Abstract
The activity of tigecycline (GAR-936), a novel glycylcycline, was investigated in vitro and in experimental endocarditis due to the susceptible Enterococcus faecalis JH2-2 strain, its VanA type transconjugant BM4316, and a clinical VanA type strain, E. faecium HB217 resistant to tetracycline. MICs of GAR-936 were 0.06 μg/ml for the three strains. In vitro pharmacodynamic studies demonstrated a bacteriostatic effect of GAR-936 that was not enhanced by increasing concentrations to more than 1 μg/ml and a postantibiotic effect ranging from 1 to 4.5 h for concentrations of 1- to 20-fold the MIC. Intravenous injection of [14C]GAR-936 to five rabbits with enterococcal endocarditis sacrificed 30 min, 4 h, or 12 h after the end of the infusion evidenced a lower clearance of GAR-936 from aortic vegetations than from serum and a homogeneous diffusion of GAR-936 into the vegetations. In rabbits with endocarditis, GAR-936 (14 mg/kg of body weight twice a day [b.i.d.]) given intravenously for 5 days was bacteriostatic against both strains of E. faecalis. Against E. faecium HB217, bacterial counts in vegetations significantly decreased during therapy (P < 0.01), and the effect was similar with GAR-936 at 14 mg/kg b.i.d., 14 mg/kg once a day (o.d.), and 7 mg/kg o.d., which provided concentrations in serum constantly above the MIC. Mean serum elimination half-life ranged from 3.3 to 3.6 h. No GAR-936-resistant mutants were selected in vivo with any regimen. We concluded that the combination of prolonged half-life, significant postantibiotic effect, and good and homogeneous diffusion into the vegetations may account for the in vivo activity of GAR-936 against enterococci susceptible or resistant to glycopeptides and tetracyclines, even when using a o.d. regimen in rabbits.
Tetracyclines have been widely used in clinical practice because of their broad spectrum of activity, high tissue and intracellular penetration, and low toxicity. However, acquisition of resistance by numerous gram-negative and gram-positive bacteria has significantly limited their use. Glycylcyclines are new tetracycline analogs derived from minocycline that show the same spectrum of activity as tetracyclines and remain active against many pathogens resistant to tetracyclines, since they were shown to overcome the two major mechanisms responsible for tetracycline resistance, i.e., ribosomal protection and active efflux (2, 5, 6, 13, 14, 18). Preliminary studies suggest that tigecycline (GAR-936, 9-t-butylglycylamido-minocycline), a member of this class, is active against most susceptible and resistant bacteria, among which glycopeptide-resistant enterococci (5, 8, 11-13; D. Milatovic, A. C. Fluit, and J. Verhoef, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 521, 2001). This may be of particular interest since glycopeptide resistance in enterococci, particularly in Enterococcus faecium, is often associated with multidrug resistance, which drastically limits the therapeutic options. However, few studies have evaluated the impact of glycopeptide resistance in enterococci on GAR-936 in vivo activity. Moreover, little is known about the pharmacodynamic properties of GAR-936. The aims of the present work were to study (i) the pharmacodynamic profile of GAR-936 against enterococci with various phenotypes of resistance to glycopeptides and tetracyclines and (ii) the pharmacokinetics and autoradiographic distribution patterns of GAR-936 throughout a deep seat of infection, using the rabbit model of endocarditis.
MATERIALS AND METHODS
Bacterial strains and media.
Enterococcus faecalis JH2-2 is devoid of acquired resistance to glycopeptides, β-lactams, and aminoglycosides (7). E. faecalis BM4316 is a JH2-2 transconjugant harboring a 50-kb genetic element conferring VanA type resistance and was obtained by conjugal transfer from clinical isolate E. faecium HM1074 to JH2-2 (1). E. faecium HB217 is a multidrug-resistant clinical isolate obtained from blood cultures of a 24-year-old female who had undergone a liver transplantation and had received multiple courses of broad-spectrum antibiotics. All cultures and susceptibility testings were performed in brain heart infusion (BHI) broth or agar (Difco Laboratories, Detroit, Mich.).
In vitro studies.
The MICs of GAR-936 (Wyeth-Ayerst Research, Pearl River, NY), vancomycin (Eli Lilly & Co., Saint-Cloud, France), teicoplanin (Aventis, Vitry sur Seine, France), doxycycline (Pfizer, Orsay, France), tetracycline (Aventis), and minocycline (Wyeth Lederle, Pearl River, N.Y.) were determined by the method of Steers et al. (17) with 105 CFU per spot on BHI agar after 24 h of incubation. For time-kill curves, exponentially growing E. faecalis or E. faecium was diluted in glass tubes containing 10 ml of BHI to obtain 107 CFU/ml and incubated with GAR-936 at various concentrations (0.06 to 2 μg/ml). Aliquots were taken after 0, 3, 6, and 24 h of incubation and plated on BHI agar to enumerate the surviving bacteria. A bactericidal effect was defined as a ≥3-log10-CFU/ml killing between the initial inoculum and the bacterial count after 24 h of incubation. For determination of the postantibiotic effect (PAE) of GAR-936, exponentially growing E. faecalis or E. faecium (108 CFU/ml) was exposed to GAR-936 at 0-, 1-, 10-, or 20-fold the MIC for 1 h. Aliquots were then centrifuged, washed, and 1,000-fold diluted to lower the concentration of GAR-936 well below its MIC. PAE was defined as the difference in time required for the exposed organisms and unexposed controls to grow 1 log10 CFU/ml (3).
In vivo studies. (i) Experimental enterococcal endocarditis.
Aortic endocarditis was induced in female New Zealand White rabbits (2.2 to 2.5 kg) by insertion of a polyethylene catheter through the right carotid artery into the left ventricle (10). The rabbits were inoculated via the ear vein 24 h after catheter insertion with 108 CFU of E. faecalis JH2-2, BM4316, or E. faecium HB217 in 1 ml of 0.9% NaCl. The catheter was left in place throughout the experiment. Treatment was started 48 h after inoculation. Animals infected with any of the three strains received GAR-936 intravenously twice daily (b.i.d.) for 5 days at 14 mg/kg of body weight per injection. In order to evaluate the impact of lowering the dosage of GAR-936 on the activity of the drug, rabbits infected with E. faecium HB217 received a 5-day course of GAR-936 at 7 mg/kg b.i.d. or once daily (o.d.). Treated animals were sacrificed 12 h after the last dose for the b.i.d. regimens and 24 h after the last dose for the o.d. regimen. Control animals were sacrificed either 48 h after inoculation (start-of-therapy controls) or at the same time as treated animals (end-of-therapy controls). Animals were killed by intravenous injection of pentobarbital. At the time of sacrifice, the heart was removed and the chambers on the left side were examined to confirm vegetative endocarditis. For each rabbit, vegetations were excised, pooled, weighed and homogenized in 1 ml of sterile distilled water. Vegetation homogenates were plated on agar to count surviving bacteria and on agar containing GAR-936 at two- to eightfold the MIC to detect for the selection of resistant mutants after 48 h of incubation. Results were expressed as log10 CFU per gram of vegetation.
(ii) Serum pharmacokinetic studies. (a) Blood sampling. For determination of GAR-936 pharmacokinetics in serum, 2 ml of blood was sampled 10, 15 and 30 min and 1, 2, 4, 8, 12, 24, and 48 h after a single 7- or 14-mg/kg intravenous injection to four uninfected rabbits.
(b) Assay. Determination of GAR-936 concentrations was performed by reverse-phase liquid chromatography. The chromatographic system consisted of a Wisp 717+ device (Waters, Inc., St-Quentin-en-Yvelines, France), a Shimadzu LC-6A pump (Touzart Matignon, Les Ulis, France), and a Shimadzu SPD 10A device (Touzart Matignon, Les Ulis, France). The chromatographic column was an Ultrasphere ODS column (Beckman, Roissy, France), and UV detection was performed at 243 nm. The eluate (flow rate, 1 ml/min) was composed of oxalic acid (0.01 M)-acetonitrile-methanol (12:56:32, vol/vol/vol). The volume injected was 50 μl. Doxycycline (internal standard) and GAR-936 were well separated, with retention times of, respectively, 4.6 and 11.3 min. Reverse-phase liquid chromatography was chosen for determination of GAR-936 concentrations since it allowed a better sensitivity (0.1 μg/ml) than the microbiological method (0.2 μg/ml) using Sarcina lutea.
(c) Pharmacokinetic study. Pharmacokinetic constants were calculated after a single injection of 7 or 14 mg/kg by using noncompartmental analysis. The WinNonlin program (Scientific Consulting, Inc., Apex, N.C.) was used to fit the data. The area under the plasma concentration-time curve from 0 h to infinity (AUC0-∞) was calculated by trapezoidal rule with extrapolation.
(iii) Autoradiography studies. Five rabbits with experimental endocarditis due to JH2-2 received intravenously, 8 days after bacterial inoculation, 52 to 207 μCi of [14C]GAR-936 in a volume of 10 ml over 30 min. Rabbits were sacrificed 30 min (one rabbit), 4 h (two rabbits), or 12 h (two rabbits) after the end of the infusion so that we could study the influence of the time of sacrifice on the diffusion pattern of the antibiotic. Blood and serum samples were collected at the end of the infusion and at the time of sacrifice. Entire vegetations were excised for autoradiography, and others were used for measurement of antibiotic concentrations. Labeled antibiotic concentrations were counted in samples of blood, plasma, cardiac muscles, and vegetations by liquid scintillation counting, as previously described (15) and expressed as disintegrations per minute per gram. Results were expressed as mean radioactivity concentration in vegetation/injected radioactivity and mean vegetation/serum, vegetation/blood, and vegetation/cardiac tissue concentration ratios. Frozen vegetation samples were cut on a cryostat, thaw mounted onto gelatin-coated microscope slides, and freeze-dried at −25°C for 24 h, as previously described (15). A radioimager (InstantImager; Packard Instruments, Meriden, Conn.) was used for quantitative autoradiography. The principle of detection has been previously described (4). Vegetation sections were imaged for 20 h, and 14C microscale standards were simultaneously included for quantitation. Digital images were stored in a 400-by-480-pixel matrix (pixel area, 0.5 by 0.5 mm), and regions of interest were then manually drawn in order to obtain the different concentrations within the diffusion pattern of the antibiotic.
Statistics.
Comparison of bacterial counts into the vegetations of rabbits treated with various regimens was performed by the Fisher test after analysis of variance (16). Ratios of mean antibiotic radioactivity according to the time of sampling were compared by an unpaired t test. A P of <0.05 was considered to be significant.
RESULTS
In vitro activity of GAR-936.
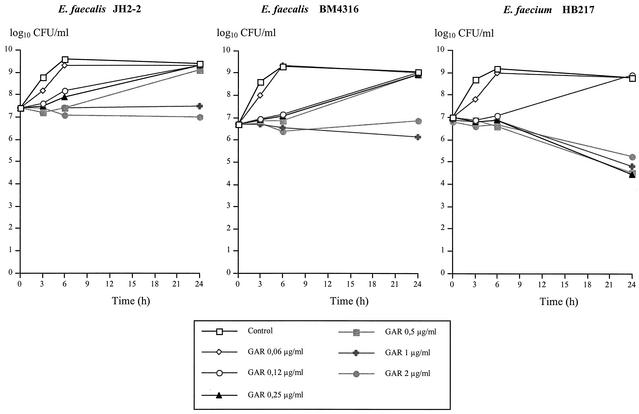
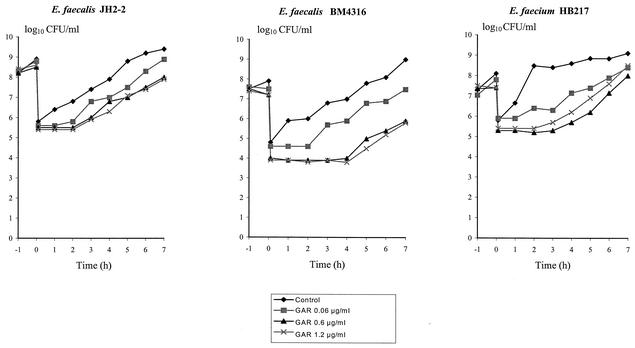
As shown in Table 1, MICs of GAR-936 were as low as 0.06 μg/ml and were similar for the three strains, despite acquired resistance to glycopeptides in E. faecalis BM4316 and E. faecium HB217 and to tetracycline in E. faecium HB217. Time-kill curves demonstrated that GAR-936 was bacteriostatic against E. faecalis JH2-2 and E. faecalis BM4316 for concentrations up to 1 μg/ml, whereas the maximal reduction in bacterial titers at 24 h (−2.0 log10 CFU/ml) was observed against E. faecium HB217 for GAR-936 concentrations of 0.25 μg/ml or more (Fig. 1). Against the three strains, no benefit was obtained from concentrations above 1 μg/ml (16-fold the MIC). A PAE of GAR-936 at 1- to 20-fold the MIC was evidenced against the three strains, ranging from 1 to 2 h for E. faecalis JH2-2, 2 to 4.5 h for E. faecalis BM4316, and 2.5 to 3.3 h for E. faecium HB217, as shown in Fig. 2.
TABLE 1.
MICs of various drugs against glycopeptide-susceptible and -resistant enterococci
| Strain | Glycopeptide phenotype | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| GAR-936 | Vancomycin | Teicoplanin | Tetracycline | Doxycycline | Minocycline | ||
| E. faecalis JH2-2 | Susceptible | 0.06 | 1 | 2 | 0.5 | 0.25 | 0.12 |
| E. faecalis BM4316 | VanA | 0.06 | 256 | 64 | 0.5 | 0.25 | 0.12 |
| E. faecium HB217 | VanA | 0.06 | 512 | 256 | 16 | 1 | 0.12 |
FIG. 1.
Antibacterial activity of GAR-936 (GAR) against the susceptible strain E. faecalis JH2-2 and the VanA type strains E. faecalis BM4316 and E. faecium HB217.
FIG. 2.
PAE of GAR-936 (GAR) against susceptible E. faecalis JH2-2 and VanA type E. faecalis BM4316 and E. faecium HB217.
Experimental endocarditis. (i) Serum pharmacokinetics of GAR-936.
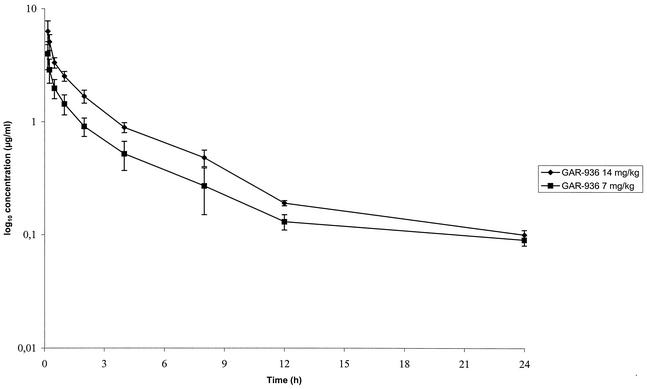
The mean serum levels ± standard deviations after a single intravenous injection of GAR-936 at 7 or 14 mg/kg to uninfected rabbits are shown in Fig. 3. The pharmacokinetic parameters of GAR-936 are given in Table 2. At 24 h, regardless of the dose injected (7 or 14 mg/kg), GAR-936 was still detected in serum (≥0.1 μg/ml), indicating that concentrations of GAR-936 in serum were constantly above the MIC (0.06 μg/ml) within the interval between two intravenous injections. However, these concentrations were near of the limit of quantification of GAR-936 and the number of samples (n = 4) was low, suggesting that these data should be confirmed in additional studies. Forty-eight hours after injection, GAR-936 was not detectable in the serum of any rabbit.
FIG. 3.
Mean serum concentrations (in micrograms per milliliter) of GAR-936 after a single intravenous injection of 7 or 14 mg/kg to uninfected rabbits. Error bars, standard deviations.
TABLE 2.
Pharmacokinetic data for GAR-936 administered by intravenous injection
| GAR-936 dose (mg/kg) | Cmax (mg/liter) | AUC0-∞ (mg/liter/h) | t1/2β (h) | Clearance (ml/h/kg) |
|---|---|---|---|---|
| 7 | 3.7 | 9.5 | 3.6 | 0.7 |
| 14 | 4.4 | 15.3 | 3.3 | 0.9 |
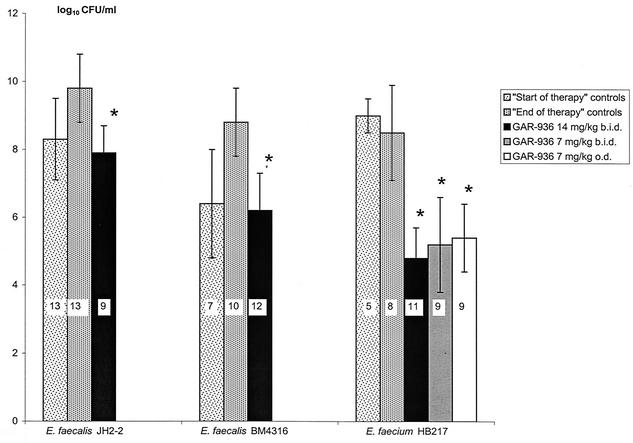
(ii) In vivo activity of GAR-936. Compared to start-of-therapy controls, GAR-936 at 14 mg/kg b.i.d. for 5 days showed a bactericidal activity (reduction of 4.2 log10 CFU/g of vegetation; P < 0.01) against E. faecium HB217 and a bacteriostatic activity against E. faecalis JH2-2 and E. faecalis BM4316 (Fig. 4). Indeed, for E. faecalis JH2-2 and E. faecalis BM4316, bacterial counts from treated rabbits were not different from the start-of-therapy controls. For the three strains, bacterial counts from treated animals were significantly lower than those from untreated animals (end-of-therapy controls) (P < 0.01). In rabbits infected with E. faecium HB217, treatment with GAR-936 7 mg/kg b.i.d. or 7 mg/kg o.d. showed activity similar to that of the 14 mg/kg b.i.d. regimen (Fig. 4). All three regimens produced a bactericidal activity compared with the starting inoculum (start-of-therapy controls). None of the animals treated with GAR-936 retained GAR-936-resistant mutants.
FIG. 4.
Results of treatment with GAR-936 for 5 days in rabbits with experimental endocarditis due to glycopeptide-susceptible and -resistant enterococci. The number of rabbits in each group is indicated inside the columns. *, P < 0.01 versus end-of-therapy controls. Error bars, standard deviations.
(iii) [14C]GAR-936 studies. Counts of [14C]GAR-936 concentrations in samples of blood, serum, cardiac muscles, and vegetations 30 min, 4 h, and 12 h after the intravenous injection of labeled antibiotic to rabbits with enterococcal endocarditis showed an increase in vegetation/plasma and cardiac tissue/serum ratios with time (Table 3), demonstrating that clearance of GAR was lower from the vegetations and cardiac tissue than from plasma. A quantitative autoradiograph of a vegetation representative of [14C]GAR-936 in a rabbit sacrificed 4 h after radiolabeled antibiotic injection is shown in Fig. 5. [14C]GAR-936 diffused homogeneously into the vegetations of rabbits with enterococcal endocarditis. [14C]GAR-936 concentrations in the core of the vegetation were approximately three fold higher than those of the periphery of the vegetation and more than fivefold higher than those of the aortic structure (Fig. 5). The autoradiographic pattern of [14C]GAR-936 was not modified when vegetations were examined 30 min or 12 h after the antibiotic infusion (data not shown).
TABLE 3.
Tissue ratios of [14C]-GAR-936 concentrations according to time of sampling after a 30-min intravenous injection of [14C]-GAR-936
| Time of sacrifice (h) after antibiotic infusion | Dose injected (μCi) (no. of animals) | Ratio of mean radioactivity
|
||
|---|---|---|---|---|
| Vegetation/ serum | Cardiac tissue/ serum | Vegetation/ cardiac tissue | ||
| 0.5 | 100 (1) | 1.1 | 1.9 | 0.6 |
| 4 | 190 (2) | 5.3 | 4.8 | 1.2 |
| 12 | 54 (2) | 9.3 | 15.7 | 2.7 |
FIG. 5.
Quantitative autoradiography of a cardiac vegetation taken from a rabbit treated with 190 μCi of [14C]GAR-936, 4 h after the end of intravenous infusion. Zones (radioactivity data): 1, aortic lumen (70 nCi/g); 2, aortic wall (83 nCi/g); 3, periphery of vegetation (134 nCi/g); 4, core of vegetation (451 nCi/g).
DISCUSSION
We showed in vitro and in experimental endocarditis that glycopeptide resistance in enterococci did not affect the activity of GAR-936, as demonstrated by similar activities of the drug against two isogenic strains differing by their glycopeptide susceptibility. This result, which was already reported by others (11; S. M. Mikels, E. B. Lenoy, W. Allen, S. Compton, and W. J. Weiss, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 135, 1998), could be anticipated from the absence of relation between mechanisms of resistance to glycopeptides and to tetracyclines. GAR-936 was not less active against a tetracycline-resistant VanA type E. faecium isolate than against a tetracycline-susceptible VanA type E. faecalis strain, showing that the ability of GAR-936 to overcome the mechanisms responsible for tetracycline resistance is relevant in vivo. Since glycopeptide resistance is often associated to multidrug resistance, including tetracycline resistance (9), our results suggest that GAR-936 may be considered for the treatment of infections due to glycopeptide-resistant enterococci, regardless of their susceptibility to tetracyclines. Indeed, comparisons with previous studies done in our laboratory in the same experimental model indicate that GAR-936 was as active as vancomycin and teicoplanin against the susceptible strain E. faecalis JH2-2 but more active against the VanA strain E. faecalis BM4316, as expected by in vitro data (15). Of course, the impact of tetracycline resistance in enterococci on the in vivo activity of GAR-936 would be more precisely evaluated by testing isogenic pairs of strains, harboring or not the determinants responsible for resistance.
As for tetracycline antibiotics, GAR-936 was found to be bacteriostatic rather than bactericidal, even though in vitro and in vivo antimicrobial activities were more pronounced against E. faecium HB217 than against strains of E. faecalis (Fig. 1 and 4). We have no explanation for this phenomenon since there is no known natural mechanism of resistance to tetracycline in E. faecalis that would not be present in E. faecium. Additional time-kill studies against numerous strains are needed in order to know if this differential activity against E. faecalis and E. faecium is a general behavior of GAR-936.
Pharmacodynamic parameters predictive of GAR-936 efficacy could not be clearly extrapolated from our results since, despite o.d. injection, concentrations of GAR-936 in serum remained above the MICs for the strains during the entire interval between two injections,even with the lowest dose tested. However, the fact that the increase in GAR-936 concentrations above 1 μg/ml in vitro, as well as the in vivo total daily dose of GAR-936 above 7 mg/kg, did not enhance the in vitro and in vivo activity suggests that GAR-936 does not exhibit a concentration- or dose-dependent antimicrobial activity as long as concentrations remain above the MIC. Such a pharmacodynamic profile was suggested by van Ogtrop et al. who found, in a murine thigh infection model, that the pharmacokinetic parameter that correlated best with efficacy was the time above a certain factor times the MIC for most of the organism-drug combinations studied and that the efficacy of GAR-936 was dependent on the frequency of dosing. However, due to the relatively long half-life (t1/2) and the long PAE, the AUC was also reasonably predictive (18). A time-dependent antimicrobial activity of GAR-936 is also suggested by Mikels et al. (Mikels et al., 38th ICAAC), who demonstrated in a murine model of pneumococcal pneumonia that an 8-mg/kg o.d. regimen was less active than a 4-mg/kg b.i.d. regimen. It is important to outline that AUCs achieved in uninfected rabbits with the 7- and the 14-mg/kg doses were in the range of those reported in healthy subjects with an intravenous infusion of doses of 100 to 200 mg and 200 mg, respectively, that are likely to be used in humans (G. Muralidharan, J. Getsy, P. Mayer, I. Paty, M. Micalizzi, J. Speth, B. Wester, and P. Mojaverian, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 416, 1999).
Although experimental endocarditis is a difficult-to-treat infection characterized by difficulties of antibiotic diffusion and emergence of antibiotic-resistant mutants, the in vivo activity of GAR-936 correlated with in vitro results. The combination of a prolonged PAE of GAR-936, a relatively long t1/2, and the excellent homogeneous tissular diffusion and accumulation of GAR-936 we and others found (18; P. J. Petersen, W. J. Weiss, P. Labthavikul, and P. A. Bradford, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 132, 1998; N. L. Tombs, I. Chaudhary, R. Conant, and J. Kantrowitz, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 413, 1999) may lead to a prolonged inhibition of bacterial growth into the vegetations. In addition, we did not evidence the emergence of GAR-936-resistant mutants despite the high bacterial inoculum present in the vegetations at the start of therapy, and to our knowledge such mutants have never been reported in the literature to date. Since the t1/2 of GAR-936 in humans is longer than in rabbits (G. Muralidharan, J. Getsy, P. Mayer, I. Paty, M. Micalizzi, J. Speth, B. Wester, and P. Mojaverian, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 416, 1999), our results suggest that o.d. tigecycline (GAR-936) may be of clinical interest for the treatment of enterococcal infections due to strains susceptible or resistant to glycopeptides and tetracyclines.
Acknowledgments
This work was supported by Wyeth-Ayerst Research, Pearl River, N.Y.
We thank Patrice Courvalin, Unité des Agents Antibactériens, Institut Pasteur, Paris, France, for providing E. faecalis JH2-2 and E. faecalis BM4316, and Catherine Branger, Laboratoire de Bactériologie, Hôpital Beaujon, Clichy, France, for providing E. faecium HB217.
REFERENCES
- 1.Arthur, M., F. Depardieu, G. Gerbaud, M. Galimand, R. Leclercq, and P. Courvalin. 1997. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546 and related elements in the absence of induction. J. Bacteriol. 179:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher, H. W., C. B. Wennersten, and G. M. Eliopoulos. 2000. In vitro activities of the glycylcycline GAR-936 against gram-positive bacteria. Antimicrob. Agents Chemother. 44:2225-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, Md.
- 4.Englert, D., N. Roessler, A. Jeavons, and S. Fairless. 1995. Microchannel array detector for quantitative electronic radioautography. Cell. Mol. Biol. (Noisy-le-grand) 41:57-64. [PubMed] [Google Scholar]
- 5.Gales, A. C., and R. N. Jones. 2000. Antimicrobial activity and spectrum of the new glycylcycline, GAR-936 tested against 1,203 recent clinical bacterial isolates. Diagn. Microbiol. Infect. Dis. 36:19-36. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein, E. J., D. M. Citron, C. V. Merriam, Y. Warren, and K. Tyrrell. 2000. Comparative in vitro activities of GAR-936 against aerobic and anaerobic animal and human bite wound pathogens. Antimicrob. Agents Chemother. 44:2747-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, R. N. 1999. Disk diffusion susceptibility test development for the new glycylcycline, GAR-936. Diagn. Microbiol. Infect. Dis. 35:249-252. [DOI] [PubMed] [Google Scholar]
- 9.Leclercq, R. 1997. Enterococci acquire new kinds of resistance. Clin. Infect. Dis. 24(Suppl. 1):80-84. [DOI] [PubMed] [Google Scholar]
- 10.Lefort, A., and B. Fantin. 1999. Rabbit model of bacterial endocarditis, p. 611-617. In O. Zak and M. Sande (ed.), Handbook of animal models of infection. Academic Press, London, United Kingdom.
- 11.Murphy, T. M., J. M. Deitz, P. J. Petersen, S. M. Mikels, and W. J. Weiss. 2000. Therapeutic efficacy of GAR-936, a novel glycylcycline, in a rat model of experimental endocarditis. Antimicrob. Agents Chemother. 44:3022-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel, R., M. S. Rouse, K. E. Piper, and J. M. Steckelberg. 2000. In vitro activity of GAR-936 against vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:177-179. [DOI] [PubMed] [Google Scholar]
- 13.Petersen, P. J., N. V. Jacobus, W. J. Weiss, P. E. Sum, and R. T. Testa. 1999. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (GAR-936). Antimicrob. Agents Chemother. 43:738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Projan, S. J. 2000. Preclinical pharmacology of GAR-936, a novel glycylcycline antibacterial agent. Pharmacotherapy 20:219S-223S. [DOI] [PubMed]
- 15.Saleh-Mghir, A., A. Lefort, Y. Petegnief, S. Dautrey, J. M. Vallois, D. Le Guludec, C. Carbon, and B. Fantin. 1999. Activity and diffusion of LY333328 in experimental endocarditis due to vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 43:115-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steel, R. G. D., and J. H. Tovrie. 1980. Multiple comparisons, p. 172-194. In C. Napier and J. W. Maisel (ed.), Principles and procedures of statistics: a biometrical approach. McGraw-Hill, New York, N.Y.
- 17.Steers, E., E. L. Foltz, B. S. Graves, and J. Riden. 1959. An inocula replicating apparatus for routine testing of bacterial susceptibility to antibiotics. Antibiot. Chemother. (Basel) 9:307-311. [PubMed] [Google Scholar]
- 18.van Ogtrop, M. L., D. Andes, T. J. Stamstad, B. Conklin, W. J. Weiss, W. A. Craig, and O. Vesga. 2000. In vivo pharmacodynamic activities of two glycylcyclines (GAR-936 and WAY 152,288) against various gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 44:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]