Abstract
Growth of Legionella pneumophila on buffered charcoal-yeast extract (BCYE) medium is dependent on l-cysteine (but not l-cystine), which is added in excess over what is required for nutrition. We investigated the biochemical and genetic bases for this unusual requirement and determined that much of the l-cysteine in BCYE medium is rapidly oxidized to l-cystine and is unavailable to the bacteria. Analysis of cysteine consumption during bacterial growth indicated that of the 11% consumed, 3.85% (∼0.1 mM) was incorporated into biomass. The activities of two key cysteine biosynthetic enzymes (serine acetyltransferase and cysteine synthase) were not detected in cell extracts of L. pneumophila, and the respective genes were not present in the genome sequences, confirming cysteine auxotrophy. Kinetic studies identified two energy-dependent cysteine transporters, one with high affinity (apparent Km, 3.29 μM) and the other with low affinity (apparent Km, 93 μM), each of which was inhibited by the uncoupling agent carbonyl cyanide m-chlorophenylhydrazone. Cystine was not transported by L. pneumophila; however, a mutant strain capable of growth on l-cystine (CYS1 mutant) transported l-cystine with similar kinetics (Km, 4.4 μM and 90 μM). Based on the bipartite kinetics, requirement for proton motive force, and inhibitor studies, we suggest that a high-affinity periplasmic binding protein and a major facilitator/symporter (low affinity) mediate uptake. The latter most likely is functional at high cysteine concentrations and most likely displays altered substrate specificity in the CYS-1 mutant. Our studies provide biochemical evidence to support a general view that L. pneumophila is restricted to an intracellular lifestyle in natural environments by an inability to utilize cystine, which most likely ensures that the dormant cyst-like transmissible forms do not germinate outside suitable protozoan hosts.
Members of the genus Legionella reside in aquatic environments as intracellular parasites of protozoa. When these bacteria are aerosolized into the lungs of susceptible humans, some species such as Legionella pneumophila infect and propagate within alveolar macrophages, leading to an atypical pneumonia referred to as Legionnaires' disease (13, 35). L. pneumophila and most likely other species are dimorphic, cycling between intracellular replicating forms (RFs) and extracellular planktonic forms that resemble spore-like cysts, referred to as mature intracellular forms, observed in HeLa cells (11, 15, 16). Compared with vegetative RFs, cyst-like mature intracellular forms were near dormant metabolically, displayed heightened resistance to detergents and antibiotics, and exhibited enhanced virulence in cell-based assays (15). The resilience of cysts is most likely attributable to the thickened cell wall architecture, laminations of intracytoplasmic membranes, and accumulations of inclusions of poly-β-hydroxybutyrate (11, 15). Cysts do not appreciably form in macrophages due to early apoptotic lysis, and their morphogenesis in vitro aborts in stationary phase (15). As survival forms, cysts and endospores must be programmed to germinate when growth conditions are favorable and, in the case of L. pneumophila, following entry into a susceptible eukaryotic host cell or in vitro on rich media. We are interested in understanding what nutritional factors promote or prevent cysts from germinating outside of host cells in natural environments.
Most species of Legionella can be grown in vitro on buffered charcoal-yeast extract (BCYE) agar that has been supplemented with l-cysteine and ferric pyrophosphate in amounts that are in great excess over what would be required for nutrition (12). Cysteine supplementation is not required for intracellular growth in macrophages, mammalian cell lines, or natural amoebic hosts, indicating that cysteine and other essential amino acids are obtained from the host (34). In contrast to other cysteine-requiring organisms such as Neisseria gonorrhoeae (6) and Bordetella pertussis (30), Legionella spp. are unable to utilize the oxidized dipeptide cystine (17, 27). Therefore, the inability to utilize l-cystine would be expected to impose restrictions on the extracellular growth of L. pneumophila in aerated aquatic environments, such as biofilms. Interestingly, the apparent cysteine auxotrophy of B. pertussis and N. gonorrhoeae is not due to an absence of genes encoding the biosynthetic enzymes for cysteine but rather is due to a deficiency in sulfide production (22). Little is known about the cysteine biosynthetic pathways in L. pneumophila or the nature and specificity of cysteine transporters.
In other bacteria, such as Salmonella enterica serovar Typhimurium, three transporter systems for l-cystine are predicted based on kinetic studies (2), and in Escherichia coli two transporters are predicted, one that is rather nonspecific and also transports diaminopimelic acid and one that is more specific (4). The former is an ABC transporter that interacts with a periplasmic l-cystine binding protein and is susceptible to osmotic shock inhibition (4). In Bacillus subtilis, three transporters are involved in l-cystine uptake; these include the YhcL symporter and two ATP binding ABC transporters (5). Other families of secondary transporters, such as the ATP-independent periplasmic transporters (31), are indicative of the great diversity of transporter systems that exist in microorganisms (10). Genome-based analyses of bacterial transporter systems suggest that 15 to 20% of all transport proteins are associated with uptake of amino acids (26), and most bacteria are able to transport a wide range of sulfur-containing compounds as a source for l-cysteine. Thus, the dependence of L. pneumophila solely on l-cysteine for growth is unique in the microbial world.
In the present study we have examined the cysteine requirement of L. pneumophila and have shown that l-cysteine, but not l-cystine, is taken up by an energy (proton motive force)-dependent transporter (inhibited by carbonyl cyanide m-chlorophenylhydrazone [CCCP]). Biosynthetic enzymes for l-cysteine were not detected in cell extracts, and the structural genes were not present in the completed genome sequences of L. pneumophila (7, 8). Chemical analysis for cysteine in BYE medium revealed that most of the supplemented l-cysteine is rapidly oxidized (ferric iron catalyzed) to l-cystine and at equilibrium; a steady-state level of available cysteine of ∼0.2 to 0.5 mM is sufficient to promote growth. While a cystine-utilizing mutant (CYS-1) was isolated in the course of these studies, this capability most likely is not advantageous. We suggest that in natural environments, the scarcity of l-cysteine most likely restricts L. pneumophila to an intracellular lifestyle and ensures that the highly infectious planktonic cysts do not germinate outside suitable host cells.
MATERIALS AND METHODS
Bacterial strains and cultivation.
Legionella pneumophila serogroup 1 strains (Philadelphia 1, SVir, and Knoxville-1), Legionella jordanis, and Legionella oakridgensis OR10 were obtained from the Centers for Disease Control and Prevention, Atlanta, Ga., and were maintained on BCYE agar as previously described (12). Unless otherwise indicated, l-cysteine and l-cystine were used in all studies. L. pneumophila CYS-1 (a cystine-utilizing mutant of SVir) was maintained on BCYE agar supplemented with 0.02% l-cystine in place of l-cysteine. All cultures were routinely tested for purity by microscopic examination and by culture on LB agar, on which the bacteria do not grow. Batch cultures were grown in BYE broth (without charcoal) as described elsewhere (18, 20) and harvested in late log phase (A660 = 0.5 to 1.0) by centrifugation, and following a phosphate-buffered saline wash, pellets were stored at −20°C.
Isolation of a cystine-utilizing mutant.
Mutagenesis was carried out on BCYE agar by spreading 0.1 ml of a bacterial suspension (ca. 108 bacteria/ml) on the surface followed by seeding a few crystals of N-methyl-N′-nitro-N-nitrosoguanidine centrally. Following incubation for 24 to 48 h, the peripheral growth at the edge of the zone of inhibition was spread onto BCYE agar plates containing 0.02% cystine in place of cysteine. Colonies were picked and propagated on l-cystine-containing medium and validated as L. pneumophila by indirect immunofluorescence with serogroup 1 antibody reagents obtained from the CDC.
Cystine-dependent growth.
Growth rates for the wild-type (WT) and CYS-1 strains of L. pneumophila on l-cysteine and l-cystine were determined in liquid chemically defined medium (CDM [ABCD medium without glutathione and thiotic acid]) as described previously (27, 28), in BYE broth, and on BCYE agar plates supplemented with different concentrations of ferric pyrophosphate, cysteine, and cystine. In the case of wild-type L. pneumophila to be used as inoculum for growth studies involving l-cystine, the bacteria were harvested by centrifugation from l-cysteine-containing medium and washed once in phosphate-buffered saline prior to inoculation of the medium. Growth curves were determined in duplicate and repeated three times, and the results from a single experiment are depicted.
Cysteine oxidation studies.
The cysteine concentration in BYE broth was determined colorimetrically with acid ninhydrin as described by Gaitonde (14). In this procedure, 2.3 mM (0.04%) l-cysteine dissolved in distilled water was added to BYE broth (100 ml in a 250 ml flask with or without 0.02% ferric pyrophosphate). The pH of the medium was adjusted so that following the addition of cysteine the final pH would be 6.9. Duplicate flasks were shaken at 100 rpm at 37°C in a rotary shaker (New Brunswick), and the flasks were protected from light by aluminum foil. Samples (1 ml) were taken hourly, and the cysteine concentration was determined colorimetrically at 560 nm and read from a standard curve (ɛ = 28 mM−1 cm−1). The l-cystine concentration in BYE broth was determined following reduction to cysteine with 5 mM dithiothreitol. Ferrous iron concentrations were similarly determined hourly by the ferrozine method as described by Dailey and Lascelles (9). Each experiment was repeated three times, and the mean and standard deviation were computed.
Cysteine carbon balance studies.
To determine the extent of cysteine consumption during bacterial growth, 1 μCi of uniformly labeled [14C]cysteine was added to 50 ml of BYE medium containing ∼2.3 mM cold cysteine. The medium was inoculated with L. pneumophila to a starting turbidity (A600) of 0.1 and grown for 22 h at 37°C with shaking. Three 0.1-ml aliquots were filtered onto 0.45-μm membrane filters (Gelman Sciences; Ann Arbor, Mich.) and washed, and the radioactivity was counted by liquid scintillation. The bacteria were collected by sedimentation, 20-μl aliquots were obtained from the spent medium, and the remaining radioactivity was counted. The percent incorporation of cysteine was determined from the ratio of the mean counts per minute obtained from membrane filters to total radioactivity added to BYE medium times 100.
Radiotracer studies.
A membrane vacuum filtration method was used to measure time-dependent uptake of radiolabeled cysteine or cystine (20). Mutant and wild-type strains were grown in BYE medium supplemented with either cysteine or cystine and harvested from late log phase by centrifugation. The cells were washed once in 50 mM potassium phosphate buffer containing 1 mM MgSO4 (PPB) and suspended in the reaction mixture to a final turbidity (A660) of 0.5. The reaction mixture contained (per 5 ml) bacteria suspended in PPB, 100 μl of carrier cysteine or cystine (0.1 to 100 μM), 1 μCi of uniformly labeled [14C]cysteine (33.8 mCi/mmol; Amersham) or [35S]cystine (307 mCi/mmol; Amersham), and 250 μl of chloramphenicol (15 μg/ml) to inhibit protein synthesis. The bacterial suspension was allowed to equilibrate for 5 min at 37°C with shaking, and the reaction was initiated by the addition of carrier and labeled substrates. Samples (200 μl) were removed at various intervals, filtered onto 0.45-μm filters (Gelman), and washed with 10 ml of ice cold PPB. The filters were placed in scintillation fluor, and radioactivity was counted in a liquid scintillation counter. l-Serine and l-glutamate (1 mM final concentration) were added as energy sources, and in other experiments the uncoupling agent CCCP (4 to 10 μM) was added to collapse the energized membrane. Nonspecific binding of isotopes to whole cells was determined at 0 to 4°C. Kinetic constants were determined from the initial velocities of uptake at each carrier concentration of cysteine or cystine. All radiotracer studies were repeated independently three times, and the results from representative experiments are presented.
To determine if l-cystine could inhibit cysteine transport, L. pneumophila CYS-1 was suspended in 5 ml of PPB (A660 = 0.5) containing 10 μM l-cysteine, and the l-cystine concentration was varied from 0 to 100 μM. The reaction was started by adding 2 μCi [35S]cysteine (Amersham), 0.5-ml samples were removed at 1 h and filtered, and the radioactivity was counted by liquid scintillation. The control value (without cystine) was set at 100%, and the mean percentages for triplicate samples were used to determine the percent inhibition of cysteine uptake.
Enzyme assays.
Cell extracts were prepared from 1 g of cell paste of bacteria grown in BYE medium (plus cysteine) as described previously (20). Serine transacetylase (EC 2.3.1.30) was assayed by the method of Kredich et al. (21). Briefly, this reaction measures the absorbance of the chromophore produced by the reaction of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) with free reduced coenzyme A at 412 nm (ɛ = 13.6 mM−1 cm−1). Since thiols react with DTNB, the reference cuvette contained crude cell extract and all reactants with the exception of acetyl coenzyme A. Both reactions were initiated simultaneously, and the difference was recorded on a modified Cary 14 spectrophotometer (OLIS, Bogart, Georgia). To ensure the reproducibility of both sample and reference reactions, each was recorded separately several times against a reference that contained no DTNB and the reference trace was subtracted from the sample trace and compared with real-time data collection results. Cysteine synthase (o-acetyl-serine sulfhydrylase) (EC 2.5.1.47) activity was determined colorimetrically as described by Becker et al. (3). In this method, cysteine formation is determined chemically in a reaction involving sulfanilamide and N-1-naphthylethylenediamine dihydrochloride, and the color is read as A540. Specific activities were determined using concentrations determined from a standard curve. Protein concentration was estimated by the Bradford dye binding assay (Bio-Rad). Cystine reductase activity in extracts from L. pneumophila was measured either by following the oxidation of NADH (A340) or by reaction of the thiol with DTNB (A412). Escherichia coli was grown in cysteine-deficient medium and used as a positive control for enzymes of cysteine biosynthesis (21). Enzyme assays for L. oakridgensis were done with bacteria grown in the absence of cysteine (25). All enzyme assays were repeated at least three times, and the mean and standard deviation for each set was computed.
RESULTS
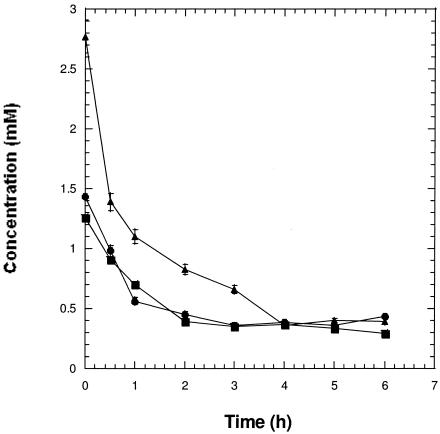
The ability of most species of Legionella to grow in vitro requires supplementation of BCYE agar medium with ∼2.3 mM l-cysteine, a concentration that is far in excess of what would be required for nutrition. In contrast, the ability of L. pneumophila to grow intracellularly in cell-based systems does not depend on supplementation of tissue culture media with l-cysteine or l-cystine. In a previous study, we reported that the high rate of hydrogen peroxide decomposition in CYE medium was partly due to the presence of cysteine, which was oxidized to cystine (18). To address whether the excess cysteine supplement was necessary for nutrition or for scavenging of toxic forms of oxygen, the fate of l-cysteine was followed by measuring the cysteine concentration by its reaction with acid ninhydrin under aerobic conditions. Ninhydrin interacts with amino acids to produce a purple color. Under acidic conditions, acid ninhydrin interacts specifically with l-cysteine to produce a pink to red color reaction whose absorbance at 560 nm is proportional to cysteine concentration (14). As seen in Fig. 1, cysteine was rapidly oxidized to cystine in BYE broth and by 4 h had reached equilibrium with cystine in the 0.35 to 0.5 mM range, which remained stable for several days. Ferric pyrophosphate enhanced the rate of cysteine oxidation (23 ± 5 nmol per min per ml) in a 3:1 stoichiometry with reduction of ferric pyrophosphate to the ferrous form (7 ± 2 nmol per min per ml of medium) and resulted in a slightly lower equilibrium value of 0.25 to 0.35 mM (Fig. 1). These values were above the minimum cysteine concentration (0.1 mM) that was previously shown to partly support growth of L. pneumophila (27). The addition of lower starting concentrations of l-cysteine (e.g., 0.57 mM) resulted in a final steady-state level below 0.1 mM, which did not support bacterial growth (Table 1). The addition of excess l-cystine to BYE medium also leads to an equilibrium that provides sufficient l-cysteine to support growth of L. pneumophila (Table 1). However, in the absence of ferric pyrophosphate, l-cystine was a poor substitute for cysteine in supporting growth of L. pneumophila, suggesting that iron may catalyze the interconversion of cysteine forms. Our studies also confirmed the requirement for excess ferric iron, as the WT strain grew poorly on BCYE medium at a low ferric pyrophosphate concentration (Table 1).
FIG. 1.
Oxidation of cysteine in BYE broth. l-Cysteine, sterilized by filtration, was aseptically added to BYE broth. Samples were removed, and the concentration of cysteine was determined by the acid ninhydrin method as described in the text. The starting concentration of cysteine was estimated at 2.7 mM, which included the 2.3 mM supplement. No added iron, ▴; 0.025% ferric pyrophosphate, •; and 0.05% ferric pyrophosphate, ▪.
TABLE 1.
Effect of ferric pyrophosphate concentration on L. pneumophila growth on BCYE supplemented with l-cysteine or cystine
| Ferric pyrophosphate concn (μg/ml) | Strain | Growtha with the indicated concn (mM) of:
|
|||||
|---|---|---|---|---|---|---|---|
| Cysteine
|
Cystine
|
||||||
| 2.27 | 1.14 | 0.57 | 1.67 | 0.83 | 0.42 | ||
| 250 | WT | +++ | +++ | − | +++ | + | − |
| CYS-1 | +++ | +++ | +++ | +++ | +++ | ++ | |
| 125 | WT | +++ | +++ | − | +++ | + | − |
| CYS-1 | +++ | +++ | +++ | +++ | +++ | ++ | |
| 62.5 | WT | +++ | +++ | − | +++ | + | − |
| CYS-1 | +++ | +++ | +++ | +++ | +++ | ++ | |
| 6.25 | WT | ++ | ++ | − | ++ | +/− | − |
| CYS-1 | +++ | +++ | +++ | +++ | ++ | + | |
The relative growth of bacteria on BCYE agar medium was scored visually as follows: +++, normal growth; ++, slow growth; +, poor growth; +/−, microcolonies; and −, no growth. The score is based on triplicate plating for each condition.
To determine how much cysteine is consumed during the growth of L. pneumophila in BYE broth, the total radioactivity of the medium was determined before and after 22 h of growth, and the amount of incorporated radiolabel (biomass) was determined (optical density at 600 nm = 0.8). Based on the 50-ml starting volume, we estimated that the bacteria incorporated ∼3.85% of the label. The radioactivity in the BYE spent medium was 88.5% of the starting value. Of the 11.5% putatively consumed during bacterial growth, 3.85% could be accounted for as biomass, while the remaining 7.65% may have been lost as CO2 (cysteine is a respirable substrate; 0.067 nmol O2/min/mg [dry weight]) or during sedimentation as insoluble cystine. Since we do not know how much cysteine is present in yeast extract, we can only estimate that ∼0.1 mM cysteine was consumed during the 22-h growth period, consistent with previous estimates based on growth studies (27).
Isolation of a cystine-utilizing mutant.
Since most cysteine auxotrophs will grow on media supplemented with cystine, we wished to know why l-cystine could not be utilized by L. pneumophila. We considered the possibility that transporters of cysteine, dipeptides, or even glutathione most likely lacked specificity for cystine. Since the substrate specificity for some enzymes can be altered via mutation (1), we attempted to isolate variants that would grow on BCYE agar containing cystine in place of cysteine. According to an agar-based mutagenesis protocol, L. pneumophila were spread plated onto BCYE medium containing 0.02% l-cystine. Several colonies were picked and propagated on BCYE-cystine medium. One of these, CYS-1, was studied further. The CYS-1 mutant was verified as L. pneumophila by its inability to grow on LB agar, oxidase-positive reaction, poor growth on BYE agar (requirement for charcoal for optimal growth), and requirement for either cysteine or cystine for growth in BCYE medium. There was no change in protein profiles (data not shown), and both PCR (ompS and htpAB) and serological tests confirmed CYS-1 as serogroup 1 L. pneumophila (data not presented). As seen in Table 1, the cystine mutant (CYS-1) grew well on lower concentrations of l-cystine (compared with the WT), and ferric iron had little effect on growth. The ability of this mutant to grow on cystine-supplemented BCYE medium might be due to activation of a cryptic transporter or to modification of the cysteine transporter to either transport cystine or more efficiently transport l-cysteine (e.g., lower Km) produced at equilibrium from cystine.
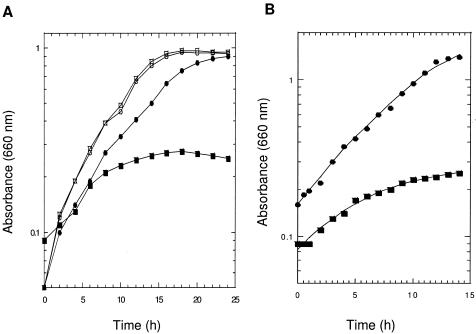
Growth kinetics in BYE and CDM.
Figure 2 depicts the growth curves for the CYS-1 and WT strains of L. pneumophila in BYE broth with l-cysteine or l-cystine (Fig. 2A) or in CDM broth supplemented with l-cystine at pH 6.5 (Fig. 2B). The parent strain grew poorly in BYE in the presence of l-cystine, while the CYS-1 mutant grew equally well with either substrate. Similarly, the CYS-1 mutant grew well with l-cystine in CDM broth and exhibited no other nutritional requirements. The l-cystine-supplemented CDM also supported growth of the parental strain to some extent, and growth was dependent on the starting pH of the medium. Apparently, the ratio of cystine to cysteine is pH dependent, and more available l-cysteine (0.08 to 0.1 mM detected chemically) was present in cystine-supplemented CDM when the pH was adjusted to between 6.2 and 6.5.
FIG. 2.
Growth kinetics of L. pneumophila wild-type and CYS-1 mutant strains. A. Growth of wild type with l-cysteine (•) or l-cystine (▪) and of CYS-1 with l-cysteine (○) or l-cystine (□) in BYE broth. B. Growth of wild type (▪) and CYS-1 mutant (•) in CDM medium supplemented with l-cystine. Turbidities were measured with a Spectronic 20 spectrometer in 300-ml sidearm flasks containing 100 ml of medium. Each time point represents the average of two determinations.
Uptake of l-cysteine and l-cystine.
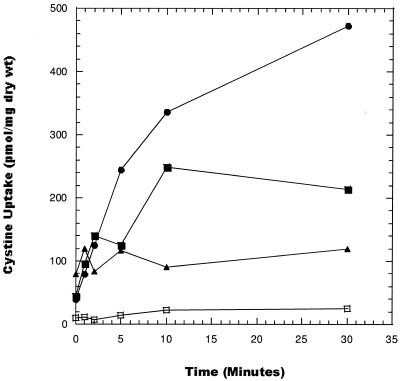
To determine more precisely the kinetics of l-cysteine and l-cystine uptake by the L. pneumophila CYS-1 mutant and wild-type strains, uptake studies were performed with PPB buffer in the presence of chloramphenicol to inhibit incorporation of the amino acid into protein. Preliminary studies examined the energy dependence of l-cysteine and l-cystine transport. As seen in Fig. 3 (results for cystine are depicted), addition of amino acids (l-serine and l-glutamate) stimulated uptake of l-cystine and transport was inhibited by the uncoupling agent CCCP, suggesting that an energized membrane is required for uptake. The radiotracer study confirmed that the WT L. pneumophila strain does not transport cystine. This finding diminishes the possibility that cystine uptake by the CYS-1 mutant is due to increased affinity for cysteine. However, further kinetic studies are required to distinguish between activation of a cryptic transporter and modification of the cysteine transporter system to bind cystine.
FIG. 3.
Energy-dependent uptake of l-[35S]cystine by the CYS-1 mutant and the WT and effect of CCCP. Cystine uptake was determined in PPB in the absence of energy sources (▪), in the presence of 1 mM l-serine and l-glutamate (•), and with energy sources in the presence of 10 μM CCCP (▴). WT L. pneumophila did not transport cystine (□).
We next investigated the pH dependence of transport and found that with the WT strain a marginal uptake of cysteine was observed at or above pH 6.9, a pH that is optimal for growth on BCYE agar (12). When the pH of the PPB was decreased to 6.2, cysteine uptake dramatically increased. The optimal pH for these studies was determined to be 6.2, which is similar to that of the partly acidified phagosome in which the bacteria replicate in macrophages (33).
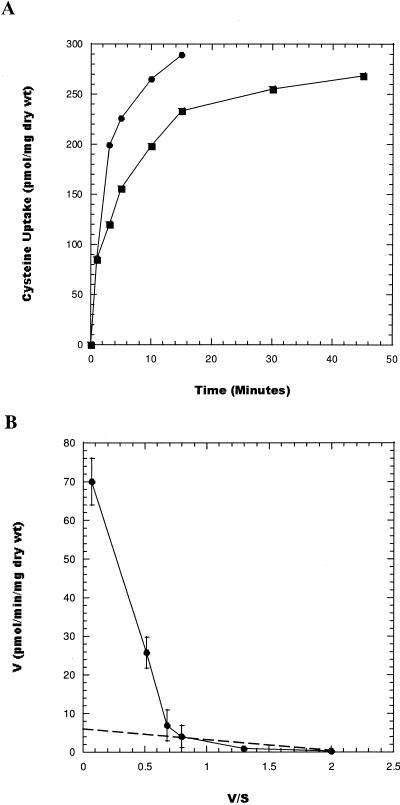
As seen in Fig. 4A, the kinetics of l-cysteine uptake were similar in the wild type and the CYS-1 mutant as determined from measures of the initial velocity of transport. The greater incorporation of label noted for the CYS-1 mutant relative to the WT might reflect the ability of the CYS-1 mutant to transport both cystine and cysteine. By varying the cysteine or cystine carrier concentration between 100 nM and 100 μM, we derived an apparent Km for cysteine uptake of 3.29 ± 1.7 μM and a Vmax of 6.25 ± 2 pmol/min/mg (dry weight) (Fig. 4B). The Hofstee plot accentuates the biphasic nature of the kinetics of cysteine transport. At higher concentrations of l-cysteine (10 to 100 μM), the Km for cysteine shifted to 93 μM, with a Vmax of 75 pmol/min/mg (dry weight). Multiple kinetic constants have been reported for the transport of cystine/cysteine in other microorganisms and in some cases may reflect the participation of a periplasmic amino acid binding protein in the high-affinity system (2, 4, 5, 31). The kinetics of cystine uptake was similar, with an apparent Km for l-cystine uptake for the CYS-1 mutant of 4.4 ± 2.1 μM (Vmax of ∼6 pmol/min/mg [dry weight]), and the lower-affinity transporter displayed a Km of 92 μM (Vmax of 70 pmol/min per mg [dry weight]). The kinetic analysis supports the notion that a common transporter(s) has been modified to recognize cystine.
FIG. 4.
Cysteine and cystine uptake by L. pneumophila strains. A. Uptake of l-[14C]cysteine by the wild type (•) and the CYS-1 mutant (▪). B. Hofstee plot of [14C]cysteine uptake by wild-type L. pneumophila. The standard deviation determined for each time point is based on three independent determinations. The dashed line indicates the high-affinity transporter and the solid line the low-affinity transporter. V/S was calculated from the initial velocities and is in picomoles/minute/milligram (dry weight)/μM cysteine, and the substrate concentration is in nanomoles per milliliter.
To confirm that l-cystine and l-cysteine are transported by common transporter, a substrate inhibition experiment was performed. As seen in Table 2, l-cysteine uptake decreased with increasing concentrations of l-cystine, with 10 μM l-cystine reducing uptake of l-cysteine by nearly 80%. The study did not account for oxidation of the unlabeled l-cysteine during the course of the experiment. The reciprocal experiment was not performed due to problems with cysteine oxidation. The inhibition results are consistent with a common transporter shared by these substrates but do not distinguish between mutations to a binding protein or in a component of the transporter system.
TABLE 2.
Inhibition of cysteine uptake by cystinea
| Concn (μM) | [35S]cysteine uptake (%)b | |
|---|---|---|
| Cystine | Cysteine | |
| 0 | 10 | 100 |
| 1 | 10 | 60 |
| 10 | 10 | 21 |
| 50 | 10 | 16 |
| 100 | 10 | 14 |
Inhibition studies were performed with the CYS-1 mutant of L. pneumophila as detailed in the text. Radiolabel and l-cysteine were held constant, and the concentration of cystine was varied.
The percentages represent the means for three replicates.
Genetic and enzymatic analysis of cysteine biosynthetic enzymes.
Recent publication of the whole genome sequences for three strains of serogroup 1 L. pneumophila has permitted searches for cysteine biosynthetic genes (7, 8). Within the lipopolysaccharide locus of L. pneumophila is an acetyltransferase gene with weak similarity (27% identity) to the serine acetyltransferase gene of Salmonella enterica serovar Typhimurium. Mutational analysis of this acetyltransferase demonstrated its role in acetylation of lipopolysaccharide (23). Two genes encoding cystathionine beta-synthase (CBS) and cystathionine beta-lyase (metC) present in L. pneumophila exhibit similarities with homologues in the enteric bacteria (e−38, and e−39, respectively). Interestingly the CBS of L. pneumophila shares 72% identity with a similar gene in Coxiella burnetii and, based on BLAST and phylogenetic analyses, shares ∼60% identity with the mammalian enzyme. Because CBS and cysteine synthase share some homology and a requirement for pyridoxal phosphate, we examined cell extracts prepared from various Legionella species for activities of l-cysteine synthase and acetyltransferase (Table 3). As controls, these enzymes were assayed for in cell extracts of E. coli and of L. oakridgensis, one of the few species of Legionella capable of growing on media in the absence of l-cysteine (25). As seen in Table 3, none of the L. pneumophila strains or L. jordanis contained measurable activities (<0.1 nmol/min/mg protein) of the biosynthetic enzymes. In contrast, extracts of L. oakridgensis contained serine acetyltransferase activity (79 ± 12 nmol per min per mg protein) and cysteine synthase activity (21 ± 6 nmol per min per mg protein). These activities were similar to those measured in extracts from E. coli (54 ± 7 and 48 ± 8 nmol per min per mg protein, respectively, for these enzymes). The absence of cysteine biosynthetic enzymatic activities, together with genetic findings, supports the conclusion that cysteine is an essential amino acid for L. pneumophila. Since production of sulfide is required for cysteine synthesis, cell extracts from the CYS-1 and wild-type strains were examined for NAD(P)H l-cystine reductase, sulfite reductase, and sulfate reductase activities. Activities of these enzymes were not detected in these organisms, and whole-genome searches did not find orthologues of these genes in L. pneumophila.
TABLE 3.
Activities of cysteine biosynthetic enzymes in Legionella spp.
| Organism | Sp acta (mean ± SD)
|
|
|---|---|---|
| Serine transacetylase | Cysteine synthase | |
| L. pneumophila Philadelphia-1 | <0.1 | <0.11 |
| L. pneumophila Knoxville-1 | <0.1 | <0.1 |
| L. jordanis ABB9 | <0.1 | <0.1 |
| L. oakridgeneis OR10 | 79 ± 12 | 21 ± 6 |
| E. coli K-12 | 54 ± 7 | 48 ± 8 |
Specific activity is expressed as nanomoles per minute per milligram of protein. Each enzyme activity was determined in triplicate.
DISCUSSION
Most members of the genus Legionella exhibit a unique requirement for the amino acid l-cysteine, which must be added in excess to laboratory media along with ferric iron salts to support in vitro growth (12, 17, 27, 32). In contrast, intracellular growth of L. pneumophila is not dependent on excess cysteine or iron salts, as the bacteria obtain these from the host (34). Early studies aimed at optimizing the BCYE primary isolation medium provided explanations for the functional role of charcoal (18) and for the requirements for a low sodium ion concentration (12), excess ferric pyrophosphate (19), and alpha keto acids (28), but an explanation for the excess cysteine requirement has not been fully addressed (17). Here we show that cysteine is rapidly oxidized to cystine in BYE medium and that this oxidation is accelerated by ferric pyrophosphate (reduced to ferrous iron) and at chemical equilibrium (pH dependent) establishes a growth-supporting steady-state concentration of l-cysteine in the 0.2 to 0.5 mM range. The addition of less l-cysteine to the medium resulted in equilibrium levels of l-cysteine below growth-supporting levels (<0.1 mM) that have been previously documented (27). Indeed, our studies indicate that L. pneumophila consumes ca. 0.1 mM cysteine during growth in BYE medium. As demonstrated for CDM medium supplemented with l-cystine, a lower pH (6.2 to 6.5) favored growth of L. pneumophila by shifting the equilibrium in favor of l-cysteine (pKa = 8 for the thiol/disulfide). Our studies also confirmed the iron dependency of L. pneumophila for growth on BCYE agar (19), particularly at a low cysteine or cystine concentration. Since the CYS-1 mutant had only slightly improved growth at low iron concentrations compared to the WT, we suggest that most of the excess iron may be unavailable to the bacteria, perhaps being sequestered in coordination complexes with cysteine or charcoal. Finally, radiotracer studies confirmed a long-held view that the absolute requirement for l-cysteine was due to an inability to transport the oxidized dipeptide l-cystine (17). This unique nutritional requirement accounts for the inability of most laboratory media to support the growth of L. pneumophila and, in natural aerobic environments where most of the l-cysteine exists as l-cystine, provides an explanation for why bacterial growth is restricted to suitable protozoan hosts.
Our ability to isolate a cystine-utilizing mutant exhibiting energy-dependent uptake kinetics similar to that for l-cysteine suggests that the binding specificity for l-cysteine can be modified by mutation (1). This conclusion is supported by kinetic data and by the inhibitory effect of cystine on uptake of [35S]cysteine by the CYS-1 mutant. We had considered the possibility that the phenotype for cystine utilization by the CYS-1 mutant might have resulted from increased expression of a periplasmic cystine reductase capable of converting exogenous cystine to cysteine, which would then be transported by a cysteine transporter(s). However, cystine reductase activity was not detected in extracts or with whole cells of the mutant or WT L. pneumophila.
Our studies provide evidence for two cysteine transporters, one with high affinity (Km of ∼3 to 4 μM) and the other with low affinity (Km of ∼90 μM). Alternatively, the kinetic constants might reflect two transport states of the same enzyme, which is supported by the absence of uptake of l-cystine by the wild-type strain and by the unlikelihood that favorable mutations have occurred simultaneously in two distinct transporter genes. Since both cysteine uptake and cystine uptake are dependent on proton motive force, it is unlikely that ABC transporter systems mediate transport or that a single major facilitator family transporter is involved (single Km). Given the unique nature of cysteine-specific transport in L. pneumophila, it seems likely that a hybrid transport system, perhaps similar to the novel ATP-independent periplasmic transporter systems, may be involved (31). In this regard, two kinetic constants might be explained by a periplasmic amino acid binding protein that binds either cystine or cysteine and interacts with a cysteine-specific transporter that is altered in the CYS-1 mutant. High-affinity transport would define the action of the binding protein, while the low-affinity transport observed at high concentrations of cysteine (or cystine for the CYS-1 mutant) would result from direct transport of substrate by the transporter. Cystine/cysteine transport has been studied in only a few bacteria (2, 5) and is mediated by periplasmic binding proteins and ATP-dependent ABC transporters which are resistant to the effects of uncoupling agents (2). Bioinformatic analyses reveal over 40 transporter genes in L. pneumophila, 16 of which belong to the major facilitator family (29), and 11 encode periplasmic binding proteins. One potential symporter exhibiting similarity at e−24 with YhcL of Bacillus subtilis, which has recently been demonstrated to transport l-cystine (5), was sequenced from the CYS-1 mutant and found to contain no nucleotide sequence changes from published sequences (data not presented). We also found no DNA sequence differences for two genes encoding amino acid binding proteins and one encoding another transporter. With little protein structural information to guide our studies, we decided not to pursue the identification of transporter genes at this time. Future studies might include random transposon mutagenesis to identify cysteine transporter genes, since L. pneumophila can be adapted to grow on medium containing the tripeptide glutathione (different transport system) as the cysteine source (17, 27).
We also searched the three published genome sequences of L. pneumophila for cysteine biosynthetic genes (7, 8). Two genes annotated as cystathionine beta synthase and cystathionine beta lyase were identified, but the deduced protein functions are associated with interconversions of homoserine and cystathionine, respectively. The deduced products share some similarity with l-cysteine synthase enzymes as well as the common requirement for pyridoxyl phosphate for activity. The formation of homoserine from cystathionine is necessary for methionine synthesis, as it is methylated by a 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase to produce methionine. The gene encoding this enzyme is absent in L. pneumophila and most likely is responsible for methionine auxotrophy. The gene whose product converts cystathionine to l-cysteine (cystathionine gamma lyase) is apparently absent in the genome of L. pneumophila. The absence of serine acetyltransferase and cysteine synthase activities in extracts from several strains of L. pneumophila supports genetic evidence for l-cysteine auxotrophy of L. pneumophila. Since we cannot grow L. pneumophila in the absence of cysteine, we cannot formally rule out the possibility that cysteine represses the biosynthesis of these putative enzymes, as is the case in E. coli (3). The presence of these activities in L. oakridgensis confirms earlier findings indicating that this organism does not require exogenous cysteine for growth (25). A source of sulfide is also essential for cysteine biosynthesis from o-acetyl-serine, and the activities of various sulfur and sulfite reductases were not detected in extracts and a search of the L. pneumophila genome found no orthologues of these genes. Taken together, these studies indicate that l-cysteine auxotrophy of L. pneumophila is due to an absence of key cysteine biosynthetic genes.
Our ability to isolate cystine-utilizing mutants of L. pneumophila suggests the potential for these mutants to occur naturally. While there is no history of such mutants being isolated from natural environments, one might predict that l-cystine utilization might enable L. pneumophila to grow outside host cells in associations with other bacteria in biofilms. Since such variants have not been isolated from natural environments, an ability to utilize cystine might be a disadvantage for L. pneumophila. In this regard, attempts to demonstrate replication of L. pneumophila in artificial biofilms in the absence of amoebal hosts have not been successful (24). We suggest that l-cysteine auxotrophy is necessary to ensure that these bacteria do not multiply outside of suitable protozoan hosts. This view is further supported by discovery of a developmental cycle in which intracellular replicating bacteria differentiate postexponentially into cyst-like survival forms that can persist for extended periods in water in a highly infectious dormant state while between hosts (15). It is likely that these organisms have evolved mechanisms that prevent accidental germination of cysts outside host cells. Additionally, since cystine is rapidly reduced to cysteine in eukaryotic hosts, there would be no selection for cystine-utilizing variants in situ, perhaps guaranteeing that L. pneumophila remains an obligate intracellular parasite. Further studies will be required to determine if availability of l-cysteine, either singly or together with other essential amino acids, such as threonine (29), detected within host cells delivers germination signals that promote differentiation of dormant cysts into RFs.
Acknowledgments
We thank M. G. Keen, E. D. Street, and C. A. Butler for contributions to this work. We also thank the late Robert (Bob) J. Kadner for critical reading of the manuscript and constructive comments.
This work was supported by CIHR grant MOP 14443 and Public Health Service grant RO1 AI20867 to P.S.H.
REFERENCES
- 1.Antikainen, N. M., and S. F. Martin. 2005. Altering protein specificity: techniques and applications. Bioorg. Med. Chem. 13:2701-2716. [DOI] [PubMed] [Google Scholar]
- 2.Baptist, E. W., and N. M. Kredich. 1977. Regulation of l-cystine transport in Salmonella typhimurium. J. Bacteriol. 131:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, M. A., N. M. Kredich, and G. M. Tompkins. 1969. The purification and characterization of o-acetylserine sulfhydrylase-A from Salmonella typhimurium. J. Biol. Chem. 244:2418-2427. [PubMed] [Google Scholar]
- 4.Berger, E. A., and L. A. Heppel. 1972. A binding protein involved in transport of cystine and diaminopimelic acid in Escherichia coli. J. Biol. Chem. 247:7684-7694. [PubMed] [Google Scholar]
- 5.Burguiere, P., S. Auger, M.-F. Hullo, A. Danchin, and I. Martin-Verstraete. 2004. Three different systems participate in l-cystine uptake in Bacillus subtilis. J. Bacteriol. 186:4875-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carifo, K., and W. Catlin. 1973. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl. Microbiol. 26:223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazalet, C., C. Rusniok, H. Bruggemann, N. Zidane, A. Magnier, L. Ma, M. Tichit, S. Jarraud, C. Bouchier, F. Vandenesch, F. Kunst, J. Etienne, P. Glaser, and C. Buchrieser. 2004. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat. Genet. 36:1165-1173. [DOI] [PubMed] [Google Scholar]
- 8.Chien, M., I. Morozova, S. Shi, H. Sheng, J. Chen, S. M. Gomez, G. Asamani, K. Hill, J. Nuara, M. Feder, J. Rineer, J. J. Greenberg, V. Steshenko, S. H. Park, B. Zhao, E. Teplitskaya, J. R. Edwards, S. Pampou, A. Georghiou, I. C. Chou, W. Iannuccilli, M. E. Ulz, D. H. Kim, A. Geringer-Sameth, C. Goldsberry, P. Morozov, S. G. Fischer, G. Segal, X. Qu, A. Rzhetsky, P. Zhang, E. Cayanis, P. J. De Jong, J. Ju, S. Kalachikov, H. A. Shuman, and J. J. Russo. 2004. The genomic sequence of the accidental pathogen Legionella pneumophila. Science 305:1966-1968. [DOI] [PubMed] [Google Scholar]
- 9.Dailey, H. A., Jr., and J. Lascelles. 1977. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J. Bacteriol. 129:815-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dassa, E., M. Hofnung, I. T. Paulsen, and M. H. Saier. 1999. The Escherichia coli ABC transporters: an update. Mol. Microbiol. 32:87-889. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner, G., and R. A. Garduno. 2002. Ultrastructural analysis of differentiation in Legionella pneumophila. J. Bacteriol. 184:7025-7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feeley, J. C., R. J. Gibson, G. W. Gorman, N. C. Langford, J. K. Rasheed, D. C. Mackel, and W. B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaitonde, M. K. 1967. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 104:627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garduno, R. A., E. Garduno, M. Hiltz, and P. S. Hoffman. 2002. Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infect. Immun. 70:6273-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greub, G., and D. Raoult. 2003. Morphology of Legionella pneumophila according to their location within Hartmanella vermiformis. Res. Microbiol. 154:619-621. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman, P. S. 1984. Bacterial physiology, p. 61-67. In C. Thornsberry, A. Balows, J. C. Feeley, and W. Jakubowski (ed.) Legionella: proceedings of the 2nd International Symposium. American Society for Microbiology, Washington, D.C.
- 18.Hoffman, P. S., L. Pine, and S. Bell. 1983. Production of superoxide and hydrogen peroxide in medium used to culture Legionella pneumophila: catalytic decomposition by charcoal. Appl. Environ. Microbiol. 45:784-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, W., L. Varner, and M. Poch. 1991. Acquisition of iron by Legionella pneumophila: role of iron reductase. Infect. Immun. 59:2376-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keen, M. G., and P. S. Hoffman. 1984. Metabolic pathways and nitrogen metabolism in Legionella pneumophila. Curr. Microbiol. 11:81-88. [Google Scholar]
- 21.Kredich, N. M., M. A. Becker, and G. M. Tompkins. 1969. Purification and characterization of cysteine synthetase, a bifunctional protein complex from Salmonella typhimurium. J. Biol. Chem. 244:2428-2439. [PubMed] [Google Scholar]
- 22.Le Faou, A. 1984. Sulphur nutrition and metabolism in various species of Neisseria. Ann. Microbiol. (Paris) 135B:3-11. [DOI] [PubMed] [Google Scholar]
- 23.Luck, P. C., T. Freier, C. Steudel, Y. A. Knirel, E. Luneberg, U. Zahringer, and J. H. A. Helbig. 2001. Point mutation in the active site of Legionella pneumophila O-acetyltransferase results in modified lipopolysaccharide but does not influence virulence. Int. J. Med. Microbiol. 291:345-352. [DOI] [PubMed] [Google Scholar]
- 24.Murga, R., T. S. Forster, E. Brown, J. M. Pruckler, B. S. Fields, and R. M. Donlan. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121-3126. [DOI] [PubMed] [Google Scholar]
- 25.Orrison, L. H., W. B. Cherry, R. L. Tyndall, C. B. Fliermans, S. B. Gough, M. A. Lambert, L. K. McDougal, W. F. Bibb, and D. J. Brenner. 1983. Legionella oakridgensis: unusual new species isolated from cooling tower water. Appl. Environ. Microbiol. 45:36-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulsen, I. T., M. K. Sliwinski, and M. H. Saier. 1998. Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J. Mol. Biol. 277:573-592. [DOI] [PubMed] [Google Scholar]
- 27.Pine, L., J. R. George, M. W. Reeves, and W. K. Harrell. 1979. Development of a chemically defined liquid medium for growth of Legionella pneumophila. J. Clin. Microbiol. 9:615-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pine, L., P. S. Hoffman, G. B. Malcolm, R. F. Benson, and M. J. Franzus. 1986. Role of keto acids and reduced-oxygen-scavenging enzymes in the growth of Legionella species. J. Clin. Microbiol. 23:33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer, J. D., M. A. Bachman, and M. S. Swanson. 2005. The phagosomal transporter A couples threonine acquisition to differentiation and replication of Legionella pneumophila in macrophages. Proc. Natl. Acad. Sci. USA 102:9924-9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stainer, D. W., and M. J. Scholte. 1971. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 31.Thomas, G. H., T. Southworth, M. R. Leon-Kempis, A. Leech, and D. J. Kelly. 2006. Novel ligands for the extracellular solute receptors of two bacterial TRAP transporters. Microbiology 152:187-198. [DOI] [PubMed] [Google Scholar]
- 32.Warren, W. J., and R. D. Miller. 1979. Growth of Legionnaires' disease bacterium (Legionella pneumophila) in chemically defined medium. J. Clin. Microbiol. 10:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieland, H., F. Goetz, and B. Neumeister. 2004. Phagosomal acidification is not a prerequisite for intracellular multiplication of Legionella pneumophila in human monocytes. J. Infect. Dis. 189:1610-1614. [DOI] [PubMed] [Google Scholar]
- 34.Wieland, H., S. Ullrich, F. Lang, and B. Neumeister. 2005. Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter SLC1A5. Mol. Microbiol. 55:1528-1537. [DOI] [PubMed] [Google Scholar]
- 35.Winn, W. C., Jr. 1988. Legionnaire's disease: historical perspective. Clin. Microbiol. Rev. 1:60-81. [DOI] [PMC free article] [PubMed] [Google Scholar]