti-acetaldehyde dehydrogenase antibody. (B) Sugar consumption in the same experiment. The error bars indicate standard deviations.
Monitoring of fermentation was carried out as a control for the process. The fermentation began 6 days after inoculation of the base wine, and sugar was exhausted after 11 days of incubation (Fig. 3B). Induction of autophagy by S. cerevisiae EC1118, indicated by depletion of Ald6p, was detected on the sixth day of incubation in the base wine, at the same time as the beginning of the fermentation (Fig. 3). One possible reason for detection of the beginning of autophagy before sugar was exhausted is that nitrogen starvation triggered autophagy in these experimental conditions; however, addition of a rich nitrogen source did not noticeably change the time course of Ald6p depletion (data not shown).
Although this result showed that there was induction of autophagy by industrial yeasts during the second fermentation of base wine, we wanted to confirm the biochemical data using a morphological approach based on the detection of autophagic bodies. S. cerevisiae EC1118 was inoculated into the base wine and then incubated for 8 days at 17°C before PMSF addition. The cells were incubated for an additional 8 h with PMSF before examination by TEM. Under these conditions, the vacuole of S. cerevisiae EC1118 was packed with autophagic bodies (a representative example is shown in Fig. 4), similar to the results obtained previously under nitrogen starvation conditions for this strain (Fig. 2C).
FIG. 4.
Transmission electron micrographs of EC1118 cells during the second fermentation of base wine. PMSF (1 mM) was added to the base wine 8 days after inoculation. After 8 h of incubation with the protease inhibitor, cells were removed and analyzed by TEM. The arrows indicate autophagic bodies in the vacuole. V, vacuole; N, nucleus; MIT, mitochondrion.
Finally, we tried to prove that autophagy occurs under authentic second-fermentation conditions, namely, in closed bottles. Under these conditions, the fermentation proceeded more slowly (Fig. 3B and 5A); however, most of the sucrose added to the base wine was depleted at the end of the process (Fig. 5A). During the fermentation, samples were removed for detection of Ald6p by Western blotting. The results of this experiment confirmed data obtained with open flasks; Ald6p was clearly detected at the time of inoculation, but it could not be detected by the middle (day 10) or the end (day 16) of fermentation. Again, the Ald4p levels remained stable (Fig. 5B).
FIG. 5.
(A) Monitoring of a second-fermentation experiment in closed bottles with base wine and S. cerevisiae EC1118. The error bars indicate standard deviations. (B) Western blot of EC1118 protein extracts from the same experiment. The membranes were probed with rabbit monoclonal anti-acetaldehyde dehydrogenase antibody.
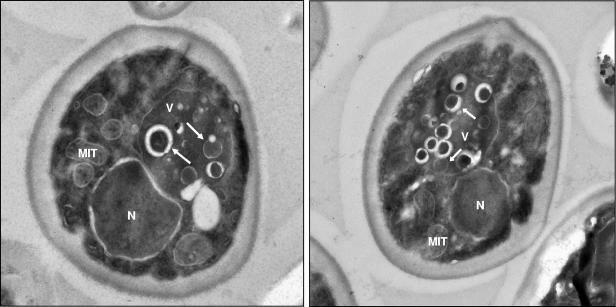
As explained above, PMSF had to be omitted in the experiments performed under real wine-making conditions. Notwithstanding this, samples that were not treated with PMSF from all the experiments were also subjected to TEM analysis in order to detect autophagic bodies and autophagosomes (we have previously shown that S. cerevisiae EC1118 cells, incubated in SD-N without PMSF, occasionally contain one or two isolated autophagic bodies in the vacuoles [Fig. 2E]). Some isolated vesicles were also observed by TEM in the vacuoles of EC1118 cells after 10 days of incubation in the base wine (one example is shown in Fig. 6A), even though no vesicles were observed in most of the vacuoles. Due to the absence of protease inhibitors, the percentage of vacuoles containing autophagic bodies in these experimental conditions was less than 5%.
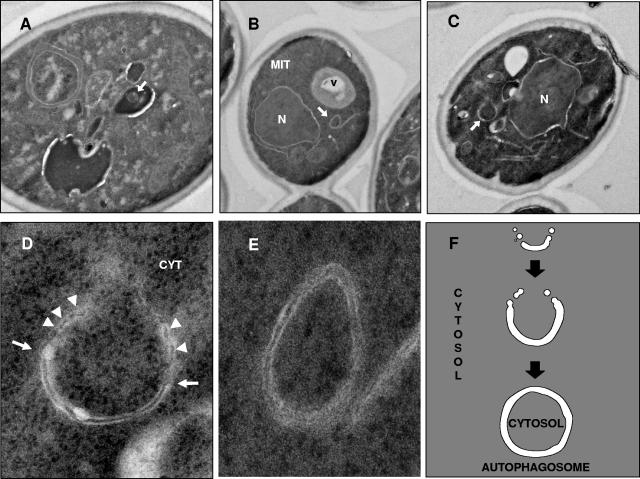
FIG. 6.
Transmission electron micrographs of EC1118 cells incubated for 10 days in a closed bottle with base wine. (A) Autophagic body. (B) Autophagosome. (C) Autophagosome precursor. (D and E) Enlarged images of the autophagosome and the autophagosome precursor. The arrows indicate the cup-shaped membrane, and the arrowheads indicate places where the cup-shaped membrane is fused to membranes. (F) Schematic representation of the autophagosome formation process according to the model of Kirisako et al. (18), shown for comparison. CYT, cytoplasm; V, vacuole; N, nucleus; MIT, mitochondrion.
Detection of autophagosomes, the precursors of autophagic bodies, might also provide morphological evidence of the progress of autophagy in industrial yeast cells during the elaboration of sparkling wine. A single autophagosome (Fig. 6B and 6E) with a characteristic double membrane and a lumen having a density identical to that of the cytoplasm (as determined by TEM) was identified in the cytoplasm of EC1118. The morphological differences between an autophagic body, a single-membrane-bound vesicle, and an autophagosome, which has a double membrane, are shown by a comparison of Fig. 2D and 6E.
A model for the development of autophagosomes was proposed by Kirisako et al. (18), based on electron microscopy observations. According to this model, autophagosomes arise from assembly of precursor membranous structures. Initially, a cup-shaped membrane arises as a consequence of fusion of membranous precursors, which leads to the formation of a spherical mature autophagosome enclosing a portion of the cytoplasm (Fig. 6F). New evidence concerning the induction of autophagy by second-fermentation yeasts under winery conditions was obtained by detection of such a cup-shaped membrane (Fig. 6C and 6D). This structure, apparently fused with membranes, surrounded a portion of the cytoplasm (Fig. 6D).
Conclusions.
Based on the biochemical and morphological results described above, we concluded that second-fermentation yeasts undergo autophagy when they are subjected to industrial conditions for elaboration of sparkling wines. In a previous report we showed that autophagy was induced in laboratory strains subjected to analogous second-fermentation conditions (8). Ape1p is synthesized in the cytoplasm as a precursor (prApe1p) that can be targeted to the vacuole either by autophagy in starvation conditions or by the CVT pathway in rich-nutrient conditions. Based on the finding that in conditions similar to those employed in the industry Ape1 could be transported to the vacuole in a mutant defective in the CVT pathway, we concluded that yeasts undergo autophagy under these conditions (8). However, the biochemical approach had two drawbacks: first, laboratory strains were used instead of industrial yeasts, and second, the greater susceptibility to ethanol of the laboratory strains resulted in the use of dealcoholized instead of commercial base wine. Both of these experimental limitations were overcome by the biochemical approach used in this study based on monitoring of Ald6p levels.
In addition, we obtained morphological data illustrating the progression of autophagy by second-fermentation yeasts. Autophagic bodies filled the vacuoles of cells that were starved or subjected to the second fermentation in the presence of PMSF, but some isolated autophagic bodies were also detected without PMSF. The fact that we were able to observe these structures was probably due to the low temperature used in this experiments (17°C). Autophagic bodies or autophagosomes have never been found at 30°C without the help of proteinase-defective mutants or protease inhibitors. The detection of an isolation membrane, a precursor of the mature autophagosome, was very relevant proof for confirming the induction of autophagy by industrial yeasts during sparkling wine elaboration.
Although reproducing a wine-making industrial process in the laboratory is usually limited by a scale factor, this is not true in the case of the second fermentation, because bottles that are the same size as those used in the industry can be used. In this way, the experimental conditions used in this study were identical to the conditions employed by the industry, including preadaptation to ethanol.
The data obtained in the present work provide the first unequivocal evidence of autophagy in industrial yeasts subjected to real enological conditions. Autophagy could be a potential target for genetically improving autolytic properties of wine yeast strains in two opposing ways. First, wine yeast cells showing increased rates of autophagy would be expected to enter a futile cycle of synthesis and degradation of macromolecules, which would finally lead to accelerated cell death and autolysis. Second, since autophagy is necessary for survival under starvation conditions and since cells defective in autophagy are known to die quickly in the stationary phase, it can be anticipated that such mutant cells would also experience accelerated autolysis when the carbon sources are exhausted. It is not known whether the autolysis products obtained by these two strategies are similar or whether they contribute in the same way to wine quality. Indeed, recent results from our laboratory indicate that laboratory strains that are constitutive for autophagy undergo accelerated autolysis under pseudoenological conditions (7), probably because all the necessary activities are already in place before the cells are starved. On the other hand, strains defective in autophagy also undergo accelerated autolysis (29). In this case, faster autolysis is probably due to a higher death rate under starvation conditions.
The next step is construction of genetically improved industrial autolytic wine yeast strains that show constitutive or defective autophagy. Such strains could be useful for improving the quality of sparkling wines or red wines aged “on lees.”
Acknowledgments
We are grateful to M. T. Rejas for assistance with electron microscopy and to M. V. Santamaría and J. M. Barcenilla for technical assistance.
This work was supported by the Spanish Ministerio de Ciencia y Tecnología (grant AGL2003-01762) and by Comunidad de Madrid (grant S-505/AGR-0153). E.C. is the recipient of an FPI fellowship from the Spanish Ministerio de Ciencia y Tecnología.
REFERENCES
- 1.Abeliovich, H., and D. J. Klionsky. 2001. Autophagy in yeast: mechanistic insights and physiological function. Microbiol. Mol. Biol. Rev. 65:463-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrés-Lacueva, C., R. M. Lamuela-Raventós, S. Buxaderas, and M. C. De la Torre-Boronat. 1997. Influence of variety and aging on foaming properties of cava (sparkling wine). J. Agric. Food Chem. 45:2520-2525. [Google Scholar]
- 3.Baba, M., K. Takeshige, N. Baba, and Y. Ohsumi. 1994. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J. Cell Biol. 124:903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba, M., M. Osumi, S. V. Scott, D. J. Klionsky, and Y. Ohsumi. 1997. Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome. J. Cell Biol. 139:1687-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernfeld, P. 1955. Amylases α and β. Methods Enzymol. 1:149-158. [Google Scholar]
- 6.Carrascosa, A. V., A. Martínez-Rodríguez, E. Cebollero, and R. González. 2005. Levaduras. Saccharomyces II. Levaduras de segunda fermentación, p. 57-77. In A. V. Carrascosa, R. González, and R. Muñoz (ed.), Microbiología enológica. A. Madrid Vicente, Ediciones, Madrid, Spain.
- 7.Cebollero, E., A. Martínez-Rodríguez, A. V. Carrascosa, and R. González. 2005. Overexpression of csc1-1. A plausible strategy to obtain wine yeast strain undergoing accelerated autolysis. FEMS Microbiol. Lett. 246:1-9. [DOI] [PubMed] [Google Scholar]
- 8.Cebollero, E., A. V. Carrascosa, and R. González. 2005. Evidence for yeast autophagy during simulation for sparkling wine aging: a reappraisal of the mechanism of yeast autolysis in wine. Biotechnol. Prog. 21:614-616. [DOI] [PubMed] [Google Scholar]
- 9.Charpentier, C., and M. Feuillat. 1992. Yeast autolysis, p. 225-242. In G. H. Fleet (ed.), Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, Switzerland.
- 10.Cheong, H., T. Yorimitsu, F. Reggiori, J. E. Legakis, C. W. Wang, and D. J. Klionsky. 2005. Atg17 regulates the magnitude of the autophagic response. Mol. Biol. Cell 16:3438-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connew, S. J. 1998. Yeast autolysis. A review of current research. Aust. N. Z. Wine Ind. J. 13:61-64. [Google Scholar]
- 12.Epple, U. D., I. Suriapranata, E. L. Eskelimen, and M. Thumm. 2001. Aut5/cvt7p, a putative lipase essential for disintegration of autophagic bodies inside the vacuole. J. Bacteriol. 183:5942-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escot, S., M. Feuillat, L. Dulau, and C. Charpentier. 2001. Release of polysaccharides by yeast and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 7:153-159. [Google Scholar]
- 14.Fornairon-Bonnefond, C., C. Camarasa, M. Moutounet, and J. M. Salmon. 2002. New trends on yeast autolysis and wine ageing on lees: a bibliographic review. J. Int. Sci. Vigne Vin 36:49-69. [Google Scholar]
- 15.Gonzalez, R., A. J. Martinez-Rodriguez, and A. V. Carrascosa. 2003. Yeast autolytic mutants potentially useful for sparkling wine production. Int. J. Food Microbiol. 84:21-26. [DOI] [PubMed] [Google Scholar]
- 16.Huang, W. P., and D. J. Klionsky. 2002. Autophagy in yeast: a review of the molecular machinery. Cell Struct. Funct. 27:409-420. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins, M. U., and D. J. Klionsky. 2001. Vacuolar localization of oligomeric alpha-mannosidase requires the cytoplasm to vacuole targeting and autophagy pathway components in Saccharomyces cerevisiae. J. Biol. Chem. 276:20491-20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirisako, T., M. Baba, N. Ishihara, K. Miyazawa, M. Ohsumi, T. Yoshimori, T. Noda, and Y. Ohsumi. 1999. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J. Cell Biol. 147:435-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leber, R., E. Silles, I. V. Sandoval, and M. J. Mazón. 2001. Yol082p, a novel CVT protein involved in the selective targeting of aminopeptidase I to the yeast vacuole. J. Biol. Chem. 276:29210-29217. [DOI] [PubMed] [Google Scholar]
- 20.Leroy, M. J., M. Charpentier, B. Duteurtre, M. Feuillat, and C. Charpentier. 1990. Yeast autolysis during champagne aging. Am. J. Enol. Vitic. 41:21-28. [Google Scholar]
- 21.Martínez-Rodríguez, A., A. V. Carrascosa, P. J. Martin-Alvarez, V. Moreno-Arribas, and M. C. Polo. 2002. Influence of the yeast strain on the changes of the amino acids, peptides and proteins during sparkling wine production by the traditional method. J. Ind. Microbiol. Biotechnol. 29:314-322. [DOI] [PubMed] [Google Scholar]
- 22.Matsuura, A., M. Tsukada, Y. Wada, and Y. Ohsumi. 1997. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192:245-250. [DOI] [PubMed] [Google Scholar]
- 23.Ohsumi, Y. 1999. Molecular mechanism of autophagy in yeast, Saccharomyces cerevisiae. Philos Trans. R. Soc. Lond. B 354:1577-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onodera, J., and Y. Ohsumi. 2004. Ald6 is a preferred target for autophagy in yeast, Saccharomyces cerevisiae. J. Biol. Chem. 279:16071-16076. [DOI] [PubMed] [Google Scholar]
- 25.Peppler, H. J. 1982. Yeast extracts, p. 293-312. In A. H. Rose (ed.), Fermented foods. Economic microbiology. Academic Press, London, Great Britain.
- 26.Scott, S. V., M. Baba, Y. Ohsumi, and D. J. Klionsky. 1997. Aminopeptidase I is targeted to the vacuole by a nonclassical vesicular mechanism. J. Cell Biol. 138:37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Straub, M., M. Bredschneider, and M. Thumm. 1997. AUT3, a serine/threonine kinase gene, is essential for autophagocytosis in Saccharomyces cerevisiae. J. Bacteriol. 179:3875-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suriapranata, I., U. D. Epple, D. Bernreuther, M. Bredschneider, K. Sovarasteanu, and M. Thumm. 2000. The breakdown of autophagic vesicles inside the vacuole depends on Aut4p. J. Cell Sci. 113:4025-4033. [DOI] [PubMed] [Google Scholar]
- 29.Tabera, L., R. Muñoz, and R. Gonzalez. 2006. Deletion of bcy1 from the Saccharomyces cerevisiae genome is semidominant and induces autolytic phenotypes suitable for improvement of sparkling wines. Appl. Environ. Microbiol. 72:2351-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshige, K., M. Baba, S. Tsuboi, T. Noda, and Y. Ohsumi. 1992. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119:301-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teter, S. A., K. P. Eggerton, S. V. Scott, J. Kim, A. M. Fischer, and D. J. Klionsky. 2001. Degradation of lipid vesicles in the yeast vacuole requires function of cvt17, a putative lipase. J. Biol. Chem. 276:2083-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tini, V., C. Zambonelli, M. Benevelli, and L. Castellari. 1995. The autolysogenic Saccharomyces cerevisiae strains for the sparkling wines production. Ind. Bevande 24:113-118. [Google Scholar]
- 33.Todd, B. E. N., G. H. Fleet, and P. A. Henscheke. 2000. Promotion of autolysis through the interaction of killer and sensitive yeasts: potential application in sparkling wine production. Am. J. Enol. Vitic. 51:65-72. [Google Scholar]