Abstract
In the C4 plant Guinea grass (Panicum maximum), phosphoenolpyruvate carboxykinase (PEPCK) is phosphorylated in darkened leaves and dephosphorylated in illuminated leaves. To determine whether the properties of phosphorylated and non-phosphorylated PEPCK were different, PEPCK was purified to homogeneity from both illuminated and darkened leaves. The final step of the purification procedure, gel filtration chromatography, further separated phosphorylated and non-phosphorylated forms. In the presence of a high ratio of ATP to ADP, the non-phosphorylated enzyme had a higher affinity for its substrates, oxaloacetate and phosphoenolpyruvate. The activity of the non-phosphorylated form was up to 6-fold higher when measured at low substrate concentrations. Comparison of proteoloytically cleaved PEPCK from Guinea grass, which lacked its N-terminal extension, from yeast (Saccharomyces cerevisiae), which does not possess an N-terminal extension, and from the C4 plant Urochloa panicoides, which possesses an N-terminal extension but is not subject to phosphorylation, revealed similar properties to the non-phosphorylated full-length form from Guinea grass. Assay of PEPCK activity in crude extracts of Guinea grass leaves, showed a large difference between illuminated and darkened leaves when measured in a selective assay (a low concentration of phosphoenolpyruvate and a high ratio of ATP to ADP), but there was no difference under assay conditions used to estimate maximum activity. Immunoblots of sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels showed no difference in the abundance of PEPCK protein in illuminated and darkened leaves. There were no light/dark differences in activity detected in maize (Zea mays) leaves, in which PEPCK is not subject to phosphorylation.
The aim of this study was to investigate whether phosphorylation of phosphoenolpyruvate (PEP) carboxykinase (PEPCK) from plants alters its properties or its activity. In plants, PEPCK is a cytosolic enzyme that catalyzes the reversible reaction:
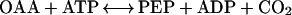 |
PEPCK occurs in a diverse range of plant tissues, including both developing and germinating seeds, flowers, fruits, trichomes, the vasculature, and leaves of some Crassulacean acid metabolism (CAM) and C4 plants (Leegood and Walker, 1999; Walker et al., 2001). In leaves of CAM plants and C4 plants and in some algae, PEPCK functions as a decarboxylase in their photosynthetic CO2-concentrating mechanisms (Reiskind and Bowes, 1991; Leegood et al., 1996). C4 plants are divided into different subtypes on the basis of the enzyme decarboxylating C4 acids in the bundle sheath. The original classification into three subtypes (PEPCK, NAD-malic enzyme, and NADP-malic enzyme; Hatch et al., 1975) is now known to be an over-simplification, since the PEPCK subtypes, such as Guinea grass (Panicum maximum), also utilize NAD-malic enzyme as a decarboxylase (Burnell and Hatch, 1988) and some monocot members of the NADP-malic enzyme subtype, such as maize (Zea mays), also utilize PEPCK to decarboxylate Asp (Walker et al., 1997; Furumoto et al., 1999; Wingler et al., 1999).
In the leaves of C4 plants, the amounts of both PEPCK protein and activity change little during a diurnal period, an observation that is at variance with the proposal that the activity of PEPCK must be regulated to prevent depletion of oxaloacetate (OAA) and/or ATP in the cytosol in the dark (Carnal et al., 1993). Similarly, in cotyledons of germinating cucumber, in which PEPCK is important in catalyzing a gluconeogenic flux from lipid and protein reserves, the reaction catalyzed by PEPCK is displaced from equilibrium despite the amount of PEPCK being in excess of that required to catalyze the flux (Leegood and ap Rees, 1978). These observations raised the possibility that the activity of PEPCK was subject to an as-yet-unknown regulatory mechanism. A clue as to the nature of this mechanism was provided by the observation that PEPCK is subject to reversible protein phosphorylation in many plant tissues (Walker and Leegood, 1995, 1996). PEPCK is phosphorylated in the cotyledons of all germinating seedlings and leaves of PEPCK-type CAM plants studied (Walker and Leegood, 1995; Walker et al., 1997). In contrast, PEPCK is subject to reversible phosphorylation in the leaves of some C4 plants, such as Guinea grass, but not in leaves of others, such as Urochloa panicoides and maize (Walker and Leegood, 1996; Walker et al., 1997), although maize PEPCK has been shown to be weakly phosphorylated by a cAMP-dependent protein kinase in vitro (Furumoto et al., 1999). In CAM plants and in C4 plants in which PEPCK is subject to phosphorylation, it is phosphorylated in darkened leaves and dephosphorylated in illuminated leaves (Walker and Leegood, 1996). These observations suggest that, if phosphorylation does modulate the activity of PEPCK, then the phosphorylated enzyme is the less active form.
In common with PEP carboxylase (Chollet et al., 1996), PEPCK from plants possesses an extension at the N terminus that is absent from enzymes with otherwise very similar sequences from bacteria or fungi (Walker et al., 1997). This N-terminal extension is rapidly lost by proteolysis upon extraction of the enzyme and is likely to be contain site(s) at which PEPCK is phosphorylated because loss of the N-terminal extension leads to the loss of 32P (Walker and Leegood, 1996). An examination of the available sequences of plant PEPCK shows that the sequence of the N-terminal extension, unlike the rest of the protein, is quite variable except for one region which often contains two potential phosphorylation sites. One is a cAMP-dependent protein kinase site. PEPCK from cucumber is a substrate for this protein kinase (Walker and Leegood, 1995). The other is a consensus sequence for the SNF-1 related protein kinases (Halford and Hardie, 1998; Leegood and Walker, 1999). The latter are thought to be global regulators of carbon metabolism in plants, implicated in the regulation of Suc-P synthase, nitrate reductase, and 3-hydroxy-3-methyl glutaryl coenzyme A reductase (Halford and Hardie, 1998). Both these potential sites are absent from U. panicoides leaf PEPCK (Finnegan and Burnell, 1995), which is not subject to phosphorylation (Walker and Leegood, 1996).
In this paper, we describe procedures for the purification of intact phosphorylated and non-phosphorylated PEPCK from Guinea grass leaves and show how phosphorylation modulates its activity. We then show how a selective assay based on these changes in properties can be used to estimate its phosphorylation state in crude leaf extracts.
RESULTS
Purification of PEPCK
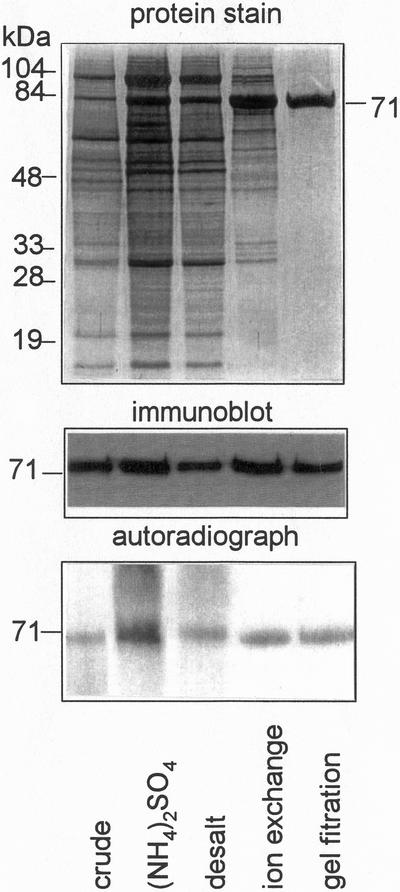
The N-terminal extension of PEPCK from both cotyledons of germinating cucumber and darkened leaves of Guinea grass, contains the site(s) at which the protein is phosphorylated in vivo (Walker and Leegood, 1995, 1996). This N-terminal extension is rapidly lost by proteolysis after extraction of the enzyme (Walker et al., 1995). To prevent proteolysis, all purification procedures were done at pH 9.8 (Walker et al., 1995). PEPCK is much more heavily phosphorylated in darkened leaves of Guinea grass (Walker and Leegood, 1996). To obtain phosphorylated and non-phosphorylated enzyme, PEPCK was therefore purified from both darkened and illuminated leaves. In both cases, a 150-fold purification resulted in a single polypeptide of 71 kD after SDS-PAGE (Table I; Fig. 1), the same size as the intact polypeptide from leaves of Guinea grass (Walker and Leegood, 1996). The yield of pure PEPCK from crude extracts of both illuminated and darkened leaves was between 20% and 25%. To determine whether phosphate was lost from the enzyme during purification, PEPCK was purified from darkened leaves in which it had been labeled with 32Pi. Loss of phosphate was determined by comparing the abundance of PEPCK at different stages of purification on an immunoblot of a SDS-PAGE gel with the extent of phosphorylation shown by autoradiography of the immunoblot. The ratio of intensity of PEPCK on the immunoblot to the autoradiograph did not change greatly during purification, showing that the phosphorylation state of the enzyme remained largely unchanged.
Table I.
Purification of PEPCK from leaves of Panicum maximum
| Purification Stage | Total Activity | Protein | Specific Activity | Purification | Yield |
|---|---|---|---|---|---|
| μmol min−1 | mg | units mg−1 protein | -fold | % | |
| Crude extract | 172 | 627 | 0.27 | 1 | 100 |
| 30–50% (NH4)2SO4 | 80 | 27 | 3.0 | 11 | 47 |
| Ion-exchange chromatography | 50 | 5 | 10.0 | 37 | 29 |
| Gel-filtration chromatography | 38 | 0.9 | 41.6 | 154 | 22 |
PEPCK activity at different stages of the purification was measured in the carboxylation direction under conditions that allowed measurement of its maximum activity (saturating Mn2+).
Figure 1.
Analysis of fractions from different stages of the purification of 32P-labeled PEPCK from Guinea grass leaves. After separation by SDS-PAGE, polypeptides were visualized by Coomassie Brilliant Blue dye, and radiolabeling of PEPCK assessed by autoradiography. Polypeptides in an identically loaded gel were transferred to Immobilon P membrane and PEPCK visualized using an antiserum specific for PEPCK.
In darkened leaves a proportion of PEPCK was not phosphorylated and gel filtration chromatography was effective in separating two forms (Fig. 2). The molecular mass of the phosphorylated enzyme was 320 kD and that of the non-phosphorylated was 280 kD, which is consistent with the enzyme being a tetramer. This difference in mass was likely due to a conformational change rather than a change in subunit composition. It was not due to proteolytic cleavage of the enzyme since the molecular mass of the subunits remained constant at 71 kD (Fig. 2). PEPCK that eluted earlier from the column was strongly radiolabeled, whereas the enzyme eluting after the main peak of PEPCK activity was only weakly labeled.
Figure 2.
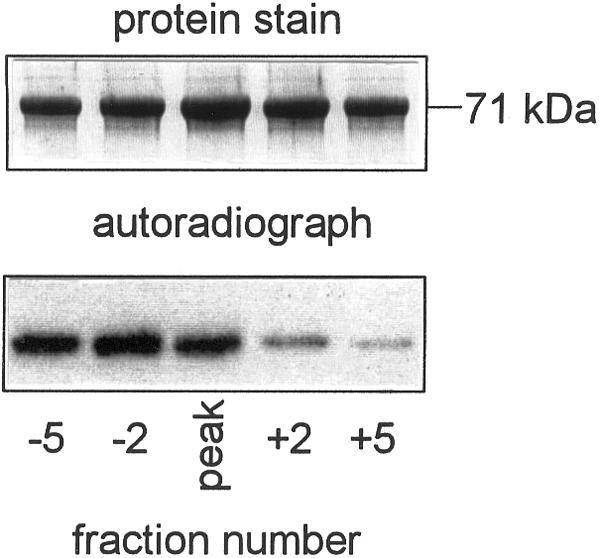
Separation of phosphorylated and non-phosphorylated forms of PEPCK by gel filtration chromatography. The gel filtration column was calibrated using molecular mass markers and elution times for fraction −5, peak and +5 corresponded to molecular masses of 320, 300, and 280 kD respectively. Fractions containing equal amounts of PEPCK activity were subjected to SDS-PAGE, polypeptides visualized by Coomassie Brilliant Blue dye, and radiolabeling assessed by autoradiography.
Effect of Phosphorylation on PEPCK Activity
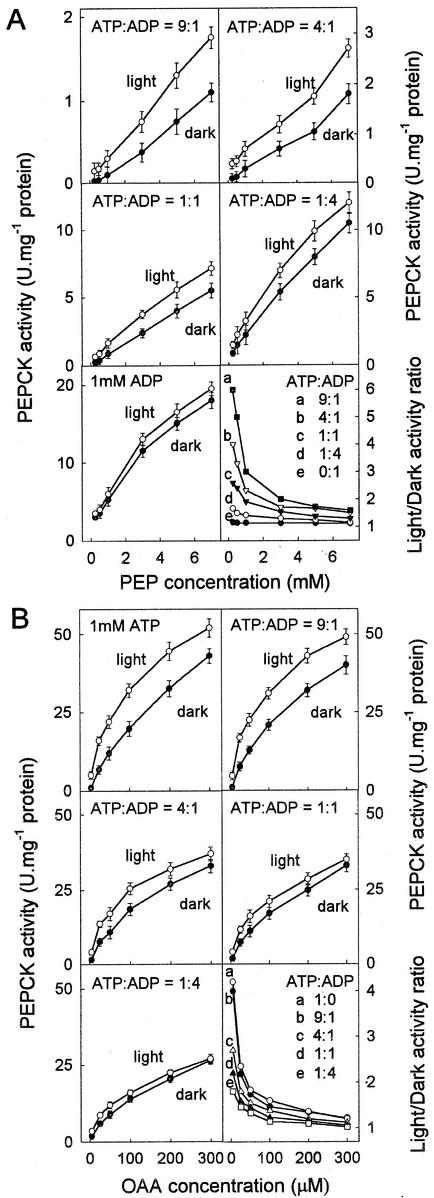
To determine how phosphorylation of PEPCK affects its activity, the properties of the phosphorylated and largely non-phosphorylated forms of the enzyme from darkened and illuminated leaves were compared in in vitro assays. In addition, phosphorylated and non-phosphorylated PEPCK purified from darkened leaves that were separated by gel filtration were compared. Kinetic differences between these two forms of the enzyme were similar to those observed for the enzymes purified separately from illuminated and darkened leaves (data not shown). Phosphorylation of PEPCK more or less doubled its Km for its substrates in the decarboxylation direction (Km [OAA] 156 ± 20 and 304 ± 25 μm; Km [ATP] 26 ± 4 and 44 ± 3 μm in the light and dark forms, respectively), but affinities for its substrates in the carboxylation reaction were little changed (Km [PEP] 2.1 ± 0.3 and 2.7 ± 0.3 mm; Km [ADP] 36 ± 3 and 44 ± 3 μm in the light and dark forms, respectively; under these measurement conditions all responses to substrates were hyperbolic). However, these changes in affinity were more complex because the concentration of each substrate and the phosphorylation state affected its affinity for other substrates. In the decarboxylation direction, both the concentration of ADP and ATP and the ratio of ADP to ATP greatly affected the affinity of the enzyme for both ATP and for OAA and these interactions were modulated by phosphorylation (Fig. 3B). The ratio of the activity of non-phosphorylated to phosphorylated PEPCK was compared at different concentrations of OAA and different ratios of ATP to ADP (Fig. 3B). The total concentration of adenylates was kept constant to minimize perturbations in free Mn2+ and Mg2+. The difference in activity was greatest at lower concentrations of OAA and at higher ratios of ATP to ADP.
Figure 3.
Effects of phosphorylation on the activity of PEPCK. A, The effect of phosphorylation on the carboxylase activity of PEPCK. The affinity of the phosphorylated and non-phosphorylated forms of the enzyme for PEP was determined at different ratios of ATP:ADP. B, The effect of phosphorylation on the decarboxylase activity of PEPCK. The affinity of the phosphorylated and non-phosphorylated forms of the enzyme for OAA was determined with different ratios of ATP to ADP. In both A and B the total concentration of adenylates was 1 mm. The rate at zero substrate concentration was always zero. To illustrate the interaction between phosphorylation state, ATP:ADP ratio and concentration of OAA or PEP the ratio of the activity of the non-phosphorylated (light) to phosphorylated (dark) enzyme for each value of ATP:ADP was plotted against the OAA or PEP concentration.
Similarly, in the carboxylation direction both the concentration of ATP and ADP and the ratio of ADP to ATP altered the affinity of the enzyme for ADP and PEP, and these interactions were modulated by phosphorylation (Fig. 3A). The ratio of the activity of non-phosphorylated to phosphorylated PEPCK was compared at different concentrations of PEP and at different ratios of ATP to ADP (Fig. 3A). There was essentially no difference between the two forms in the absence of ATP. The difference in activity was greatest at lower concentrations of PEP and higher ratios of ATP to ADP. Assayed in the carboxylation direction in the absence of ATP, the response to increasing PEP was hyperbolic, but increasing the amount of ATP strongly inhibited the rate and induced sigmoidal behavior.
Effect of Loss of N-Terminal Extension Site on PEPCK Activity
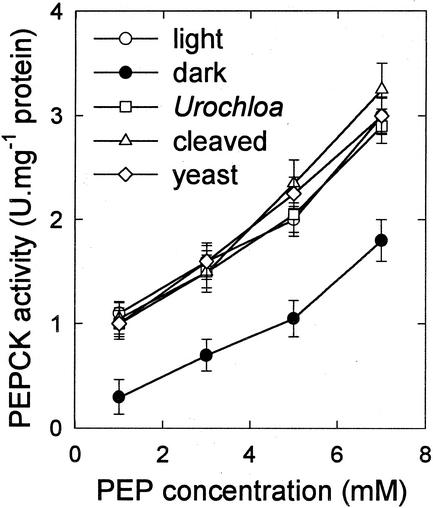
We investigated the effect of loss of the N-terminal region and its phosphorylation site by comparing the properties of (a) the proteolysed, truncated, form of PEPCK from Guinea grass; (b) PEPCK from leaves of the C4 grass, U. panicoides, which possesses a shorter N-terminal extension and which is not phosphorylated in vivo (Finnegan and Burnell, 1995; Walker et al., 1997); and (c) the enzyme from yeast (Saccharomyces cerevisiae), which does not possess the N-terminal extension (Krautwurst et al., 1995). A comparison of the affinity of the different forms of PEPCK for PEP is shown in Figure 4. Truncated PEPCK and PEPCK from yeast and from U. panicoides all behaved similarly to intact dephosphorylated PEPCK in showing a hyperbolic response to PEP in the carboxylation assay when measured in the absence of ATP (data not shown) and a remarkably similar slightly sigmoidal response to PEP when assayed at a high ATP to ADP ratio (Fig. 4, compare with the response curves in Fig. 3). By comparison, intact phosphorylated PEPCK showed a decrease in activity over the whole range of PEP concentrations (see also Fig. 3), however this difference was much more pronounced at lower concentrations of PEP.
Figure 4.
The effect of N-terminal phosphorylation on the response of PEPCK activity to the concentration of PEP in the assay. PEPCK from illuminated and darkened leaves of Guinea grass is compared with the proteolytically cleaved form of PEPCK from leaves of Guinea grass, intact PEPCK from U. panicoides, and the enzyme from yeast. All assays contained 0.8 mm ATP and 0.2 mm ADP.
Diurnal Changes in Phosphorylation State and Properties
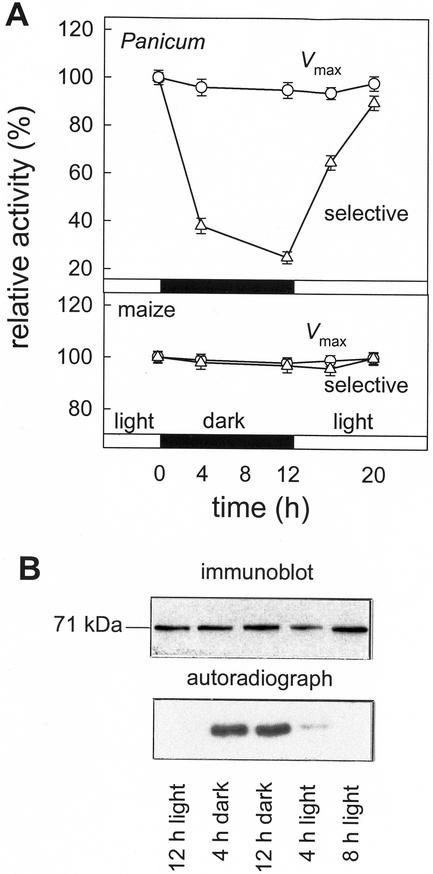
We investigated whether changes in the properties of PEPCK activity were correlated with changes in phosphorylation state in Guinea grass leaves during a light-dark cycle. Detached leaves were fed 32Pi and harvested at different times during a light-dark cycle. A western blot showed no change in the abundance of PEPCK protein (Fig. 5B), whereas autoradiography of a similarly loaded SDS-PAGE gel showed a large difference in the phosphorylation state. Similar changes in phosphorylation state were shown in PEPCK immunoprecipitated from these extracts. PEPCK was much more phosphorylated in darkened leaves than illuminated leaves. The degree of phosphorylation increased markedly after darkening leaves. Upon illumination the extent of phosphorylation decreased over a period of 8 h. The carboxylase activity of PEPCK was measured in crude extracts of leaves using (a) assay conditions that allowed measurement of maximum activity (non-selective, approximating to Vmax) and (b) under assay conditions that revealed a large difference in activity between phosphorylated and non-phosphorylated enzyme by the inclusion of ATP (selective). There was little change in PEPCK activity under non-selective conditions, but when activity was measured under conditions that discriminate between phosphorylated and non-phosphorylated forms of the enzyme, much less activity was present in extracts of darkened leaves (Fig. 5A). In contrast, similar measurements made on PEPCK extracted from leaves of maize (Fig. 5A) or U. panicoides (data not shown) showed no change in activation state. PEPCK in these plants is not phosphorylated (Walker et al., 1997).
Figure 5.
Diurnal changes in the amount and activity of PEPCK in leaves of Guinea grass and maize. A, Changes in the phosphorylation state of PEPCK were assessed by feeding detached illuminated leaves 32Pi by the transpiration stream. Leaves were placed in darkness for 12 h, then illuminated (500 μmol m−2 s−1, 25°C) for 12 h, darkened for 12 h, and then re-illuminated, during which time samples were taken. PEPCK activity was measured under conditions that estimate maximum activity (Vmax; 5 mm PEP, 5 mm Mn2+, and 0.5 mm ADP) and a selective assay (0.25 mm PEP, 10 μm MnCl2, 4 mm MgCl2, 0.8 mm ATP, and 0.2 mm ADP) that discriminates between the phosphorylated and non-phosphorylated forms of the enzyme. B, Diurnal changes in the amount of PEPCK protein and its phosphorylation state in Guinea grass leaves. Proteins were separated by SDS-PAGE and polypeptides transferred to immobilon P membrane. PEPCK was visualized using an antiserum specific for PEPCK and its phosphorylation state assessed by autoradiography of the blot.
DISCUSSION
We developed a purification procedure that results in minimal proteolysis of PEPCK from Guinea grass, and thus the N-terminal region, together with its phosphorylation site, is retained (Walker et al., 1995). Previous purification of the enzyme from the C4 plant, Urochloa panicoides, yielded PEPCK with a molecular mass of between and 62 and 64 kD (Burnell, 1986) as a result of proteolytic cleavage of the native 68-kD polypeptide (Finnegan and Burnell, 1995). It is likely that previous purifications of the enzyme from other C4 plants, including Guinea grass (Ray and Black, 1976; Urbina and Avilan, 1989) and Chloris gayana (Hatch and Mau, 1977), also yielded a cleaved enzyme (Walker et al., 1995, 1997).
Our results are consistent with a phosphorylation-based mechanism for the light-dark regulation of PEPCK in the leaves of C4 plants and are the first evidence from any organism for changes in the regulatory properties as a result of covalent modification of PEPCK. First, the enzyme isolated from darkened tissues showed kinetic differences to the enzyme isolated from illuminated tissues. Second, these two kinetic forms, with different phosphorylation states, could be separated by gel filtration. Third, there was a diurnal interconversion between the two kinetic forms that was associated with changes in the phosphorylation state of immunoprecipitated PEPCK. Fourth, the enzymes from maize and U. panicoides, that are not phosphorylated in vivo, showed no diurnal changes in activity when measured under selective assay conditions. In addition, the form purified from illuminated leaves could also be partially converted, by phosphorylation catalyzed by cAMP-dependent protein kinase, into a form with similar kinetic properties to the form purified from darkened leaves (data not shown).
Although diurnal changes in PEPCK activity have previously been reported for leaves of the CAM plants aloe (Aloe vera; Lin et al., 1991) and pineapple (Ananas comosus; Lin et al., 1994), with a 2- to 4-fold increase in activity recorded during the daytime deacidification (decarboxylation) phase, these assays were made under non-selective assay conditions, and changes in the amount of PEPCK were not measured. However, we measured similar changes in activation state of PEPCK in pineapple to those reported for Guinea grass in Figure 5 (data not shown), indicating that the properties of the pineapple enzyme are similar to those of PEPCK from Guinea grass.
The diurnal changes in the properties of PEPCK are the combined result of differences in substrate affinities and in sensitivity to adenylates of the phosphorylated and dephosphorylated enzyme. It seems unlikely that the difference in sensitivity of these two forms of the enzyme to adenylates was a simple mass-action effect (Wood et al., 1966; Walker et al., 1997). It has been suggested that adenylates interact with PEPCK at an allosteric site (Burnell, 1986; Urbina and Avilan, 1989). In this study we found that the ratio of ATP to ADP modulated the affinity of PEPCK for OAA and PEP. Assayed in the carboxylation direction in the absence of ATP, the response to increasing PEP was hyperbolic, but increasing the amount of ATP strongly inhibited the rate and induced sigmoidal behavior. A similar response to PEP was shown by PEPCK from yeast, by PEPCK from U. panicoides that lacks the phosphorylation site, and by PEPCK from Guinea grass that lacked the N-terminal extension. Sequence comparisons of PEPCKs from flowering plants show that they are closely related to the ATP-dependent enzymes from yeast, Rhizobium sp., Escherichia coli, and trypanosomes (Leegood and Walker, 1999). These results indicate that modulation of the kinetic properties of PEPCK by adenylates is a fundamental property of ATP-dependent PEPCKs and that in plants the possession of the N-terminal extension together with phosphorylation of a residue(s) within it enhances an existing mechanism of adenylate regulation (Fig. 4). It should also be noted that assay of recombinant maize PEPCK revealed a specific activity nearly 2-fold higher in the N-terminal truncated compared with the intact protein (Furumoto et al., 1999).
Regulation of PEPCK by adenylates could be an effective means of regulation in vivo. In leaves and protoplasts of C3 plants, the cytosolic ATP to ADP ratio is high (approximately 6; see Fig. 3B) and relatively constant between light and dark conditions (Stitt et al., 1982). If such conditions obtained in the bundle-sheath cytosol, phosphorylation of PEPCK could act as an off-switch in darkness, acting together with the decrease in the supply of OAA (and hence malate and Asp) from the mesophyll that would result from the dephosphorylation-dependent inactivation of PEP carboxylase in the dark (Chollet et al., 1996). During photosynthesis in PEPCK-type C4 species, NAD-malic enzyme operates in tandem with PEPCK, and mitochondrial respiration provides the ATP necessary to drive the PEPCK reaction (Burnell and Hatch, 1988; Carnal et al., 1993; Agostino et al., 1996). NAD-malic enzyme in plants is also regulated by adenylates (Furbank et al., 1991). Coordination of these decarboxylation reactions in C4 photosynthesis is therefore likely to be achieved at least partially by changes in adenylates. However, much more needs to be known about changes of pH and metabolite effectors in the bundle sheath before a theory of regulation can be formulated.
Why has regulation by phosphorylation been lost in many other C4 species? The smaller, non-phosphorylated, forms of PEPCK observed in many C4 plants (Walker et al., 1997) suggests that regulation by phosphorylation has been lost to suit its role in the relatively recently evolved C4 plants. This may be connected with substrate concentrations. Modulation of the affinity of PEPCK for OAA by phosphorylation only occurs at low, micromolar OAA concentrations (Fig. 3B). However, in leaves of Asp-forming C4 plants, the concentration of OAA appears to be very much higher than in C3 plants, in the millimolar range in leaves of Amaranthus edulis (Leegood and von Caemmerer, 1988). This is because OAA is generated from Asp by Asp aminotransferase, that has an equilibrium constant that favors OAA formation. If the concentration of OAA in Guinea grass leaves were also high, the modulation of the activity of PEPCK by phosphorylation would be much less effective than in C3 tissues. This indicates that PEPCK might also be effectively regulated by metabolites in C4 plants. Evidence for such regulation of PEPCK was provided by Carnal et al. (1993), who showed that the reaction catalyzed by PEPCK was not at equilibrium in the darkened leaves of U. panicoides (in which PEPCK is not phosphorylated).
In conclusion, this work describes how phosphorylation alters the kinetic properties of PEPCK to decrease its activity in darkened leaves. PEPCK joins a small number of other plant enzymes whose catalytic activity is known to be directly regulated by phosphorylation, such as PEP carboxylase, Suc phosphate synthase and pyruvate dehydrogenase (Huber et al., 1994; Chollet et al., 1996). Further work is now required to characterize the kinase and its phosphorylation site and to integrate the regulatory phosphorylation of PEPCK into the global regulation of carbon and nitrogen metabolism in plants (Walker et al., 1999).
MATERIALS AND METHODS
Plant Material
Seeds of Guinea grass (Panicum maximum) and Urochloa panicoides were obtained from the Kew Seed Bank (Royal Botanical Gardens, Kew, UK). Maize seeds (Zea mays L. cv DeKalb XL81) were a gift from Dr. R.T. Furbank (Commonwealth Scientific and Industrial Research Organization, Division of Plant Industry, Canberra, Australia). Plants were grown in a greenhouse in summer under ambient light. Baker's yeast (Saccharomyces cerevisiae) was grown in yeast peptone dextrose (bacto-yeast extract 10 g L−1, bacto-tryptone 20 g L−1, dextrose 20 g L−1, and bacto-agar 20 g L−1).
SDS-PAGE and Immunoblotting
SDS-PAGE and immunoblotting was done as described previously (Walker and Leegood, 1996). Immunoreactive polypeptides were visualized using an antiserum raised to purified Guinea grass PEPCK. Protein was measured as in Walker et al. (1995).
Extraction and Measurement of PEPCK Activity
For measurement of PEPCK activity in crude extracts, leaf samples were extracted in 5 volumes of ice-cold 200 mm Bicine-KOH (pH 9.8) containing 5 mm dithiothreitol (DTT). For preparation of proteolytically cleaved PEPCK, leaves of Guinea grass were extracted in 200 mm Tris-HCl (pH 7.0), containing 25 mm DTT, then left at room temperature for 8 h. Unless stated otherwise, the carboxylation activity of PEPCK was measured in a continuous assay at 25°C, including 100 mm HEPES (pH 7.0), 4% (v/v) 2-mercaptoethanol, 100 mm KCl, 90 mm KHCO3, 5 mm PEP, 1 mm ADP, 10 μm MnCl2, 4 mm MgCl2, 0.14 mm NADH, 6 units of malate dehydrogenase (Walker et al., 1995; Chen et al., 2002). Decarboxylase activity was measured in a continuous assay at 25°C including 65 mm Tris-acetate (pH 7.4), 100 mm KCl, 0.3 mm OAA, 1 mm ATP, 10 μm MnCl2, 4 mm MgCl2, 71.5 mm mecaptoethanol, 0.1 mm NADH, 2 units of pyruvate kinase, and 5 units of lactate dehydrogenase (Lee et al., 1981). After adding pyruvate kinase and lactate dehydrogenase, the change in absorbance was measured for 10 min before the addition of PEPCK to correct for the nonenzymatic decarboxylation of OAA to pyruvate. One unit of PEPCK activity corresponds to the production of 1 μmol product min−1 at 25°C.
Purification of PEPCK
The light form of PEPCK was purified from leaves illuminated for 6 h at 500 to 1,000 μmol m−2 s−1 in a greenhouse at 25°C to 30°C. For purification of the dark-form of PEPCK, leaves were fed 32Pi (Walker and Leegood, 1996) under illumination at 500 μmol m−2 s−1 for 2 h and then left in darkness for 8 h at 25°C to 30°C. Leaves were immediately frozen in liquid N2 after harvest. Frozen leaves were homogenized in 5 volumes of ice-cold 200 mm Bicine-KOH (pH 9.8) containing 5 mm DTT and then clarified by centrifugation at 20,000g for 30 min. The same buffer was used for all subsequent procedures. There was no loss of PEPCK activity during prolonged storage (5 h) in this buffer at 25°C. Protein in the supernatant precipitating between 30% and 50% saturation with (NH4)2SO4 was collected by centrifugation at 20,000g for 30 min. The pellet was resuspended and applied to a Hi-Trap Q ion-exchange column (Pharmacia, Piscataway, NJ) at a flow rate of 1.5 mL min−1. Proteins were eluted using a 0 to 1 m linear gradient of NaCl. Fractions containing PEPCK were pooled and applied to a Pharmacia Superose 12-gel filtration column at a flow rate of 0.2 mL min−1. Malate dehydrogenase, which interferes with the decarboxylation assay, was completely removed from the preparation at the final gel filtration step. Fractions containing PEPCK were stored at −20°C with no loss of activity for at least 3 months.
Antibody to PEPCK
A polyclonal antiserum was generated in a New Zealand rabbit (Walker et al., 1995). Four injections, each containing 250 μg of purified PEPCK, were used.
In Vivo Phosphorylation of PEPCK
PEPCK was phosphorylated in vivo as described by Walker and Leegood (1996). For immunoprecipitation (Walker and Leegood, 1996), 10 μL of antiserum raised against the protein from Guinea grass was used.
Footnotes
This research was supported by the Biotechnology and Biological Sciences Research Council, UK (research grant nos. CO5229 and RSP07804), by a David Phillips Research Fellowship to R.P.W., by a research studentship to R.M.A.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010432.
LITERATURE CITED
- Agostino A, Heldt HW, Hatch MD. Mitochondrial respiration in relation to photosynthetic C4 acid decarboxylation in C4 species. Aust J Plant Physiol. 1996;23:1–7. [Google Scholar]
- Burnell JN. Purification and properties of phosphoenolpyruvate carboxykinase from C4 plants. Aust J Plant Physiol. 1986;13:577–587. [Google Scholar]
- Burnell JN, Hatch MD. Photosynthesis in phosphoenolpyruvate carboxykinase-type C4 plants: pathways of C4 acid decarboxylation in bundle sheath cells of Urochloa panicoides. Arch Biochem Biophys. 1988;260:187–199. doi: 10.1016/0003-9861(88)90440-7. [DOI] [PubMed] [Google Scholar]
- Carnal NW, Agostino A, Hatch MD. Photosynthesis in phosphoenolpyruvate carboxykinase-type C4 plants: mechanism and regulation of C4 acid decarboxylation in bundle sheath cells. Arch Biochem Biophys. 1993;306:360–367. doi: 10.1006/abbi.1993.1524. [DOI] [PubMed] [Google Scholar]
- Chen Z-H, Walker RP, Acheson RM, Leegood RC. Phosphoenolpyruvate carboxykinase assayed at physiological concentrations of metal ions has a high affinity for CO2. Plant Physiol. 2002;128:160–164. [PMC free article] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O'Leary MH. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- Finnegan PM, Burnell JN. Isolation and sequence analysis of cDNAs encoding phosphoenolpyruvate carboxykinase from the PCK-type C4 grass Urochloa panicoides. Plant Mol Biol. 1995;27:365–376. doi: 10.1007/BF00020190. [DOI] [PubMed] [Google Scholar]
- Furbank RT, Agostino A, Hatch MD. Regulation of C4 photosynthesis: modulation of mitochondrial NAD-malic enzyme by adenylates. Arch Biochem Biophys. 1991;289:376–381. doi: 10.1016/0003-9861(91)90426-j. [DOI] [PubMed] [Google Scholar]
- Furumoto T, Hata S, Izui K. cDNA cloning and characterization of maize phosphoenolpyruvate carboxykinase, a bundle sheath cell-specific enzyme. Plant Mol Biol. 1999;41:301–311. doi: 10.1023/a:1006317120460. [DOI] [PubMed] [Google Scholar]
- Halford NG, Hardie DG. SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol. 1998;37:735–748. doi: 10.1023/a:1006024231305. [DOI] [PubMed] [Google Scholar]
- Hatch MD, Kagawa T, Craig S. Subdivision of the C4-pathway species based on differing C4 acid decarboxylating systems and ultrastructure. Aust J Plant Physiol. 1975;2:111–128. [Google Scholar]
- Hatch MD, Mau S. Properties of phosphoenolpyruvate carboxykinase operative in C4 pathway photosynthesis. Aust J Plant Physiol. 1977;4:207–216. [Google Scholar]
- Huber SC, Huber JL, McMichael RW. Control of plant enzyme activity by reversible protein phosphorylation. Int Rev Cytol. 1994;149:47–98. [Google Scholar]
- Krautwurst H, Encinas MV, Marcus F, Latshaw SP, Kemp RG, Frey PA, Cardemil E. Saccharomyces cerevisiae phosphoenolpyruvate carboxykinase: revised amino acid sequence, site directed mutagenesis and microenvironment characteristics of cysteines 365 and 458. Biochemistry. 1995;34:6382–6388. doi: 10.1021/bi00019a017. [DOI] [PubMed] [Google Scholar]
- Lee MH, Hebda CA, Nowak T. The role of cations in avian liver phosphoenolpyruvate carboxykinase catalysis. J Biol Chem. 1981;256:12793–12801. [PubMed] [Google Scholar]
- Leegood RC, ap Rees T. Phosphoenolpyruvate carboxykinase and gluconeogenesis in cotyledons of Curcurbita pepo. Biochim Biophys Acta. 1978;524:207–218. doi: 10.1016/0005-2744(78)90119-5. [DOI] [PubMed] [Google Scholar]
- Leegood RC, von Caemmerer S. The relationship between contents of photosynthetic intermediates and the rate of photosynthetic carbon assimilation in leaves of Amaranthus edulis. Planta. 1988;174:253–262. doi: 10.1007/BF00394779. [DOI] [PubMed] [Google Scholar]
- Leegood RC, von Caemmerer S, Osmond CB. Metabolite transport and photosynthetic regulation in C4 and CAM plants. In: Dennis DT, Turpin DH, Layzell DD, Lefebvre DK, editors. Plant Metabolism. London: Longman; 1996. pp. 341–369. [Google Scholar]
- Leegood RC, Walker RP. Phosphoenolpyruvate carboxykinase in plants: its role and regulation. In: Bryant JA, Burrell MM, Kruger NJ, editors. Plant Carbohydrate Biochemistry. Oxford: BIOS Scientific Publishers; 1999. pp. 201–213. [Google Scholar]
- Lin ZF, Lin GZ, Sun GC. The relation between activity of phosphoenolpyruvate carboxykinase and photosynthesis in Aloe vera leaves. Acta Bot Sin. 1991;33:273–279. [Google Scholar]
- Lin ZF, Peng C-L, Lin GZ, Li S-S. Diurnal changes of phosphoenolpyruvate carboxykinase activity, oxaloacetate content and adenosine pool in pineapple leaves. Acta Phytophys Sin. 1994;20:353–359. [Google Scholar]
- Ray TB, Black CC., Jr Characterization of phosphoenolpyruvate carboxykinase from Panicum maximum. Plant Physiol. 1976;58:603–607. doi: 10.1104/pp.58.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiskind JB, Bowes G. The role of phosphoenolpyruvate carboxykinase in a marine macroalga with C4-like photosynthetic characteristics. Proc Natl Acad Sci USA. 1991;88:2883–2887. doi: 10.1073/pnas.88.7.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RMC, Heldt HW. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982;70:971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina JA, Avilan L. The kinetic mechanism of phosphoenolpyruvate carboxykinase from Panicum maximum. Phytochemistry. 1989;28:1349–1353. [Google Scholar]
- Walker RP, Acheson RM, Técsi LI, Leegood RC. Phosphoenolpyruvate carboxykinase in C4 plants: its role and regulation. Aust J Plant Physiol. 1997;24:459–468. [Google Scholar]
- Walker RP, Chen Z-H, Johnson KE, Famiani F, Técsi LI, Leegood RC. Using immunohistochemistry to study plant metabolism: the examples of its use in the localization of amino acids in plant tissues, and of phosphoenolpyruvate carboxykinase and its possible role in pH regulation. J Exp Bot. 2001;52:565–576. [PubMed] [Google Scholar]
- Walker RP, Chen Z-H, Técsi LI, Famiani F, Lea PJ, Leegood RC. Phosphoenolpyruvate carboxykinase plays a role in interactions of carbon and nitrogen metabolism during grape seed development. Planta. 1999;210:9–18. doi: 10.1007/s004250050648. [DOI] [PubMed] [Google Scholar]
- Walker RP, Leegood RC. Purification, and phosphorylation in vivo and in vitro, of phosphoenolpyruvate carboxykinase from cucumber cotyledons. FEBS Lett. 1995;362:70–74. doi: 10.1016/0014-5793(95)00212-r. [DOI] [PubMed] [Google Scholar]
- Walker RP, Leegood RC. Phosphorylation of phosphoenolpyruvate carboxykinase in plants: studies in plants with C4 photosynthesis and Crassulacean acid metabolism and in germinating seeds. Biochem J. 1996;317:653–658. doi: 10.1042/bj3170653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RP, Trevanion SJ, Leegood RC. Phosphoenolpyruvate carboxykinase from higher plants: purification from cucumber and evidence of rapid proteolytic cleavage in extracts from a range of plant tissues. Planta. 1995;195:58–63. [Google Scholar]
- Wingler A, Walker RP, Chen Z-H, Leegood RC. Phosphoenolpyruvate carboxykinase is involved in the decarboxylation of aspartate in the bundle-sheath of maize. Plant Physiol. 1999;120:539–545. doi: 10.1104/pp.120.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood HG, Davies JJ, Lochmüller H. The equilibria of reactions catalyzed by carboxytransphosphorylase, carboxykinase and pyruvate carboxylase and the synthesis of phosphoenolpyruvate. J Biol Chem. 1966;241:5692–5704. [PubMed] [Google Scholar]