Abstract
Simple methods of reducing the microbial load on surfaces in hospitals are needed to reduce the risk of hospital-associated infections. Here we report on the ability of a cellulose acetate coating containing the photosensitizers toluidine blue and rose bengal to kill microbes (Staphylococcus aureus, Escherichia coli, Clostridium difficile, a bacteriophage, and Candida albicans) on its surface when illuminated with white light.
In the United Kingdom, approximately 1 in 11 inpatients at any given time has an infection acquired in a hospital (9). Internationally, figures suggest a similar trend, with prevalence rates ranging from 4 to 10% (10). Hospital-acquired infections can often lead to complications with existing illnesses, may cause anxiety and discomfort, and can also lead to the death of the patient. Furthermore, infected patients remain in hospital on average 2.5 times longer than uninfected patients (11), resulting in increased costs. Although it was previously believed that the inanimate environment played little or no role in the transmission of infectious disease, this concept is now being reconsidered (3). The Centers for Disease Control and Prevention has now listed contact transmission—direct, from body to surface, or indirect, via contaminated inanimate objects—as one of the main routes of microbe transmission (6). The Department of Health (4) has also stated that “good hospital hygiene is an integral and important component of a strategy for preventing hospital-acquired infections.” Contaminated environmental surfaces can contribute to the spread of microbes by acting as reservoirs from which personnel and patients can contaminate their hands (14). The problem is not only that such reservoirs exist but also that they can persist for long periods due to the ability of some microorganisms to survive on surfaces for days, weeks, and even months (8). It is widely recognized that the usual means of attempting to reduce the microbial load on environmental surfaces are ineffective. Detergent solutions, for example, often become contaminated during use, thus allowing the spread of microbes throughout the hospital via mopheads and cleaning cloths (14). There is a need, therefore, to develop new approaches to reducing microbial contamination of the hospital environment. One possible approach is to use light-activated antimicrobial agents incorporated into polymers which could be applied as coatings (either permanently or on a renewable basis) to hospital surfaces, provided that these could be activated by the ambient light conditions found in hospitals. A number of studies have shown that photosensitizers can retain their antimicrobial properties when attached to polymers (1, 17). In this study, the ability of such coatings to kill a range of microbes under lighting conditions likely to be present in hospitals has been investigated.
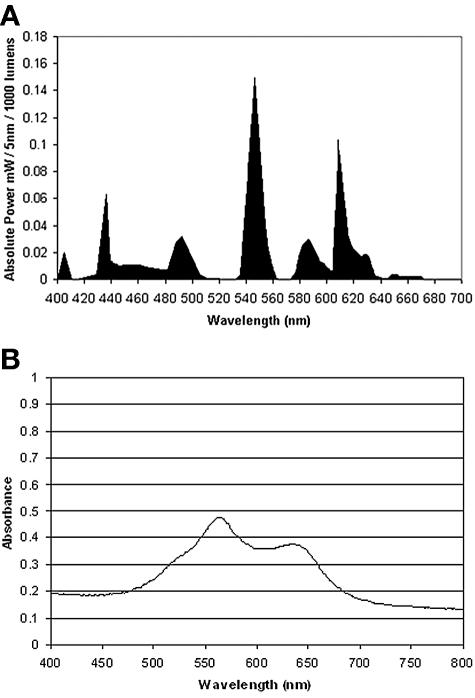
The light source used in this study was a General Electric 28-W Biax 2D compact fluorescent lamp that emits light across the visible spectrum. This lamp has the same color-rendering properties and spectral power distribution as the fluorescent luminaires used in hospitals in the United Kingdom. Prominent peaks were present at 435, 495, 545, 588, and 610 nm (Fig. 1A). The lamp was fitted into a refrigerated incubator (LTE Scientific Ltd., Oldham, United Kingdom) that maintained the temperature at a constant 22°C. The light intensity was measured using a digital luxmeter (Hagner Photometric Instruments Ltd., Bosham, United Kingdom). The photosensitizers toluidine blue O (TBO) and rose bengal (RB) were purchased from Sigma (Poole, United Kingdom). The coatings were prepared as follows. Cellulose acetate (Sigma) was dissolved in acetone (50 mg/ml), and stock solutions of the photosensitizers in acetone (100 μg/ml) were added to give a final concentration of 25 μM for each photosensitizer. Aliquots (450 μl) of each mixture were transferred to flat-bottom glass containers (diameter, 18 mm), and the acetone was left to evaporate overnight. The thickness of the coatings was measured using a Starrett (Athol, Mass.) micrometer and was found to be 43.2 ± 6 μm. The absorption spectrum of the coatings was determined using a UNICAM UV 500 UV/visible spectrophotometer (ThermoSpectronic) over the range 250 to 800 nm and is shown in Fig. 1B, where it can be seen that strong absorbance occurs between 500 nm and 675 nm, which includes three of the main emission peaks of the light source.
FIG. 1.
(A) Emission spectrum of the 28-W fluorescent lamp used in the study. (B) Absorption spectrum of cellulose acetate coatings containing toluidine blue (25 μM) and rose bengal (25 μM).
The organisms used were Staphylococcus aureus NCTC 6571, a methicillin-resistant strain of S. aureus (NCTC 13143), Escherichia coli NCTC 10418, Candida albicans (clinical isolate), Clostridium difficile 630 (clinical isolate), and bacteriophage φX174 (host organism, E. coli ATCC 13706). This particular bacteriophage was used because it has previously been used as a model virus in transmission studies and it has a stability comparable to the most resistant human-pathogenic viruses, such as the poliovirus (12). All bacteria (except for C. difficile) were maintained by weekly subculture on nutrient agar (Oxoid, Basingstoke, United Kingdom), while Candida albicans was subcultured weekly on Sabouraud dextrose agar (Oxoid). C. difficile was subcultured every 5 days onto brain heart infusion agar (Oxoid) supplemented with 5% horse blood, Clostridium supplement (Oxoid), and 10 μg/ml tetracycline. The phage was propagated and titered according to ATCC guidelines, and the resulting stock suspension was stored at 4°C. For experimental purposes, bacteria (except for C. difficile) were grown aerobically in nutrient broth, while Candida albicans was grown in Sabouraud dextrose liquid medium (Oxoid); all were incubated at 37°C for 16 h. C. difficile was grown overnight in brain heart infusion (Oxoid) at 37°C in an anaerobic cabinet (Don Whitley Scientific Ltd., United Kingdom). All organisms (except for the phage) were centrifuged, resuspended in an equal volume of phosphate-buffered saline (PBS), and then further diluted 1:1,000 in PBS. The resulting bacterial suspensions contained approximately 106 CFU/ml, while the count for Candida albicans was approximately 105 CFU/ml. In some experiments, S. aureus was resuspended in human saliva instead of PBS. The saliva was used undiluted without any further processing. For the bacteriophage, a 1:1,000 dilution of the stock suspension in PBS was used to give a PFU-per-milliliter count of around 106.
To determine the effectiveness of the photosensitizer-containing coatings, aliquots (250 μl) of each microbial suspension were placed on each of four photosensitizer-containing coatings in the glass containers, the mouths of which were then covered with transparent cling film. Two of these were exposed to light (L+ S+), while the other two were kept in the dark (L− S+). In addition, microbial suspensions were also inoculated onto four control coatings (containing no photosensitizer), two of which were exposed to light (L+ S−) and two of which were kept in the dark (L− S−). After incubation of the coatings in the refrigerated incubator at 22°C for 2, 4, 6, or 16 h, survivors were enumerated by viable counting of serial dilutions of the microbial suspension. Serial dilutions were prepared in sterile PBS, and 25-μl aliquots were plated on nutrient agar (for S. aureus and E. coli), Sabouraud dextrose agar (for Candida albicans), brain heart infusion agar supplemented with 5% horse blood, Clostridium supplement, and tetracycline (for C. difficile), or mannitol salt agar (for S. aureus when suspended in human saliva). Following incubation at 37°C under aerobic conditions (except for C. difficile, which was incubated anaerobically), the resulting colonies were enumerated. In the case of the bacteriophage, the number of PFU was determined as follows. Aliquots (30 μl)of each dilution were added to 300 μl of the host organism, E. coli ATCC 13706 (mid-exponential phase: 0.5 ml of overnight culture inoculated in 10 ml of nutrient broth and grown to an optical density of around 0.7 at 600 nm) in polypropylene phage tubes. Following incubation of the tubes at room temperature for 30 min, 3 ml of 0.5% nutrient agar (kept at 42 to 45°C) was added to each. Tubes were inverted and then poured evenly onto prewarmed nutrient agar plates. Plaques were counted after overnight incubation of the plates at 37°C.
In order to assess whether there had been any leaching of the photosensitizers out of the coating, in each experiment 1.0 ml of PBS was placed on a photosensitizer-containing coating, and following illumination, the absorbance of this solution at the peak absorbance of the photosensitizers (632 nm and 545 nm for TBO and RB, respectively) was measured. In all of the experiments, the absorbance of the solutions was found to be extremely low (A632, <0.009; A545, <0.003).
The Mann-Whitney U test was used to compare the number of survivors in the various suspensions (L+ S+, L+ S−, L− S+) with the number of survivors from the control samples (L− S−). A P value of <0.05 was considered statistically significant. In none of the experiments did exposure of the microbial suspensions to the coatings in the dark result in a significant reduction in the viable count of the suspension. Similarly, illumination of the microbial suspensions on the surfaces of the photosensitizer-free cellulose acetate coatings did not exert a microbicidal effect.
Following illumination for 2 h, the TBO-RB coating was able to achieve a 99.6% reduction in the viable count of a suspension containing approximately 2 × 106 CFU/ml of S. aureus (Table 1). After 6 h of illumination, a 100% kill was obtained (Table 1). A 100% kill of a methicillin-resistant strain of the organism was also achieved after 6 h of illumination (Table 1). S. aureus was also susceptible to killing when suspended in human saliva; no viable cells remained in a suspension containing 4.1 × 106 CFU/ml following 16 h of illumination. This is encouraging, because aerosols and droplets derived from oral and respiratory secretions are an important mode of transmission of infectious agents in a clinical setting (15).
TABLE 1.
Effects on viable counts of contact with a cellulose acetate coating containing toluidine blue and rose bengala and exposed to light from a 25-W fluorescent lamp
| Organism | Light exposure time (h) | Viable count (CFU/ml)b under the following conditionc:
|
% Reduction in viable count (L+ S+ vs L− S−) | Log10 reduction in viable count (L+ S+ vs L− S−) | |||
|---|---|---|---|---|---|---|---|
| L− S− | L− S+ | L+ S− | L+ S+ | ||||
| Staphylococcus aureus | 2 | 1.84 × 106 | 1.99 × 106 | 1.58 × 106 | 7.71 × 103* | 99.6 | 2.4 |
| Staphylococcus aureus | 6 | 2.21 × 106 | 1.8 × 106 | 1.86 × 106 | 0* | 100 | 6.3 |
| Methicillin-resistant Staphylococcus aureus | 6 | 2.69 × 106 | 2.81 × 106 | 3.02 × 106 | 0* | 100 | 6.4 |
| Clostridium difficile | 4 | 5.19 × 106 | 2.63 × 106 | 1.57 × 106 | 0* | 100 | 6.7 |
| Candida albicans | 16 | 1.99 × 105 | 2.18 × 105 | 2.33 × 105 | 2.39 × 104* | 88 | 0.9 |
| Bacteriophage φX174 | 16 | 1.34 × 106 | 9.13 × 105 | 8.15 × 105 | 1.2 × 105* | 91 | 1.1 |
| Escherichia coli | 6 | 1.96 × 106 | 1.85 × 106 | 1.9 × 106 | 1.48 × 106* | 24 | 0.1 |
| Escherichia coli | 16 | 1.92 × 106 | 1.79 × 106 | 2.09 × 106 | 0* | 100 | 6.3 |
Each at 25 μM.
Asterisks indicate that the viable count was significantly different (P < 0.05) from that for the L− S− condition by the Mann-Whitney U test.
L− S−, microbial suspension in contact with photosensitizer-free coatings and not illuminated; L− S+, microbial suspension in contact with photosensitizer-containing coatings and not illuminated; L+ S−, microbial suspension in contact with photosensitizer-free coatings and illuminated; L+ S+, microbial suspension in contact with photosensitizer-containing coatings and illuminated.
C. difficile also proved to be susceptible to killing by the illuminated coating: a 100% kill of a suspension of the organism (consisting mainly of vegetative cells) containing 5.19 × 106 CFU/ml was achieved after 4 h of illumination (Table 1). In contrast, the gram-negative organism E. coli appeared less susceptible, and little killing was observed after 6 h of illumination. However, illumination for 16 h resulted in 100% kills of a suspension containing approximately 2 × 106 CFU/ml of the organism (Table 1). It has been reported repeatedly that gram-positive bacteria are more susceptible to photodynamic inactivation than gram-negative bacteria, irrespective of which photosensitizer is used (16). The results obtained using photosensitizer-containing coatings in the current study support these findings. It is thought that the lower susceptibility of gram-negative bacteria is attributable to the presence of the outer membrane, which intercepts photogenerated reactive oxygen species (7). Candida albicans also appeared to be less susceptible than S. aureus to killing: an 88% reduction in the viable count of a suspension containing 1.99 × 105 CFU/ml was achieved after 16 h of illumination (Table 1).
Bacteriophage φX174 was susceptible to killing when the coatings were illuminated; a 91% reduction in the viable count of a suspension containing 1.34 × 106 PFU/ml was achieved after 16 h of illumination (Table 1). In terms of its ability to persist in the environment in an infectious state, bacteriophage φX174 has been shown to resemble the most resilient human-pathogenic viruses i.e., parvoviruses and polioviruses (12).
One possible problem associated with the use of such coatings is “photobleaching” of the photosensitizers, which can result from degradation of the photosensitizer by the singlet oxygen generated (5). However, when the photosensitizer-containing coating was exposed to seven cycles of alternating light and dark periods (16 h of light and 8 h of darkness), no reduction in its bactericidal activity was detectable; a 5.7 log10 reduction in the viable count of a suspension of S. aureus (2.3 × 106 CFU/ml) was obtained after 6 h of illumination. These findings suggest that photobleaching, at least in the short term, would not be a problem. If longer periods of light/dark or continuous light exposure did result in photobleaching, then it would be necessary to renew the coating on a regular basis, perhaps by spraying with a solution of the coating in a volatile solvent.
In this study we investigated the ability of a fluorescent lamp, similar to those used in United Kingdom hospitals, to activate photosensitizers embedded in a coating and thereby kill microbes in its vicinity. The results obtained demonstrate that it is possible to use light emitted by a fluorescent lamp to render cellulose acetate coatings containing TBO and RB effective at killing a range of microbes. TBO and RB were chosen for incorporation into the coating because together they are able to absorb strongly at many of the prominent wavelengths emitted by fluorescent lamps of the type commonly used in hospitals in the United Kingdom. The coatings were formed following evaporation of the solvent (acetone), and it is assumed that each photosensitizer molecule is embedded in the cellulose acetate layer, thereby trapping it but allowing the 1O2 molecules generated upon exposure to light to diffuse out and thereby interact with, and kill, any microbes associated with the surface of the coating. The leakage experiments carried out in the study demonstrated that the amounts of TBO and RB released from the coatings were extremely small. It is unlikely, therefore, that the microbicidal effect observed could be attributed to the photosensitizers leaching out of the coatings and being activated while in the bacterial suspension.
The data presented here demonstrate the feasibility of using a coating containing photosensitizers to photoinactivate microbes. Moreover, the levels of killing achieved (up to a 6.7 log10 reduction) should be more than sufficient for surface disinfection, since microbial densities encountered on hospital surfaces are generally much lower. One study, for example, showed that between 4 and 7 CFU/cm2 of S. aureus were present on the surfaces of rooms occupied by patients infected with the organism (13). Regulations governing lighting in hospitals in the United Kingdom specify minimum light levels for various locations within hospitals (2). For example, ward corridors need to have a minimum light intensity of 200 lx, while in Accident and Emergency examination rooms and operating theaters, light intensities of 1,000 and 50,000 lx, respectively, are necessary. Since the light intensity used in this study was 3,700 ± 20 lx, the light-activated coating described and tested here would be of particular use in examination rooms and operating theaters, where light intensities are highest. However, it is also possible that these coatings could achieve appreciable kills under lower light intensities if the illumination time were increased to produce higher light energy doses, e.g., by leaving lights on for 24 h per day. Alternatively, lamps emitting a higher light intensity could be used. Overall, these coatings show potential as self-disinfecting surfaces. The next step will be to perform a detailed evaluation of the activity of the coatings in the hospital environment.
Acknowledgments
This work was supported by a grant from the Charles Wolfson Charitable Trust.
REFERENCES
- 1.Bozja, J., J. Sherrill, S. Michielsen, and I. Stojiljkovic. 2003. Porphyrin-based, light-activated antimicrobial materials. J. Polymer Sci. Part A 41:2297-2303. [Google Scholar]
- 2.Chartered Institution of Building Services Engineers. 1989. CIBSE lighting guide LG2: hospitals and healthcare buildings. Chartered Institution of Building Services Engineers, London, United Kingdom.
- 3.Cozad, A., and R. D. Jones. 2003. Disinfection and the prevention of infectious disease. Am. J. Infect. Control 31:243-254. [DOI] [PubMed] [Google Scholar]
- 4.Department of Health. 2001. Standard principles for preventing hospital-acquired infections. J. Hosp. Infect. 47:S21-S37. [Google Scholar]
- 5.Finlay, J. C., S. Mitra, M.S. Patterson, and T. H. Foster. 2004. Photobleaching kinetics of Photofrin in vivo and in multicell tumour spheroids indicate two simultaneous bleaching mechanisms. Phys. Med. Biol. 49:4837-4860. [DOI] [PubMed] [Google Scholar]
- 6.Garner, J. S., et al. 1996. Guideline for isolation precautions in hospitals. Infect. Control Hosp. Epidemiol. 1:53-80. [DOI] [PubMed] [Google Scholar]
- 7.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hota, B. 2004. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 39:1182-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Audit Office. 17. February 2000. Report by the Comptroller and Auditor General, HC 230 session 1999-2000. The management and control of hospital acquired infection in acute NHS trusts in England. Stationery Office, London, United Kingdom. [Online.] http://www.nao.org.uk/publications/nao_reports/9900230.pdf.
- 10.National Audit Office. 14. July 2004. Report by the Comptroller and Auditor General, HC 876 session 2003-2004. Improving patient care by reducing the risk of hospital acquired infection: a progress report. Stationery Office, London, United Kingdom. [Online.] http://www.nao.org.uk/publications/nao_reports/03-04/0304876.pdf.
- 11.Plowman, R. 2000. The socioeconomic burden of hospital acquired infection. Euro. Surveill. 5:49-50. [DOI] [PubMed] [Google Scholar]
- 12.Rheinbaben, F., S. Schünemann, T. Gross, and M. H. Wolff. 2000. Transmission of viruses via contact in a household setting: experiments using bacteriophage φX174 as a model virus. J. Hosp. Infect. 46:61-66. [DOI] [PubMed] [Google Scholar]
- 13.Rutala, W. A., E. B. Katz, R. J. Sherertz, and F. A. Sarubbi, Jr. 1983. Environmental study of a methicillin-resistant Staphylococcus aureus epidemic in a burn unit. J. Clin. Microbiol. 18:683-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutala, W. A., and D. J. Weber. 2004. The benefits of surface disinfection. Am. J. Infect. Control 32:226-231. [DOI] [PubMed] [Google Scholar]
- 15.Sehulster, L. M., R. Y. W. Chinn, M. J. Arduino, J. Carpenter, R. Donlan, D. Ashford, R. Besser, B. Fields, M. M. McNeil, C. Whitney, S. Wong, D. Juranek, and J. Cleveland. 2004. Guidelines for environmental infection control in health-care facilities. Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). American Society for Healthcare Engineering/American Hospital Association, Chicago, Ill.
- 16.Usacheva, M. N., M. C. Teichert, and M. A. Biel. 2001. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 29:165-173. [DOI] [PubMed] [Google Scholar]
- 17.Wilson, M. 2003. Light-activated antimicrobial coating for the continuous disinfection of surfaces. Infect. Control Hosp. Epidemiol. 24:782-784. [DOI] [PubMed] [Google Scholar]