Abstract
The effects of erythromycin (a 14-membered ring macrolide) and rokitamycin (a 16-membered ring macrolide) on the viability of the Streptococcus pyogenes M phenotype were studied by means of flow cytometry and fluorescence microscopy by using a combination of two fluorochromes (syto 9 and propidium iodide) that stains live bacteria green and dead bacteria red. In order to apply the flow cytometry, a bacterial sonication procedure was expressly set up to separate single cells from the long, intralaced S. pyogenes chains of up to 30 to 40 cells that have previously prevented the application of flow cytometry to this type of bacteria. The association of flow cytometry using an appropriate sonication procedure, together with a combination of fluorescent probes, offered the possibility of very quickly investigating the different microbiological effects of rokitamycin at 2 μg/ml, which was active on the S. pyogenes M phenotype, and of erythromycin at doses of up to 32 μg/ml, which was not.
Flow cytometry makes it possible to study the morphological and physiological characteristics of individual cells and their distribution within large cell populations in a short period of time. It has wide-ranging clinical and experimental applications in investigations of eukaryotic cells and also looks very promising in the case of bacteria.
The application of flow cytometry in microbiology requires the use of particularly sensitive fluorochromes and a higher signal amplification and more precise instrument calibration than in the case of eukaryotic cells because the volume of bacteria and DNA content are typically 1,000 times less than those of mammalian cells (1) and because the amount of fluorescent dye that binds to DNA may be 1 order of magnitude smaller (4). Nevertheless, the application of flow cytometry in microbiology allows the study of bacterial respiratory activity (9, 17), membrane potential (11, 13), calcium ion concentrations and pH (20), enzymatic activity (3, 10), and bacterial growth and metabolism (21).
Furthermore, with the aid of particular fluorescent probes, flow cytometry can also be used to monitor antibiotic-induced injury in bacteria, which opens up interesting possibilities for investigating the antibacterial activities of different antibiotics (2, 14, 15, 18, 21, 23).
The basic requirement for flow cytometry is the presence of single cells that can flow separately along the flow channel. It is for this reason that studies of bacteria have mainly concentrated on Escherichia coli and Staphylococcus aureus (2, 6,16), which are generally present in cultures as single cells with a relatively uniform shape. Other bacteria with different structures, such as Streptococcus pyogenes, Streptococcus pneumoniae, and Bacillus cereus, have not yet been studied because they are generally not single cells but have two or more cells attached to each other to form intertwining chains of different lengths, thus making them more difficult to study using flow cytometry.
However, as flow cytometry has the unique characteristic of providing useful information about very large bacterial populations in just a few seconds, efforts should be made to apply this methodology even in studies dealing with bacteria such as S. pyogenes or S. pneumoniae, which are important because of the various types of disease for which they are responsible and because of their increasing resistance to previously active antibiotics such as macrolides and β-lactams.
The importance of streptococcal infections has increased over recent years in North America and Europe, as well as in other parts of the world (8). The incidence of S. pyogenes erythromycin resistance has reached about 40% in Italy (5, 19, 22). One interesting finding is that the presence of the mef(A) gene in S. pyogenes produces the so-called M phenotype, which is resistant to 14- and 15-membered ring macrolides (erythromycin, clarithromycin, and azithromycin) but is still susceptible to 16-membered ring macrolides (rokitamycin, josamycin, spiramycin, and myocamycin). A recent survey in Italy of 387 clinical strains of erythromycin-resistant S. pyogenes found that the distribution of erythromycin-resistant strains with an M phenotype reached on average 52.5% (7).
This study was undertaken to explore the possibility of applying flow cytometry to chaining bacteria such as S. pyogenes and to verify whether it was useful in analyzing differences in susceptibility to 14- and 16-membered ring macrolides.
We used three S. pyogenes M phenotype strains recently isolated from clinical specimens. The flow cytometry and fluorescence microscopy data obtained from the untreated strains were the control data to be compared with the data collected in the same strains treated with antibiotics. Erythromycin and rokitamycin were respectively obtained from Sigma and Prodotti Formenti. Stock solutions were prepared by dissolving 2 mg in ethanol; the working solutions were diluted to the appropriate concentration in 1 ml of Mueller-Hinton broth. The suspensions of each organism were prepared from overnight cultures in Mueller-Hinton broth (Oxoid, Milan, Italy) with fetal calf serum at 37°C under static conditions. An inoculum of 105 CFU of the organisms was added to 1 ml of Mueller-Hinton broth containing serial twofold dilutions of the antibiotics in order to determine their MICs under the same conditions as those used to culture the bacteria. After incubation at 37°C for 18 h, the MIC was recorded as the lowest concentration of antibiotic that completely inhibited visible growth of the organism (macrodilution method [NCCLS]) (15). The mean rokitamycin MIC for the S. pyogenes strains was 0.5 μg/ml, whereas no inhibition of bacterial growth was observed with up to 32 μg of erythromycin/ml.
To investigate the microbiological effects of the two macrolides by means of flow cytometry, we used the Live/Dead BacLight kit (Molecular Probes, Eugene, Oreg.) containing the two fluorochromes syto 9 and propidium iodide. syto 9 alone generally labels all of the bacteria in a population whatever the condition of their membranes, whereas propidium iodide penetrates all of the bacteria with a damaged membrane and reduces their syto 9 fluorescence when both dyes are present. An appropriate mixture of syto 9 and propidium iodide therefore stains the bacteria with intact cell membranes fluorescent green and stains the bacteria with damaged membranes fluorescent red.
All of the strains were grown in medium with or without antibiotics (rokitamycin, 2 μg/ml; and erythromycin, 32 μg/ml) at 37°C under static conditions, and at 0, 2, 4, and 6 h, a 1-ml aliquot was removed from the cultures and the constituents of the Live/Dead BacLight kit (1.5 μl of syto 9 plus 1.5 μl of propidium iodide) were simultaneously added. The bacteria were allowed to incubate with the dyes in the dark for 15 min at room temperature before flow cytometry analysis. The assays do not require a wash step.
The flow cytometry studies were carried out using a Partec Pas flow cytometer equipped with a 25-mW, 488-nm argon ion laser.
Events were collected by triggering on sideward laser light-scattering detection (side scatter), because we have found that it is more sensitive for bacteria than is forward scatter.
Syto 9 green fluorescence was detected using a 515- to 550-nm-bandwidth band- pass filter (Omega, Brattleboro, Vt.) and propidium iodide red fluorescence using a 610- nm filter (Omega, Brattleboro, Vt.). All of the parameters were detected in log scale. The sheath fluid consisted of bidistillate deionized water passed through a 0.1-μm filter (Millipore, Bedford, Mass.). All of the media and buffers were filtered through a 0.2-μm-pore-size membrane before being used. The sheath flow rate was 2 μl/s. The performance of the machine was monitored before all of the experiments by using 1-μm fluorescent beads (Polysciences, Inc., Warrington, Pa.). The data were analyzed using a built-in software package.
The samples were allowed to run for 1 min before the acquisition of data through a built-in volumetric sample injection recording.
The findings are shown in the form of density dot plot graphs (a common way of representing the flow cytometry data) and micrographs of various bacterial samples dyed with fluorochromes in order to facilitate the interpretation of the dot plots. The cytometric counts (means values plus or minus standard deviations) of the three strains were expressed as curves to highlight the different behavior of erythromycin and rokitamycin. A culture of S. pyogenes under normal conditions has a mixture of single and interlaced bacterial chains of different lengths (from 3 or 4 to 30 to 40 cells and sometimes more), but the best condition for accurate flow cytometry is a suspension of single cells; thus, a possible solution is to disaggregate the chains of S. pyogenes in order to obtain individual cells. To do this, the cells can be disaggregated by chemical or mechanical means; for bacteria, ultrasonic treatment is the most convenient. Bacterial samples were sonicated in a Sonoplus HD 70 (Bandelin Electronic, Berlin, Germany) for 20 s at 30 cycles, with the probe tip being 5 mm below the liquid surface of a 1-ml sample. During sonication, the tubes were surrounded by ice.
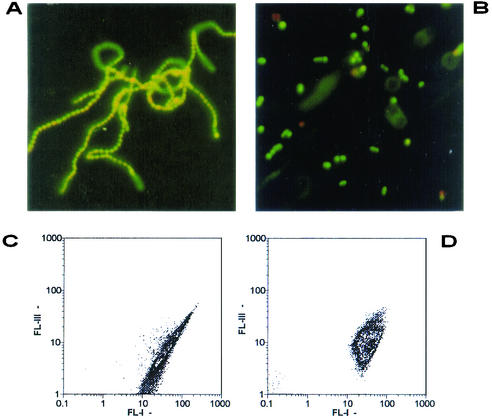
Figure 1A shows an example of the intertwined untreated S. pyogenes chains (stained using the Live/Dead BacLight kit), and Fig. 1B shows how sonication disaggregates the chains to individual cells, thus making them more suitable for flow cytometry investigation. Figure 1C shows an example of the flow cytometry dot plots of the dual parameters FL-I (syto 9) and FL-III (propidium iodide) of the S. pyogenes chains in nonsonicated samples: an elliptic distribution with an unusually long axis is a clue for the presence of a population of dishomogeneous length, shape, and fluorescence, while the distribution along the axis depends from the amount of fluorochrome adsorbed. After sonication, the dot plot is more compact (Fig. 1D), thus indicating the presence of more homogeneous particles and fluorescence.
FIG. 1.
Examples of interlaced chains of untreated S. pyogenes before sonication (A) and the individual cells obtained after sonication (B). Examples of the corresponding density dot plots (FL-I, syto 9; FL-III, propidium iodide) of the chains (C) and individual cells after sonication (D).
To ensure that the application of ultrasonic energy did not modify the number of live and/or dead bacteria before and after antibiotic treatment, the samples were monitored microscopically.
Aliquots of samples of S. pyogenes strains incubated or not with antibiotics and stained using the Live/Dead BacLight kit were observed under fluorescence microscopy, and the number of green and red bacteria present in the chains was counted in randomly chosen fields. After sonication, the green and red bacterial counts were repeated. The percentages of green and red untreated bacteria after 2 h of incubation were, respectively, 90.6 ± 4.4 and 9.3 ± 4.3 and, after sonication, 90.3 ± 3.3 and 9.6 ± 3.3. The results were similar with erythromycin-treated bacteria. In tests after 2 h of incubation with 2 μg of rokitamycin/ml, the percentages were 83.4 ± 9.2 and 16.5 ± 9.2 and, after sonication, 81.5 ± 9.3 and 18.5 ± 9.3.
These findings show that the use of low-level ultrasonic energy to separate single bacteria did not significantly interfere with their status even in the presence of active antibiotics.
Challenges of the S. pyogenes M phenotypes with erythromycin and rokitamycin revealed differences in their microbiological effects.
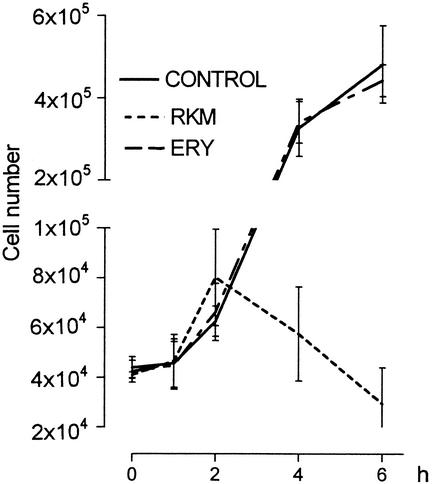
Figure 2 shows the results of the cytometry counts collected at various times in untreated bacteria (control) and in bacteria treated with erythromycin or rokitamycin. The cytometry counts (means for the three strains plus or minus standard deviations) increased up to the 2nd h of incubation with rokitamycin and then significantly and progressively decreased, thus indicating the presence of an antimicrobial effect (Fig. 2). The patterns of the counts of the bacteria treated with erythromycin overlap those of the untreated bacteria, thus indicating no antibacterial effect during the observation period.
FIG. 2.
Growth curves of S. pyogenes incubated with no drug (control), erythromycin (ERY) (32 μg/ml), and rokitamycin (RKM) (2 μg/ml).
Microscopic observations confirmed that the majority of bacteria in the three untreated S. pyogenes strains were green, as were those treated with erythromycin. However, some of the bacteria treated with rokitamycin started to become red, and this number was progressively increased after the 2nd h. It was also observed that these red cells had a short life span because, after incubation with 2 μg of rokitamycin/ml, they become bigger, lose cytoplasm, and rapidly disappear, thus reducing the number of bacteria in the culture. When individual bacteria are affected by antibiotics and lose their viability, they become part of a new subpopulation with a different distribution that can be highlighted by flow cytometry fluorescence emission (12). The possibility of recognizing the presence of different subpopulations with different dot plot distribution thus depends on the speed of disappearance of the red bacterial cells.
In order to show this more clearly, we have added a further finding obtained by incubating the bacteria with half the rokitamycin MIC. When subinhibitory concentrations are used, the process of antibiotic injury takes longer and so the presence of the population of red bacteria (injured) is more evident.
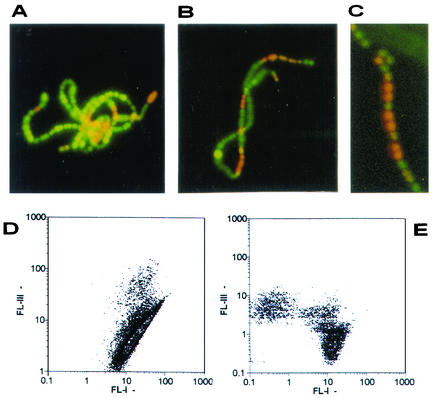
Figure 3A to C show that red bacterial cells were now present and were randomly distributed with still green bacteria inside nonsonicated chains when S. pyogenes was incubated with half the rokitamycin MIC.
FIG. 3.
Examples of the random distribution of red cells inside chains of S. pyogenes incubated with half the rokitamycin MIC (A to C). Density dot plots (FL-I, syto 9; FL-III, propidium iodide) of S. pyogenes incubated with half the rokitamycin MIC before (D) and after sonication (E).
In this situation, flow cytometry detection is working in nonoptimal conditions because the final green or red fluorescence signal is not due to a single green or red cell but actually comes from the sum of the individual signals of all of the green and red bacteria randomly trapped in the chain. The corresponding density dot plot, shown in Fig. 3D, has again the long axis typical of chained cells.
After sonication and consequent bacterial disaggregation, the fluorescence signals are recorded as single events so that the flow cytometry detection now works under optimal conditions, as is confirmed by the plot in Fig. 3E, in which distinct green and red subpopulations can be clearly seen.
In conclusion, the association of flow cytometry with an appropriate sonication procedure and a combination of fluorescent probes is a new, rapid, and sensitive alternative to other methods used for revealing differences in antibiotic susceptibility or the presence of resistant strains in bacteria such as S. pyogenes in a very short time.
Acknowledgments
We thank Prodotti Formenti for the kind gift of rokitamycin and R. Alberici for her help and fruitful comments on some technical aspects.
This study was partially supported by a grant from MURST (ex 60%).
REFERENCES
- 1.Allman, R., R. Manchee, and D. Lloyd. 1993. Flow cytometric analysis of heterogeneous bacterial populations, p. 27-47. In D. Lloyd (ed.), Flow cytometry in microbiology. Springer-Verlag, London, United Kingdom.
- 2.Alvarez-Barrientos, A., J. Arroyo, R. Canton, C. Nombela, and M. Sanchez-Perez. 2000. Applications of flow cytometry to clinical microbiology. Clin. Microbiol. Rev. 13:167-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azimi, N. T., F. E. Lytle, D. M. Huber, J. E. Whitaker, and R. P. Haugland. 1990. Multiple reagent aminopeptidase profiling of bacteria. Appl. Spectrosc. 44:400-403. [Google Scholar]
- 4.Boye, E., and H. B. Steen. 1993. The physical and biological basis for flow cytometry of Escherichia coli, p. 11-25. In D. Lloyd (ed.), Flow cytometry in microbiology. Springer-Verlag, London, United Kingdom.
- 5.Braga, P. C. 2002. Rokitamycin: bacterial resistance to a 16-membered ring macrolide differs from that to 14- and 15-membered ring macrolides. J. Chemother. 14:115-131. [DOI] [PubMed] [Google Scholar]
- 6.Davey, H. M., and D. B. Kell. 1996. Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analysis. Microbiol. Rev. 60:641-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovanetti, E., M. P. Montanari, M. Mingoia, and P. E. Varaldo. 1999. Phenotypes and genotypes of erythromycin-resistant Streptococcus pyogenes strains in Italy and heterogeneity of inducible resistant strains. Antimicrob. Agents Chemother. 47:1935-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan, E. L. 1991. The resurgence of group A streptococcal infections and their sequelae. Eur. J. Clin. Microbiol. Infect. Dis. 10:55-57. [DOI] [PubMed] [Google Scholar]
- 9.Kaprelyants, A. S., and D. B. Kell. 1993. The use of 5-cyano-2,3-ditolyl tetrazolium chloride and flow cytometry for the visualization of respiratory activity of individual cells of Micrococcus luteus. J. Microbiol. Methods 17:115-122. [Google Scholar]
- 10.Manafi, M., W. Kneifel, and S. Bascomb. 1991. Fluorogenic and chromogenic substrates used in bacterial diagnostics. Microbiol. Rev. 55:335-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason, D. J., R. Allman, and D. Lloyd. 1993. Uses of membrane potential dyes with bacteria, p. 67-81. In D. Lloyd (ed.), Flow cytometry in microbiology. Springer-Verlag, London, United Kingdom.
- 12.Mason, D. J., R. Allman, J. M. Stark, and D. Lloyd. 1994. Rapid estimation of bacterial antibiotic susceptibility with flow cytometry. J. Microsc. 176:8-16. [DOI] [PubMed] [Google Scholar]
- 13.Mason, D. J., R. Lopez-Amoros, R. Allman, J. M. Stark, and D. Lloyd. 1995. The ability of membrane potential dyes and calcafluor white to distinguish between viable and non-viable bacteria. J. Appl. Bacteriol. 78:309-315. [DOI] [PubMed] [Google Scholar]
- 14.Mortimer, F. C., D. J. Mason, and V. A. Gant. 2000. Flow cytometric monitoring of antibiotic-induced injury in Escherichia coli using cell-impermeant fluorescent probes. Antimicrob. Agents Chemother. 44:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. 1990. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A2. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 16.Pore, R. S. 1994. Antibiotic susceptibility testing by flow cytometry. J. Antimicrob. Chemother. 34:613-627. [DOI] [PubMed] [Google Scholar]
- 17.Pyle, B. H., S. C. Broadway, and G. A. McFeters. 1995. A rapid direct method for enumerating respiring enterohemorrhagic Escherichia coli O157:H7 in water. Appl. Environ. Microbiol. 61:2614-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth, B. L., M. Poot, S. T. Yue, and P. J. Millard. 1997. Bacterial viability and antibiotic susceptibility testing with SYTOX Green nucleic acid stain. Appl. Environ. Microbiol. 63:2421-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schito, G. C., A. Pesce, and E. A. Debbia. 1999. Razionale microbiologico per l'uso di antibiotici orali nella terapia delle infezioni respiratorie comunitarie alla luce dell'attuale realtà epidemiologica italiana. Gior. Ital. Microb. Med. Odont. Clinica 3(Suppl. A):SA35-SA58. [Google Scholar]
- 20.Shapiro, H. M. 1988. Practical flow cytometry, 2nd ed. Alan R. Liss, Inc., New York, N.Y.
- 21.Steen, H. B. 1990. Flow cytometric studies on microorganisms, p. 605-622. In M. R. Meland, T. Lindmo, and M. L. Mendelsohn (ed.), Flow cytometry and sorting, 2nd ed. Wiley-Liss, New York, N.Y.
- 22.Varaldo, P. E., E. A. Debbia, G. Nicoletti, D. Pavesio, S. Ripa, G. C. Schito, G. Tempera, et al. 1999. Nationwide survey in Italy of treatment of Streptococcus pyogenes pharyngitis in children: influence of macrolide resistance on clinical and microbiological outcomes. Clin. Infect. Dis. 29:869-873. [DOI] [PubMed] [Google Scholar]
- 23.Walberg, M., P. Gaustad, and H. B. Steen. 1996. Rapid flow cytometric assessment of mecillinam and ampicillin bacterial susceptibility. J. Antimicrob. Chemother. 37:1063-1075. [DOI] [PubMed] [Google Scholar]