Abstract
Exogenous proline can protect cells of Saccharomyces cerevisiae from oxidative stress. We altered intracellular proline levels by overexpressing the proline dehydrogenase gene (PUT1) of S. cerevisiae. Put1p performs the first enzymatic step of proline degradation in S. cerevisiae. Overexpression of Put1p results in low proline levels and hypersensitivity to oxidants, such as hydrogen peroxide and paraquat. A put1-disrupted yeast mutant deficient in Put1p activity has increased protection from oxidative stress and increased proline levels. Following a conditional life/death screen in yeast, we identified a tomato (Lycopersicon esculentum) gene encoding a QM-like protein (tQM) and found that stable expression of tQM in the Put1p-overexpressing strain conferred protection against oxidative damage from H2O2, paraquat, and heat. This protection was correlated with reactive oxygen species (ROS) reduction and increased proline accumulation. A yeast two-hybrid system assay was used to show that tQM physically interacts with Put1p in yeast, suggesting that tQM is directly involved in modulating proline levels. tQM also can rescue yeast from the lethality mediated by the mammalian proapoptotic protein Bax, through the inhibition of ROS generation. Our results suggest that tQM is a component of various stress response pathways and may function in proline-mediated stress tolerance in plants.
Reactive oxygen species (ROS) are produced by all aerobically respiring cells. ROS can have detrimental effects on cells by oxidizing lipids, proteins, DNA, and carbohydrates, resulting in disease and death (22, 47). It is therefore essential for aerobic organisms to modulate ROS levels and activities in order to protect against toxicity. The α-imino acid proline functions as a potent antioxidant by scavenging intracellular ROS generated by the phytopathogenic fungus Colletotrichum trifolii (7). The protective role of proline could be extended to the budding yeast Saccharomyces cerevisiae, since proline conferred cell survival in the presence of lethal levels of paraquat, a contact herbicide that uncouples electron transport by generating lethal levels of superoxide (7). Proline also is a well-known osmoprotectant, capable of mitigating the impacts of drought, salt, and temperature stress in higher plants (11). In S. cerevisiae, the cryoprotective activity of proline was established through a positive correlation between intracellular proline levels and resistance to freeze stress (33). These abiotic stresses, including drought, salinity, and cold, are tightly linked to ROS generation (2). Thus, these findings suggest a positive correlation between intracellular proline levels and resistance to oxidative stress. However, the mechanisms of proline-mediated stress protection and, in particular, the components involved in proline-dependent signal transduction pathways are still not well understood.
Intracellular proline levels are controlled by a series of key proline metabolic enzymes mediating proline synthesis and degradation. In S. cerevisiae, two enzymes, proline dehydrogenase (Put1p) and Δ1-pyrroline-5-carboxylate (P5C) dehydrogenase (Put2p), mediate the conversion of proline to glutamate in the mitochondria (5, 6, 49). Accumulating evidence has shown that these two enzymes also are active in proline-mediated stress responses. Proline accumulation by mutation or disruption of PUT1 enhances freeze tolerance and desiccation stresses (45). Increased intracellular proline levels in a put1 mutant yeast strain also were correlated with higher tolerance to hydrogen peroxide (H2O2) (46). Thus, proline acts as a compatible solute and protects cells against damage during oxidative stress. Accumulation of the proline catabolic intermediate P5C by disruption of the PUT2 gene triggers intracellular ROS generation, which suggests that proline catabolism contributes to intracellular oxidative stress (36).
The role of proline metabolic enzymes in oxidative stress also has been described in other organisms. In a human colon cancer cell line, proline dehydrogenase activity was induced by p53-dependent initiation of apoptosis and catalyzed proline-mediated ROS formation (12, 38). The antioxidant enzyme Mn-superoxide dismutase effectively inhibits apoptosis induced by proline dehydrogenase activity (27). In Arabidopsis thaliana, incompatible interactions with Pseudomonas syringae pv. tomato, which generates high amounts of ROS, resulted in proline accumulation and transcriptional activation of AtP5CS, an enzyme involved in proline biosynthesis (15). However, it remains unclear how these proline metabolic enzymes are regulated in response to oxidative stress.
The small, basic QM protein was first identified as a putative tumor suppressor from the Wilms' tumor cell line (13). It is highly conserved in mammals, plants, worms, insects, and yeasts (16). Recent studies of mammalian cells suggest that QM is a key regulator for signaling pathways involving SH3 domain-containing membrane proteins, e.g., the Src family of kinases, since the QM protein directly interacts with the SH3 domain (37). Moreover, the yeast QM homologue, GRC5, is involved in translational control of gene expression in S. cerevisiae, based on the observation that GRC5 directly participates in the recombination of 60S and 40S ribosomal protein subunits (37). Several QM homologues have been identified in plants, but their physiological functions have not yet been described.
Our objectives in this study were to examine the cellular consequences of endogenous manipulation of intracellular proline levels, particularly under conditions of oxidative stress. Since proline supplementation is cytoprotective to fungal cells (including yeast), we developed a conditional life/death in yeast to identify plant gene products that protect cells with reduced proline levels subjected to oxidative stress. In this report we show that modulation of intracellular proline is effective in ameliorating lethal levels of oxidative stress. Moreover, we describe a novel tomato gene (tQM) that rescues yeast strains with limited proline levels from a variety of ROS-inducing stimuli. Taken together, our data further demonstrate the importance of proline in stress tolerance.
MATERIALS AND METHODS
Yeast strains and culture media.
Saccharomyces cerevisiae strains EGY48 (MATα his 3 trp1 ura3 LexAop-Leu2; Clontech, Inc., Mountain View, CA) and C15-1A (put1; MATa ura3-52 trp1 put1-54) were used as the wild-type and mutant strains in this study, respectively. Yeast strains were routinely cultured in YPD (1% yeast extract, 2% peptone, 2% dextrose) or synthetic dropout (SD) media with appropriate supplements at 30°C. When indicated, 1.6 mM proline was added to the SD medium.
Plasmid construction and yeast transformation.
A Put1p expression vector was made by subcloning the PUT1 gene (48) by PCR into a pYES2 shuttle vector (Invitrogen, Carlsbad, CA) for expression from the GAL1 promoter as a C-terminal six-His tag fusion protein. A construct of the PUT1 gene also was designed that lacked the mitochondrial signaling peptide (pYES2-Put1pΔ18). Computational analysis (MitoProt II; Institute of Human Genetics, Technical University Munich/GSF National Research Center, Germany) of the Put1p primary structure predicts that the first 18 residues are involved in mitochondrial signaling. The pYES2-Put1pΔ18 construct was made from pYES2-PUT1 by using QuikChange (Stratagene, La Jolla, CA) site-directed mutagenesis. The fusion constructs pLexA-Bax and pLexA-PUT1 were prepared by amplifying DNA fragments (EcoRI-XhoI) of the PUT1 gene and the mammalian protoapoptotic bax gene by PCR and ligating the fragments into the yeast expression vector pLexA. The structures of the above constructs were confirmed by nucleic acid sequencing. Constructs were transformed into the yeast strain EGY48 (wild type) using lithium acetate (28). Transformed cells were plated on SD/Glu/-His medium, where expression of PUT1 or the bax gene was repressed by glucose. To induce expression of Put1p and the lethal effect of Bax, the transformed cells were plated on SD/Gal/Raff/-His medium, where expression of the PUT1 or bax gene, respectively, was induced by galactose.
Synthesis of Put1p and Put1pΔ18 was confirmed by Western blot analysis. Put1p was overexpressed in EGY48 at 30°C in SD/Gal medium. After harvesting, cells were resuspended in phosphate-buffered saline solution containing a protease cocktail (Sigma, St. Louis, MO) and broken by two freeze-thaw cycles in liquid N2 and by a mini bead beater (BioSpec Products, Inc., Bartlesville, OK) with a pulse sequence of 30 s on, 60 s off, and 30 s on (4). Cell debris was removed by centrifugation (5 min, 4°C, 15,000 × g), and the resulting cell extracts were separated by Tris-glycine sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto an Immuno-Blot polyvinylidene difluoride membrane (0.2-μm pore size; Bio-Rad, Hercules, CA) with an EBU-4000 Semi-Dry electrophoretic blotting system (CBS Scientific Company, Inc., Del Mar, CA). Colorimetric detection of the C-terminal six-His tags of Put1p and Put1pΔ18 was performed with a His tag monoclonal antibody kit (Novagen, San Diego, CA) and an enhanced chemiluminescence detection system (Pierce, Rockford, IL).
Stress tolerance assays.
Early-log-phase yeast cultures (optical density at 600 nm [OD600], 0.5) were diluted to an OD600 of 0.05 with appropriate SD medium. For chemical treatment, selected concentrations of H2O2 were added to the culture and incubated at 30°C with vigorous shaking for 6 h. For heat stress, yeast cells were incubated at 50°C for the indicated times. Following these treatments, cell viability was determined by counting the number of CFU. Ten-microliter aliquots of cell culture were diluted and spread on YPD plates and then incubated at 30°C for 48 h. The number of CFU from treated cells was compared to the number of CFU from untreated cells. All experiments were repeated at least three times. To evaluate yeast cell viability, yeast strains were grown in appropriate SD medium overnight and then evaluated in a spot assay. The cells were serially diluted fivefold, and 5-μl aliquots from each dilution were spotted onto SD/Glu or SD/Gal medium, incubated at 30°C for 3 days, and photographed.
Measurement of intracellular proline levels.
Yeast cells were inoculated in SD medium supplemented with 2% galactose and 1% raffinose and incubated for 3 days at 30°C. Following stress treatment, 5 ml of cell suspension was removed, washed twice with 0.9% NaCl, and suspended in 0.5 ml of distilled water. The cells were transferred to a boiling water bath, and intracellular amino acids were extracted by boiling for 10 min. After centrifugation (5 min, 4°C, 15,000×g), the supernatant was free of proteins, and intracellular proline was determined as described by Bates (3). Briefly, 200 μl of the supernatant was incubated with 200 μl of acid-ninhydrin (0.25 g ninhydrin dissolved in 6 ml glacial acetic acid and 4 ml 6 M phosphoric acid) and 200 μl of glacial acetic acid for 1 h at 100°C. The reaction was stopped by incubation on ice, and the mixture was extracted with 400 μl toluene. The toluene phase was separated, and the OD520 was used to determine the concentration of proline in the extract.
Measurement of intracellular ROS levels.
The production of ROS by yeast cells was detected by incubating 106 to 107 cells in 500 μl SD/Gal medium with 50 μM dihydrorhodamine 123 (DHR123; Molecular Probes, Eugene, OR) for 15 min at room temperature. The samples were viewed with a fluorescent microscope equipped with a rhodamine optical filter. ROS levels were quantified by measuring the green fluorescence intensity of rhodamine-123 from microscopic images with Photoshop software (Adobe Systems, Mountain View, CA).
Conditional life or death screen in yeast.
A tomato cDNA library, constructed from tobacco mosaic virus-infected tomato VF36 leaves, was cloned into the yeast expression vector pB42AD and transformed into EGY48 overexpressing Put1p. The transformed cells were plated on SD/Glu/-His/-Trp medium, where the expression of genes in the library was repressed by glucose. Growing cells were collected, washed, and plated on SD/Gal/Raf/-His/-Trp medium, where the expression of genes in the library was induced by galactose supplemented with 2 mM paraquat. After a 5-day incubation at 30°C, the surviving colonies were again streaked onto SD/Gal/Raf/-His/-Trp plates supplemented with 2 mM paraquat to confirm the resistance phenotype. Tomato cDNAs that conferred paraquat resistance were recovered, the resulting plasmid DNA was retransformed into EGY48 overexpressing Put1p, and the cells were grown on paraquat-containing SD/Gal medium to confirm the resistance phenotype. The isolated cDNA (tQM) was expressed under the control of a constitutive GAL1 promoter, and the resultant plasmid, pB42AD-tQM, was used for further analysis.
RESULTS
Overexpression of Put1p in S. cerevisiae.
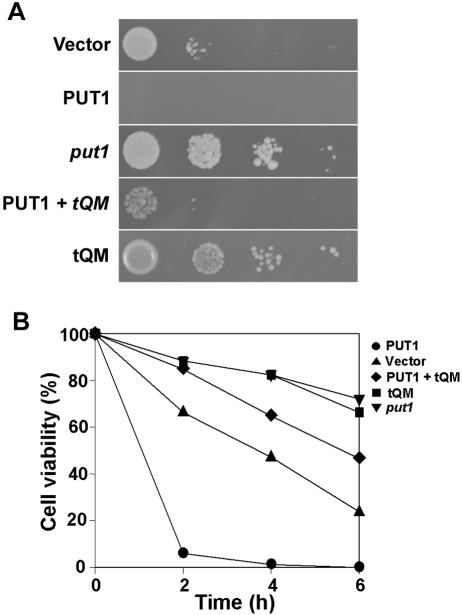
Paraquat, a contact herbicide, uncouples electron transport and generates high levels of superoxide that are lethal to EGY48. Sensitivity to paraquat is decreased following treatment with exogenous proline (7). We evaluated a Put1p-overexpressing strain (with lower proline levels) and a put1-deficient strain (with higher proline levels) to determine if paraquat sensitivity was related to proline metabolism. Put1p-overexpressing yeast cells were more sensitive to oxidants such as paraquat (Fig. 1) and H2O2 (not shown) than the control transformant. Similar results were obtained with a pYES2-PUT1 construct in EGY48, demonstrating that the Put1p fusion product from the pLexA-PUT1 construct was not contributing to the oxidative stress sensitivity (data not shown). The put1-disrupted strain had more protection from these stresses. Thus, overexpression of Put1p, which should lower intracellular proline levels, reduces the protection of cells from oxidative stress and cell death.
FIG. 1.
Altered Put1p expression in yeast affects cell viability during oxidative stress. EGY48-pLexA vector, EGY48-pLexA-PUT1, and put1 deletion strains were spotted at fivefold serial dilutions on plates containing or not containing 1 mM paraquat. Growth was monitored after 3 days incubation at 30°C.
Proline catabolism occurs in the mitochondria, where Put1p couples the oxidation of proline to the reduction of the electron transport system. Overexpression of an N-terminal deletion construct (pYES2-Put1pΔ18) that lacks the mitochondrial signaling peptide did not affect the oxidative stress sensitivity, suggesting that mislocalization of Put1p prevents its proper function in proline catabolism.
We also measured sensitivity to H2O2 in liquid medium. Strains overexpressing Put1p 2 and 4 hours after the addition of 3 mM H2O2 were more sensitive to H2O2 than control cells with vector alone (6% versus 69% viability and 2% versus 49% viability at 2 and 4 h, respectively). Accordingly, the put1 disruptant strain was more resistant to H2O2, with cell viabilities of 87% and 83% at 2 and 4 h, respectively.
Proline was measured in Put1p-overexpressing yeast cells, put1 mutant cells, and control wild-type cells (Table 1). The Put1p-overexpressing strain had the lowest levels of proline, while the put1 disruptant yeast strain accumulated the highest levels of proline (0.43 mM versus 5.3 mM, respectively). Thus, higher proline levels are correlated with increased protection from oxidative stress in yeast.
TABLE 1.
Intracellular proline levels in yeast strains with or without stress treatment
| Treatment | Proline level (mM)
|
||||
|---|---|---|---|---|---|
| Vector | PUTΔ | PUT1 | PUT1 + tQM | tQM | |
| No stress | 3.15 ± 0.30 | 5.22 ± 0.37 | 0.51 ± 0.03 | 3.34 ± 0.32 | 2.46 ± 0.15 |
| H2O2, 6 h | 2.40 ± 0.05 | 6.35 ± 0.55 | 0.62 + 0.08 | 4.35 + 0.40 | 4.31 + 0.42 |
| 50°C, 4 h | 1.05 ± 0.05 | 4.81 ± 0.30 | 0.23 ± 0.01 | 5.34 ± 0.48 | 3.50 ± 0.55 |
Expression of a tQM in Put1p-overexpressing yeast.
We screened a tomato cDNA library to identify genes that inhibit the ROS-mediated lethality of the Put1p-overexpressing yeast strain. From 5 × 106 transformants, hundreds of colonies were recovered from SD/Gal plates supplemented with 2 mM paraquat. We arbitrarily selected 95 colonies and reinoculated them onto paraquat-containing plates. Fourteen of these colonies retained paraquat resistance, and six of them contained DNA that when reintroduced to the Put1p-overexpressing strain again conferred paraquat resistance. One of these six clones encoded a predicted QM-like protein (tQM; GenBank AAY97865) that encodes a polypeptide of 179 amino acids with an estimated molecular mass of 20.3 kDa. The tQM protein shares significant sequence identity with other QM homologues, including S. cerevisiae GRC5 (73%; GenBank CAA55485) and human ribosomal protein L10 (77%; GenBank NP_006004).
EGY48 was transformed with pB42AD, pLexA-PUT1, pLexA-PUT1 plus pB42AD-tQM, and pB42AD-tQM and grown on SD medium supplemented with 2 mM paraquat. Expression of tQM enabled the Put1p-overexpressing strain to grow and survive on medium amended with lethal levels of paraquat (Fig. 2). Growth was similar on media with and without paraquat. Similar results were obtained with H2O2 treatment, suggesting that tQM can rescue yeast from oxidative stress.
FIG. 2.
The tQM protein rescues yeast from oxidant-induced cell death. (A) The EGY48 yeast strain was transformed with an empty pB42AD vector (vector), pLexA-PUT1-expressing Put1p under control of a galactose-inducible promoter (PUT1), and constructs pLexA-PUT1 and pB42AD-tQM, which encodes tQM (PUT1 + tQM). Also shown is the expression of tQM alone in the EGY48 strain (tQM). Cultures of different transformants were diluted to equal densities. Fivefold serial dilutions of each culture were directly spotted onto SD-galactose medium supplemented with 1 mM H2O2. Pictures were taken after 3 days of growth. (B) Yeast strains as shown in panel A were inoculated on liquid SD medium containing 5 mM H2O2 and then grown for different time periods. Aliquots of each yeast strain at each time point were spread on the complete medium, and the number of viable colonies that grew after 3 days was counted. Results are expressed as the percent colonies surviving after H2O2 treatment relative to colonies without H2O2 treatment. Data shown are an average of three independent experiments with standard errors of <10%.
tQM expression and heat stress.
ROS levels increase in yeast cells subjected to heat stress (50°C), and ROS production is directly involved in heat-induced cell death (10). Yeast strains were incubated at 50°C for 4 h, and culture aliquots were spotted on YPD. The Put1p-overexpressing strain was very sensitive to heat stress, but tQM reduced this sensitivity. Thus, tQM may act as a stress suppressor in response to various oxidative stresses.
tQM effects on intracellular oxidation and proline levels during stress.
Before and after heat treatment, fluorescence emission by the ROS indicator DHR123 was much higher in the Put1p-overexpressing strain (425% versus 650%) than in the control (100% versus 440%) or the tQM-overexpressing strains (20% versus 150%). tQM quenched intracellular ROS generation when coexpressed with Put1p (18% versus 240%), suggesting that tQM is scavenging the heat-induced ROS.
Intracellular proline levels also were determined in these strains following treatment with H2O2 or heat stress. The Put1p-overexpressing strain has the lowest proline levels relative to the other yeast cells (Table 1). Introduction of tQM increases proline accumulation in both the wild-type EGY48 strain and the strain carrying Put1p. Stress treatment did not alter the levels of intracellular proline in the tQM yeast strain, but the proline concentration in EGY48 decreased after stress. Thus, higher proline levels are correlated with tQM expression and lower intracellular ROS levels.
tQM interaction with Put1p.
We tested the interaction between tQM and Put1p in a yeast two-hybrid system. Put1p was expressed as a fusion to the LexA DNA binding domain (LexA:PUT1) and tQM was expressed as a fusion to the B42 activation domain (AD:tQM). Expression of both constructs was under control of the GAL promoter. When EGY48 was transformed with LexA:PUT1 and AD:tQM, the transformants grew on Leu− selection medium and turned blue on SD/Gal medium containing 5-bromo-4-chloro-3-indolyl-d-galactoside (X-Gal) but not on SD/Glu medium. A control strain containing LexA or AD alone showed no growth on Leu− medium, and no blue color was observed on X-Gal-containing medium. These results indicate that in vitro, tQM interacts directly with Put1p.
Effects of tQM on Bax-induced lethality.
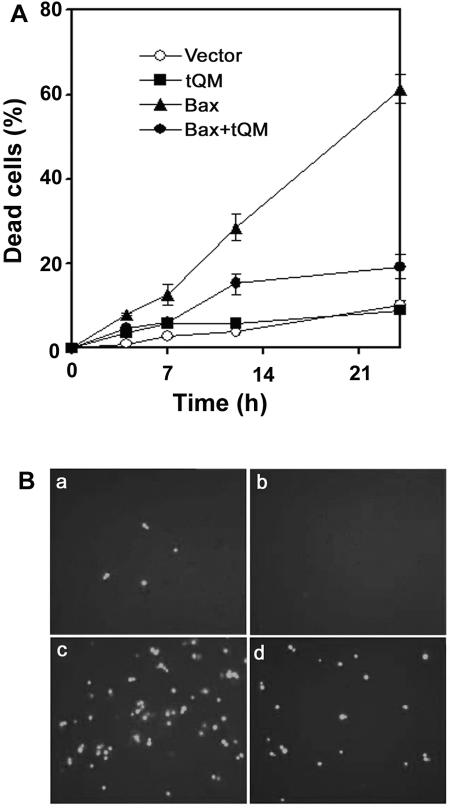
Bax is a proapoptotic member of the Bcl-2 family of proteins, is lethal when expressed in yeast (50), and may be induced by ROS production (28). To extend the generalization of our conclusion that tQM may function as a general stress protection component, we evaluated the effect of tQM expression on Bax-mediated cell death in yeast. Yeast strains were transformed with pLexA-Bax and pB42AD-tQM, cultured in SD-glucose medium for 1 day, and spotted on SD-galactose medium. After a 5-day incubation, yeast colonies transformed with tQM and Bax were visible, but there were no colonies expressing only Bax. After a 24-h incubation on SD-galactose medium, about 60% of the cells died in the Bax-expressing strain, but in a strain coexpressing tQM only 14% of the cells died (Fig. 3A). Expression of tQM also significantly inhibited Bax-induced ROS production as measured with DHR123 (Fig. 3B). Thus, tQM can reduce intracellular ROS levels and induced cell death attributable to Bax-induced oxidative stress.
FIG. 3.
The tomato tQM gene product inhibits Bax-induced cell death in yeast. (A) The EGY48 strain was individually transformed with the empty vector pB42AD, pB42AD-tQM, pLexA-Bax, or pLexA-Bax plus pB42AD-tQM, and transformants were maintained on SD-galactose medium. A time course analysis of dead cell accumulation was employed during Bax-mediated yeast lethality. Evans blue staining was performed at 0, 4, 7, 12, and 24 h after culturing on SD-galactose medium. Dead cells were scored, and data shown are means ± standard errors of the mean of at least three independent experiments. (B) Transformants were treated with 50 μM DHR123 after 24 h of growth on SD-galactose medium and then observed with an epifluorescence microscope. The EGY48 strain was transformed with empty vector pB42AD (a), pB42AD-tQM (b), pLexA-Bax (c). or pLexA-Bax plus pB42AD-tQM (d).
DISCUSSION
Proline is a compatible osmolyte and osmoprotectant (9, 11, 20, 25, 39, 42, 44, 51) and may be multifunctional, especially with respect to its ability to scavenge free radicals (23, 30, 40, 43), control redox homeostasis (1, 21, 43), and ameliorate transitions in redox potential by replenishing NADP+ (11, 18, 21). When organisms are exposed to abiotic stresses, such as salt, heat, drought, cold, and UV light, ROS are produced and proline levels increase. Thus, controlling intracellular proline accumulation confers protection to divergent stresses that all generate ROS and is consistent with previous reports that proline can scavenge ROS (7). A dominant active Ras mutant in the phytopathogenic fungus Colletotrichum trifolii, when grown on minimal medium, produced high levels of ROS resulting in aberrant hyphal morphology and ultimately apoptotic-like programmed cell death. Exogenous proline sufficed to restore wild-type hyphal morphology by inhibiting ROS-induced programmed cell death. The ROS scavenging property of proline also protects fungal cells against other stresses, such as UV light, heat, salt, and H2O2. Exogenous proline also can protect S. cerevisiae cells from paraquat-mediated lethality. These results suggest that proline acts as an antioxidant cytoprotectant during stress by scavenging ROS and maintaining redox homeostasis.
The preceding results are from experiments with exogenous proline supplements. In this study we manipulated endogenous proline levels by altering the activity of Put1p, which catalyzes the rate-limiting step of proline degradation. Overexpression of Put1p reduces intracellular proline levels and increases sensitivity to oxidative stress. In put1 mutants, which lack Put1p, the accumulated intracellular proline protects the cell from oxidative stress. These observations are consistent with those in animals and plants that also suggest that changes in intracellular proline levels are directly associated with stress tolerance. For example, overexpression of human proline dehydrogenase (PRODH2) can induce apoptosis in human tumor cell lines, suggesting a direct correlation between reduction of proline levels and loss of viability (31, 38). Moreover, suppression of proline degradation via the expression of an antisense proline dehydrogenase (AtProDH) gene in Arabidopsis thaliana improves tolerance to freezing and high salinity (34). These results are consistent with the hypothesis that control of intracellular proline levels is critical for stress tolerance.
Genes encoding the enzymes associated with the biosynthesis and degradation of proline have been cloned and partially characterized in organisms ranging from bacteria to fungi, plants, and mammals. However, the factors regulating the expression and activities of these enzymes are largely unclear. In this report, we identified and characterized a tomato QM-like protein. This protein can induce accumulation of intracellular proline and protect cells against oxidative stress. In plants and animals, QM also has a role in development and/or proliferation (13, 14, 29) and may act as a tumor suppressor (32). Phenotypic analysis of an S. cerevisiae mutant deficient in GRC5, a homologue of QM, suggested that QM is involved in multiple cellular functions, including growth control and proliferation, cytoskeletal function, and energy metabolism (35); however, there is no direct evidence on the mechanism through which QM regulates cell growth and development. In this report, we establish a link between QM expression, proline accumulation, and stress tolerance. How does tQM protect the Put1p-overexpressing strain from oxidative stress? One possibility is that tQM directly affects Put1p function. Our yeast two-hybrid analysis showed a physical interaction between tQM and Put1p that is consistent with such a hypothesis.
The observation that tQM suppresses Bax-induced cell death was unexpected. Bax is a proapoptotic member of the Bcl-2 gene family (S. cerevisiae does not contain endogenous Bcl-2 family members), and the initial events underlying Bax activity in yeast and mammalian cells are similar (17, 19, 41). Several mammalian proteins, including Bcl-2 and Bcl-xL, can suppress Bax-induced cell death in yeast, as they do in animal cells (52). Plant genes from Arabidopsis, e.g., the Bax inhibitor 1 gene (AtBI-1), and tomatoes, e.g., LePHGPx, which encodes a phospholipid hydroperoxide glutathione peroxidase, also can suppress Bax-induced lethality and protect yeast against H2O2 and heat stress (8, 24). We found that an ectopically expressed tQM protein suppressed the lethal activity of the mammalian protein Bax in yeast and that inhibition of Bax-induced cell death was correlated with reduced ROS generation. The downstream effectors of Bax-induced cell death in yeast are not yet known, but there is evidence that ROS produced in mitochondria may be included. Our results are consistent with this hypothesis and suggest that tQM can interact with endogenous yeast proteins that are downstream of Bax in a ROS-mediated signaling pathway. In addition to members of the Bcl-2 family, other mammalian proteins, such as Bax inhibitor I, an apoptotic regulator, and the mammalian prion protein (PrP), can inhibit Bax-induced cell death (26, 50, 53). Bax-induced lethality in yeast may reflect interaction with Bax-related pathways that operate in higher organisms. Future studies will center on the characterization of the yeast homologues of these or related molecules which may play a role in the tQM rescue pathway.
Acknowledgments
We thank Marjorie Brandriss at the University of Medicine and Dentistry of New Jersey-New Jersey Medical School for providing us with the Put1p-deficient S. cerevisiae strain C15-1A.
This research was supported in part by NIH pilot grant 5P-20-RR017675-03, NIH grant number P20 RR-017675-02 from the National Center for Research Resources, the University of Nebraska Biochemistry Department, and the Redox Biology Center.
This report is Nebraska Agricultural Research Division Journal Series no. 15164.
The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- 1.Alia, and P. P. Saradhi. 1993. Suppression in mitochondrial electron transport is the prime cause behind stress-induced proline accumulation. Biochem. Biophys. Res. Commun. 193:54-58. [DOI] [PubMed] [Google Scholar]
- 2.Apel, K., and H. Hirt. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55:373-399. [DOI] [PubMed] [Google Scholar]
- 3.Bates, L. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39:205-207. [Google Scholar]
- 4.Becker, D. F., and E. A. Thomas. 2001. Redox properties of the PutA protein from Escherichia coli and the influence of the flavin redox state on PutA-DNA interactions. Biochemistry 40:4714-4722. [DOI] [PubMed] [Google Scholar]
- 5.Brandriss, M. C., and B. Magasanik. 1980. Proline: an essential intermediate in arginine degradation in Saccharomyces cerevisiae. J. Bacteriol. 143:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandriss, M. C., and B. Magasanik. 1981. Subcellular compartmentation in control of converging pathways for proline and arginine metabolism in Saccharomyces cerevisiae. J. Bacteriol. 145:1359-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C., and M. B. Dickman. 2005. Proline suppresses apoptosis in the fungal pathogen of Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 102:3459-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, S., Z. Vaghchhipawala, W. Li, H. Asard, and M. B. Dickman. 2004. Tomato phospholipid hydroperoxide glutathione peroxidase inhibits cell death induced by Bax and oxidative stresses in yeast and plants. Plant Physiol. 135:1630-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csonka, L. N. 1981. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol. Gen. Genet. 182:82-86. [DOI] [PubMed] [Google Scholar]
- 10.Davidson, J. F., B. Whyte, P. H. Bissinger, and R. H. Schiestl. 1996. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delauney, A. J., and D. P. S. Verma. 1993. Proline biosynthesis and osmoregulation in plants. Plant J. 4:215-223. [Google Scholar]
- 12.Donald, S. P., X. Y. Sun, C. A. Hu, J. Yu, J. M. Mei, D. Valle, and J. M. Phang. 2001. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 61:1810-1815. [PubMed] [Google Scholar]
- 13.Dowdy, S. F., C. L. Fasching, D. Araujo, K. M. Lai, E. Livanos, B. E. Weissman, and E. J. Stanbridge. 1991. Suppression of tumorigenicity in Wilms tumor by the P15.5-P14 region of chromosome 11. Science 254:293-295. [DOI] [PubMed] [Google Scholar]
- 14.Eisinger, D. P., H. P. Jiang, and G. Serrero. 1993. A novel mouse gene highly conserved throughout evolution: regulation in adipocyte differentiation and in tumorigenic cell lines. Biochem. Biophys. Res. Commun. 196:1227-1232. [DOI] [PubMed] [Google Scholar]
- 15.Fabro, G., I. Kovacs, V. Pavet, L. Szabados, and M. E. Alvarez. 2004. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant-Microbe Interact. 17:343-350. [DOI] [PubMed] [Google Scholar]
- 16.Farmer, A. A., T. M. Loftus, A. A. Mills, K. Y. Sato, J. D. Neill, T. Tron, M. J. Yang, B. L. Trumpower, and E. J. Stanbridge. 1994. Extreme evolutionary conservation of Qm, a novel C-Jun associated transcription factor. Hum. Mol. Genet. 3:723-728. [DOI] [PubMed] [Google Scholar]
- 17.Greenhalf, W., C. Stephan, and B. Chaudhuri. 1996. Role of mitochondria and C-terminal membrane anchor of Bcl-2 in Bax induced growth arrest and mortality in Saccharomyces cerevisiae. FEBS Lett. 380:169-175. [DOI] [PubMed] [Google Scholar]
- 18.Hagedorn, C. H., and J. M. Phang. 1986. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversion of proline to Δ-pyrroline-5-carboxylate. Arch. Biochem. Biophys. 248:166-174. [DOI] [PubMed] [Google Scholar]
- 19.Hanada, M., C. Aime-Sempe, T. Sato, and J. C. Reed. 1995. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J. Biol. Chem. 270:11962-11969. [DOI] [PubMed] [Google Scholar]
- 20.Hanson, A. D., B. Rathinasabapathi, J. Rivoal, M. Burnet, M. O. Dillon, and D. A. Gage. 1994. Osmoprotective compounds in the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc. Natl. Acad. Sci. USA 91:306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hare, P. D., and W. A. Cress. 1997. Metabolic implications of stress-induced proline accumulations in plants. Plant Growth Regul. 21:79-102. [Google Scholar]
- 22.Harper, M. E., L. Bevilacqua, K. Hagopian, R. Weindruch, and J. J. Ramsey. 2004. Ageing, oxidative stress, and mitochondrial uncoupling. Acta Physiol. Scand. 182:321-331. [DOI] [PubMed] [Google Scholar]
- 23.Hong, Z., K. Lakkineni, Z. Zhang, and D. P. S. Verma. 2000. Removal of feedback inhibition of pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 122:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawai, M., L. Pan, J. C. Reed, and H. Uchimiya. 1999. Evolutionally conserved plant homologue of the Bax inhibitor-1 (BI-1) gene capable of suppressing Bax-induced cell death in yeast. FEBS Lett. 464:143-147. [DOI] [PubMed] [Google Scholar]
- 25.Le Rudulier, D., T. Bernard, G. Goas, and J. Hamelin. 1984. Osmoregulation in Klebsiella pneumoniae: enhancement of anaerobic growth and nitrogen fixation under stress by proline, betaine, γ-butyrobetaine, and other related compounds. Can. J. Microbiol. 30:299-305. [DOI] [PubMed] [Google Scholar]
- 26.Li, A., and D. A. Harris. 2005. Mammalian prion protein suppresses Bax-induced cell death in yeast. J. Biol. Chem. 280:17430-17434. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Y. M., G. L. Borchert, S. P. Donald, A. Surazynski, C. A. Hu, C. J. Weydert, L. W. Oberley, and J. M. Phang. 2005. MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis 26:1335-1342. [DOI] [PubMed] [Google Scholar]
- 28.Madeo, F., K.-U. Frohlich, M. Ligr, M. Grey, S. J. Sigrist, and D. H. Wolf. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145:757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marty, I., C. Brugidou, Y. Chartier, and Y. Meyer. 1993. Growth-related gene expression in Nicotiana tabacum mesophyll protoplasts. Plant J. 4:265-278. [DOI] [PubMed] [Google Scholar]
- 30.Matysik, J., Alia, B. Bhalu, and P. Mohanty. 2002. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 82:525-532. [Google Scholar]
- 31.Maxwell, S. A., and G. E. Davis. 2000. Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines. Proc. Natl. Acad. Sci. USA 97:13009-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteclaro, F. S., and P. K. Vogt. 1993. A Jun-binding protein related to a putative tumor suppressor. Proc. Natl. Acad. Sci. USA 90:6726-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita, Y., S. Nakamori, and H. Takagi. 2003. l-Proline accumulation and freeze tolerance in Saccaromyces cerevisiae are caused by a mutation in the PRO1 gene encoding γ-glutamyl kinase. Appl. Environ. Microbiol. 69:212-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanjo, T., M. Kobayashi, Y. Yoshiba, Y. Kakubari, K. Yamaguchi-Shinozaki, and K. Shinozaki. 1999. Antisense suppression of proline degradation improves stress tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 461:205-210. [DOI] [PubMed] [Google Scholar]
- 35.Nika, J., F. L. Erickson, and E. M. Hannig. 1997. Ribosomal protein L9 is the product of GRC5, a homolog of the putative tumor suppressor Qm in S. cerevisiae. Yeast 13:1155-1166. [DOI] [PubMed] [Google Scholar]
- 36.Nomura, M., and H. Takagi. 2004. Role of the yeast acetyltransferase Mpr1 in oxidative stress: regulation of oxygen reactive species caused by a toxic proline catabolism intermediate. Proc. Natl. Acad. Sci. USA 101:12616-12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh, H. S., H. Kwon, S. K. Sun, and C. H. Yang. 2002. Qm, a putative tumor suppressor, regulates proto-oncogene c-Yes. J. Biol. Chem. 277:36489-36498. [DOI] [PubMed] [Google Scholar]
- 38.Rivera, A., and S. A. Maxwell. 2005. The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J. Biol. Chem. 280:29346-29354. [DOI] [PubMed] [Google Scholar]
- 39.Rontein, D., G. Basset, and A. D. Hanson. 2002. Metabolic engineering of osmoprotectant accumulation in plants. Metab. Eng. 4:49-56. [DOI] [PubMed] [Google Scholar]
- 40.Saradhi, P. P., Alia, A. S. Arora, and K. V. S. K. Prasad. 1995. Proline accumulates in plants exposed to UV radiation and protects them against UV-induced peroxidation. Biochem. Biophys. Res. Commun. 209:1-5. [DOI] [PubMed] [Google Scholar]
- 41.Sato, T., M. Hanada, S. Bodrug, S. Irie, N. Iwama, L. H. Boise, C. B. Thompson, E. Golemis, L. Fong, H. G. Wang, et al. 1994. Interactions among members of the Bcl-2 protein family analyzed with a yeast two-hybrid system. Proc. Natl. Acad. Sci. USA 91:9238-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano, R. 1996. Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int. Rev. Cytol. 165:1-52. [DOI] [PubMed] [Google Scholar]
- 43.Siripornadulsil, S., S. Traina, D. P. S. Verma, and R. T. Sayre. 2002. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell. 14:2837-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart, C. R., S. F. Boggess, D. Aspinall, and L. G. Paleg. 1977. Inhibition of proline oxidation by water stress. Plant Physiol. 59:930-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi, H., K. Sakai, K. Morida, and S. Nakamori. 2000. Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and desiccation stresses in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 184:103-108. [DOI] [PubMed] [Google Scholar]
- 46.Terao, Y., S. Nakamori, and H. Takagi. 2003. Gene dosage effect of l-proline biosynthetic enzymes on l-proline accumulation and freeze tolerance in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 69:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valko, M., M. Izakovic, M. Mazur, C. J. Rhodes, and J. Telser. 2004. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell Biochem. 266:37-56. [DOI] [PubMed] [Google Scholar]
- 48.Wang, S.-S., and M. C. Brandriss. 1986. Proline utilization in Saccharomyces cerevisiae: analysis of the cloned PUT1 gene. Mol. Cell. Biol. 6:2638-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, S.-S., and M. C. Brandriss. 1987. Proline utilization in Saccharomyces cerevisiae: sequence, regulation, and mitochondrial localization of the PUT1 gene product. Mol. Cell. Biol. 7:4431-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu, Q., and J. C. Reed. 1998. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1:337-346. [DOI] [PubMed] [Google Scholar]
- 51.Yancey, P. H., M. E. Clark, S. C. Hand, R. D. Bowlus, and G. N. Somero. 1982. Living with water stress: evolution of osmolyte systems. Science 217:1214-1222. [DOI] [PubMed] [Google Scholar]
- 52.Zha, H., C. Aime-Sempe, T. Sato, and J. C. Reed. 1996. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 271:7440-7444. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, H., Q. Huang, N. Ke, S. Matsuyama, B. Hammock, A. Godzik, and J. C. Reed. 2000. Drosophila pro-apoptotic Bcl-2/Bax homologue reveals evolutionary conservation of cell death mechanisms. J. Biol. Chem. 275:27303-27306. [DOI] [PubMed] [Google Scholar]