Abstract
Lacticin 3147 is a broad-spectrum two-peptide lantibiotic whose genetic determinants are located on two divergent operons on the lactococcal plasmid pMRC01. Here we introduce each of 14 subclones, containing different combinations of lacticin 3147 genes, into MG1363 (pMRC01) and determine that a number of them can facilitate overproduction of the lantibiotic. Based on these studies it is apparent that while the provision of additional copies of genes encoding the biosynthetic/production machinery and the regulator LtnR is a requirement for high-level overproduction, the presence of additional copies of the structural genes (i.e., ltnA1A2) is not.
Lantibiotics are posttranslationally modified antimicrobial peptides produced by gram-positive bacteria (5, 9). The enormous chemotherapeutic potential of these peptides stems from the fact that a number of lantibiotics have been found to be active at nanomolar concentrations (3, 23), can inhibit multidrug-resistant pathogens (4, 13, 18), and are among a rare group of antibacterial compounds that target the lipid II component of the bacterial cell wall (2, 3). However, the study and application of lantibiotics are often compromised by limited production of these peptides by the native producer. This problem is particularly notable when working with bioengineered derivatives (8). While the optimization of fermentation processes is one option, genetic strategies offer an alternative approach which has already proved successful in the case of nisin and subtilin (6, 15). These studies demonstrated that the provision of additional copies of the regulatory (RK) or immunity [(I)FEG] genes resulted in 1.5- to 1.7-fold-greater yields of the relevant lantibiotic (6, 15). Furthermore, deletion of the Bacillus subtilis general regulator AbrB brought about an extraordinary sixfold enhancement in subtilin yields, although unfortunately this was in a less active, succinylated form (15). Here we describe a genetic system that facilitates significant overproduction of both wild-type and bioengineered derivatives of the broad-spectrum, two-peptide lantibiotic lacticin 3147 through the provision of additional copies of the genes responsible for production of, and immunity to, this antimicrobial. Through the use of 14 different subclones we also established that although the provision of additional copies of the biosynthetic/transport genes and of the regulator LtnR was required for high-level overproduction, the presence of additional copies of the two structural genes (ltnA1A2) was not.
Creation of constructs to facilitate lacticin 3147 overproduction.
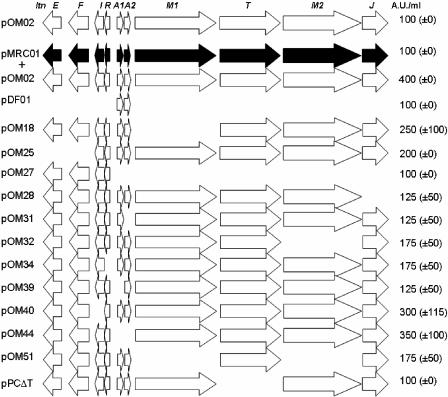
The genes responsible for production of and immunity to lacticin 3147 span a 12.6-kb region on the 60.2-kb plasmid pMRC01, originally identified in Lactococcus lactis DPC3147 (Fig. 1) (28). These genes are present on two operons, i.e., ltnRIFE—an immunity operon including genes for a regulator (ltnR), an immunity protein (ltnI), and an ABC transporter (ltnFE)—and ltnA1A2M1TM2J—a biosynthetic operon with genes for the two structural peptides (ltnA1A2) and for proteins involved in posttranslational modification (ltnM1M2J) and transport/processing (ltnT) (10, 12, 20-22, 27). It has already been reported that a subclone containing all 10 genes on a high-copy-number vector (pOM02) does not lead to higher production of lacticin 3147 than that observed from the low-copy-number parent plasmid pMRC01 (20). In this study, we sought to determine whether the introduction of the cloned region into a background containing the parent plasmid would lead to improved production. To this end, pOM02 was introduced (17) into L. lactis MG1363(pMRC01). Once it was established that the colocalization of pMRC01 and pOM02 in MG1363 did not impact the copy number of either plasmid (E. O'Connor, unpublished data), the antimicrobial activities of this strain and of MG1363 (pMRC01) were compared using a method described previously (28). This involved serial dilution of cell-free supernatant, from antibiotic-free overnight cultures (4 × 108/ml), in one-fourth-strength Ringer's solution and the addition of 50-μl volumes of these dilutions to 4.6-mm wells bored in agar plates seeded with approximately 106 cells of overnight-grown indicator bacteria (Lactococcus lactis HP)/ml. After overnight incubation of the plate, activity was determined and expressed as arbitrary units/ml (AU/ml), i.e., the reciprocal of the highest dilution which gave a definite zone multiplied by the conversion factor (i.e., 20 when 50 μl was used). For simplicity, data are presented as relative activity, i.e., activity relative to that of MG1363(pMRC01). It was found that the activity of the MG1363(pMRC01, pOM02) derivative was dramatically higher (400% activity) than that of the strains containing either plasmid alone (100% activity) (Fig. 1). To determine more specifically which of the genes introduced into pOM02 were responsible for this overproduction, we created a set of different subclones that were introduced into MG1363(pMRC01) (Table 1). PCR amplification was carried out with the Expand High-Fidelity Polymerase system (Roche) or KOD (EMD Biosciences), and restriction enzymes and T4 DNA ligase were purchased from Roche. The sequences of primers are shown in Table 2. Some of these subclones have been described previously (20, 21), while others were made during the course of this study (Table 1; Fig. 1). The plasmids were initially created in Escherichia coli DH5α or XL1 and subsequently introduced into L. lactis MG1363 (pMRC01) by electroporation. Transformants were selected on M17 agar (Oxoid) with 0.5% glucose (GM17) and 5 μg/ml chloramphenicol.
FIG. 1.
Cell-free supernatant activity (relative bioactivity) of lacticin 3147-producing strains as a percentage of that produced by MG1363(pMRC01) (black arrows). The ltn genes present in the pCI372 derivatives (pDF, pOM, and pPC) introduced into MG1363(pMRC01) are illustrated. The data presented are the averages of quadruplicate experiments (± standard deviations).
TABLE 1.
Plasmids used in this study
| Plasmid | Description and/or source (reference) |
|---|---|
| pMRC01 | 60.2-kb natural lactococcal plasmid containing ltnEFIRA1A2M1TM2J (12) |
| pMRC01S7G | Derivative of pMRC01 generated by site-directed mutagenesis (10) |
| pCI372 | High-copy-number E. coli-Lactococcus cloning vector (14) |
| pOM02 | pCI372-ltnEFIRA1A2M1TM2J (21) |
| pOM18 | Equivalent to pOM02ΔltnM1 (20) |
| pOM31 | Equivalent to pOM02ΔltnA2 (20) |
| pOM32 | Equivalent to pOM02ΔltnM2 (20) |
| pOM39 | Equivalent to pOM02ΔltnA1 (20) |
| pOM11 | 6-kb fragment from pOM02 (primers 5145 and 5146) cloned into BamHI/SalI-digested pCI372 |
| pOM27 | pCI372-ltnEFIR; 4-kb PCR product (primers 5144 and 5558) cloned into KpnI/BamHI-digested pOM11 |
| pOM25 | Equivalent to pOM02ΔltnFE; 4.3-kb product (primers 5144 and 5556) cloned into SacI/BamHI site of pOM11 |
| pOM28 | Equivalent to pOM02ΔltnJ; 4.7-kb product [primers LtnX(f) and LtnX(r)] cloned into BamHI/SalI-digested pOM02 |
| pOM34 | Equivalent to pOM02ΔltnR; 2.4-kb product [primers FP(OP)372 and 5557] cloned into SacI/KpnI-digested pOM27 |
| pOM38 | 4-kb product (primers 6163 and 5144) cloned into KpnI/BamHI-digested pOM11 |
| pOM40 | Equivalent to pOM02ΔltnI; 2-kb product [primers FP(OP)372 and 6164] cloned into SacI/KpnI-digested pOM38 |
| pOM44 | Equivalent to pOM02ΔltnA1A2; replacement of a 3.5-kb SacI/KpnI fragment from pOM31 with a 2.9-kb SacI/KpnI fragment from pOM39 |
| pOM51 | Equivalent to pOM02ΔltnM1M2; replacement of a BamHI/SacI fragment from pOM18 with the corresponding region from pOM32 |
| pPCM2J | 4.7-kb product (primers ΔT for and pOM02rev) cloned into BamHI/SalI-digested pCI372 |
| pPCΔT | Equivalent to pOM02ΔltnT; 6-kb product [primers 5144 and FP(OP)372] cloned into BamHI/SacI-digested pPCM2J |
| pDF01 | pCI372-ltnA1A2; 1-kb product (primers A1soeA and A2soeD) cloned into PstI/EcoRI-digested pCI372 |
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′ to 3′)a |
|---|---|
| 5144 | GATGGATCCATTATTATTACTGTTT |
| 5145 | ATGGATCCATCAGTAGGTAGAACTA |
| 5146 | GCTGTCGACTTAAGTATAGGGCAAT |
| LtnX(f) | ATGGATCCATCAGTAGGTAGAACTA |
| LtnX(r) | GCTGTCGACAAATTAACCACCCAAA |
| FP(OP)372 | AAAGAGCTCTGCGAATAACATCAAGGGAA |
| 5144 | GATGGATCCATTATTATTACTGTTT |
| 5556 | AGAGCTCTTTTTTTCTTATTTGA |
| 5558 | AGGTACCCAGAGTTACTAATAGAA |
| 6163 | AAGGTACCTGAGTAAAAAAGAGA |
| 6164 | TTGGTACCGGATTTATTGTCTTTT |
| A1soeA | AACTGCAGTTATATATTTGCGGC |
| A2soeD | ACGAATTCTCTTACAGAGTT |
| pOM02rev | AGCTGTCGACTTAAGTATAGGGCAAT |
| ΔT for | TTGGATCCTGGGTTCAAATTACC |
Restriction sites in oligonucleotides are underlined.
Agar well diffusion assays were again performed on all strains. It was apparent that, with the exceptions of pOM27 (which lacks all biosynthetic genes), pPCΔT (which lacks ltnT), and pDF01 (carrying structural genes only), all of the constructs facilitated lacticin 3147 overproduction at least to some extent. While the addition of pOM02 containing all 10 genes resulted in the highest levels of antimicrobial activity, the strain containing pMRC01 and pOM44 (which contains all ltn genes except ltnA1A2) also displayed high relative bioactivity (350%; Fig. 1). Although percent overproduction was lower in all other cases, the results generated gave an insight into the relative importance of each of the genes in contributing to overproduction. The assays revealed that even in the absence of additional copies of ltnFE (pOM25) and ltnI (pOM40), respective two- and threefold increases in activity were apparent. Although it has yet to be established definitively that LtnFE contribute to lacticin 3147 immunity, it may be that an inability to tolerate increased levels of lacticin 3147 limits the ability of these strains to overproduce the lantibiotic as efficiently as MG1363(pMRC01, pOM02). The absence of ltnR (pOM34), a negative regulator of the ltnRIFE operon (22), resulted in more limited overproduction (175%). It has previously been established that MG1363(pOM34) exhibits activity which is approximately 50% of that of MG1363(pOM02) (22). The cause of this reduced activity has yet to be ascertained. When individual genes involved in biosynthesis/transport were absent from the newly introduced subclones, it was apparent that the presence of ltnT (pPCΔT; 100%) and ltnJ (pOM28; 125%) was important. Based on homologies with other LanT enzymes, LtnT is responsible for the transport of the lacticin 3147 peptides from the cell and the concomitant removal of the leaders. Although it has been established that LctT is dispensable for lacticin 481 production (26, 29), the inactivation of mutT abolishes mutacin II production (7). LtnJ is required for the presence of d-alanine residues in LtnA1 and LtnA2, and an MG1363(pMRC01ΔltnJ) mutant has previously been shown to exhibit little antimicrobial activity (10). Overproduction was limited to between 175 and 250% as a consequence of not introducing additional copies of ltnM1 and/or ltnM2 (pOM18, pOM32, and pOM51). LtnM1 and LtnM2 are required for the production of fully modified LtnA1 and LtnA2, respectively (20). Previously an attenuator that impacts on the levels of ltnM1TM2J transcript was identified. Its presence undoubtedly impacts the levels of biosynthetic/transport proteins and may explain why the provision of additional copies of the corresponding set of genes is beneficial. In contrast, the presence of additional copies of the structural genes is not essential for overproduction. Curiously, while the benefits of adding pOM44 (ΔltnA1A2) were great, these benefits were almost eliminated when only one of the structural genes was missing (pOM31/39; both 125%). It may be that, as a consequence of an uneven balance of substrate, one of the enzymes that is involved in the modification/production of both peptides, i.e., LtnT or LtnJ, functions less efficiently. Taken in combination these results indicate that while the presence of additional copies of the biosynthetic/transport machinery and LtnR is required for high-level overproduction, there is no single bottleneck that can be relieved by the addition of a particular gene product.
The observation that overproduction can be achieved in the absence of the wild-type structural genes from pOM44 presented the possibility that this construct could facilitate the overproduction of bioengineered LtnA1 and LtnA2 derivatives. To test this theory, pOM44 was introduced into a strain containing a pMRC01 derivative in which the serine at position 7 in LtnA1 (normally converted to a d-alanine in the mature peptide) is replaced with an l-glycine (10). It has previously been established that although the A1S7G peptide has only 50% of the relative activity of the parent peptide, its further characterization was hampered by a 94% decrease in its production (10). It was apparent from cell-free supernatant assays that the activity associated with supernatant from the strain containing pOM44 was increased twofold (40 versus 20 AU/ml), a small but significant increase.
Quantification of lacticin 3147 overproduction.
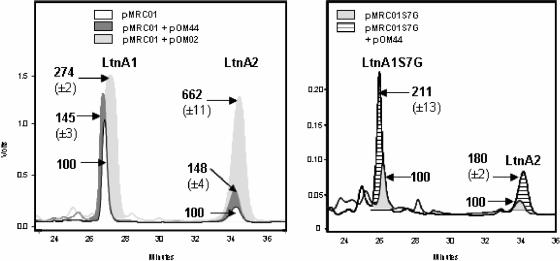
While the antimicrobial assays described above allowed us to determine the relative benefits of adding different constructs, the relationship between peptide concentration and antimicrobial activity as ascertained by the well diffusion assay is not always linear (11). To more accurately quantify the production of LtnA1 and -A2 from the strains of greatest interest, the lacticin 3147 peptides were purified from the surface of selected strains and quantified by quantitative reverse-phase high-pressure liquid chromatography (RP-HPLC) as described previously (10) (Fig. 2). The purification of LtnA1 and LtnA2 in this way results in greater peptide yields and overcomes the problems associated with contaminating peptides from culture media, which hamper the quantification of the lacticin 3147 peptides obtained from cell-free supernatant. Relative to the yield from MG1363 containing pMRC01 alone, the yield of LtnA1 from strains also containing pOM44 or pOM02 was 1.45- and 2.74-fold greater, respectively, while the yields for LtnA2 were 1.48- and 6.62-fold greater, respectively. Mass analysis (matrix-assisted laser desorption ionization-time of flight mass spectroscopy) using an AXIMA-CFRplus mass spectrometer (Shimadzu Biotech, Manchester, United Kingdom) demonstrated that the masses of LtnA1 (3,305 kDa) and LtnA2 (2,847 kDa) were as expected in all cases (data not shown), indicating the absence of unexpected modifications. It is curious that pOM02 led to differential overproduction of LtnA1 and LtnA2. In MG1363(pMRC01) and MG1363(pMRC01, pOM44), the ratios of LtnA1 to LtnA2 are similar in that there would appear to be significantly more LtnA1 than LtnA2 present. This trend was also apparent when the corresponding peptides were purified from the related two-peptide lantibiotics staphylococcin C55 and plantaricin W (16, 24). The reason for this is not apparent, but it is surprising given that these peptides function in a 1:1 ratio. However, the fact that this trend exists may explain the differential overproduction of the two peptides in MG1363(pMRC01, pOM02), in that because the levels of LtnA2 being produced by the parental strain are relatively low, there is simply more room for improvement. Conversely, as the quantities of LtnA1 that are produced by MG1363(pMRC01) are relatively high, it may be that the MG1363(pMRC01, pOM02) strain is producing the maximum amount of LtnA1 that the cell can either facilitate or tolerate.
FIG. 2.
Quantitative RP-HPLC of wild-type lacticin 3147 peptides purified from MG1363 containing pMRC01, pMRC01 plus pOM44, and pMRC01 plus pOM02 (left) and A1S7G peptide purified from LtnA1Ser7Gly and LtnA1Ser7Gly containing pOM44 (right). Values refer to peak size as a percentage of the relevant control (average of duplicate purification experiments).
RP-HPLC analysis also revealed that the yield of A1S7G from the strain possessing pOM44 was more than twofold greater than that from the strain lacking the construct. The masses of the peptides produced were also as expected (A1S7G, 3,291 kDa; LtnA2, 2,847 kDa). It should be noted that despite being more difficult to quantify accurately, the trends with respect to peak sizes from cell-free supernatant preparations mirrored those shown for cell preparations in all cases (data not shown).
Concluding remarks.
With respect to the contributions of individual components to lacticin 3147 overproduction, it has been demonstrated that one of the requirements for overproduction of both peptides of the lantibiotic lacticin 3147 is the introduction of additional copies of biosynthetic and transport genes into an existing lacticin producer. This suggests that one of the rate-limiting steps for lacticin 3147 production in the pMRC01-containing host is the abundance of modification and transport proteins but not substrate availability (i.e., unmodified peptides). These observations again raise questions about the role of the stem-loop attenuator that impacts transcription of the genes in question. The presence of a stem-loop structure between the structural gene and the downstream biosynthetic genes is a feature of a number of other lantibiotics (1, 19, 25). It has been established that in addition to allowing partial read-through to downstream synthetic genes, the stem-loop structure in Pep5 is also required for mRNA stability (25). It may be that further investigations are needed to determine whether mutagenesis of such structures in a way that improves read-through without impacting mRNA stability could facilitate lantibiotic overproduction. pOM34 (ΔltnR) is the only construct that contains all of the biosynthetic/transport genes and yet fails to improve the activity of MG1363 by at least 200%. To date, analysis of LtnR has focused on its role as a negative regulator of immunity gene expression. In light of these results, further analysis to determine how it directly or indirectly contributes to lacticin 3147 production would appear to also be merited.
This study represents the first occasion on which non-nisin-like antibiotics have been overproduced by genetic means. The extent to which overproduction occurs, i.e., 2.74-fold for LtnA1 and 6.62-fold for LtnA2, is greater than what has been observed for wild-type nisin or subtilin peptides. In fact, LtnA2 overproduction is also more dramatic than the abrB deletion-associated production of succinylated subtilin. Significantly, although deletion of abrB is an option available only to subtilin-associated applications, the subcloning of entire operons in a manner analogous to that described here for lacticin 3147 is, with the increasing availability of polymerases suited to the amplification of large products with high fidelity, a realistic option when endeavoring to overproduce other lantibiotics. As many of these peptides are of great fundamental interest and show great potential with respect to either food or clinical applications, this is particularly worthwhile. The development of the related strategy to facilitate the overproduction of bioengineered derivatives is also significant, as the low-level production of many bioengineered lantibiotics can present a major hurdle to their further characterization and utilization. While a number of new strategies involving either in vitro or heterologous production are being investigated, it remains to be seen whether they will be successful in producing large quantities of correctly modified peptide. The overproduction of a bioengineered lantibiotic for the first time is thus a welcome event.
Acknowledgments
We acknowledge Paula O' Connor for technical assistance, Eileen O'Connor for sharing unpublished data, and Desmond Field for plasmid pDF01.
This work was supported by the Irish Government under the National Development Plan (2000-2006) and Science Foundation Ireland.
REFERENCES
- 1.Altena, K., A. Guder, C. Cramer, and G. Bierbaum. 2000. Biosynthesis of the lantibiotic mersacidin: organization of a type B lantibiotic gene cluster. Appl. Environ. Microbiol. 66:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 3.Brotz, H., M. Josten, I. Wiedemann, U. Schneider, F. Gotz, G. Bierbaum, and H. G. Sahl. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30:317-327. [DOI] [PubMed] [Google Scholar]
- 4.Brumfitt, W., M. R. Salton, and J. M. Hamilton-Miller. 2002. Nisin, alone and combined with peptidoglycan-modulating antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J. Antimicrob. Chemother. 50:731-734. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee, C., M. Paul, L. Xie, and W. A. van der Donk. 2005. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105:633-684. [DOI] [PubMed] [Google Scholar]
- 6.Cheigh, C. I., H. Park, H. J. Choi, and Y. R. Pyun. 2005. Enhanced nisin production by increasing genes involved in nisin Z biosynthesis in Lactococcus lactis subsp. lactis A164. Biotechnol. Lett. 27:155-160. [DOI] [PubMed] [Google Scholar]
- 7.Chen, P., F. Qi, J. Novak, and P. W. Caufield. 1999. The specific genes for lantibiotic mutacin II biosynthesis in Streptococcus mutans T8 are clustered and can be transferred en bloc. Appl. Environ. Microbiol. 65:1356-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacterial lantibiotics: strategies to improve therapeutic potential. Curr. Protein Pept. Sci. 6:61-75. [DOI] [PubMed] [Google Scholar]
- 9.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 10.Cotter, P. D., P. M. O'Connor, L. A. Draper, E. M. Lawton, L. H. Deegan, C. Hill, and R. P. Ross. 2005. Posttranslational conversion of l-serines to d-alanines is vital for optimal production and activity of the lantibiotic lacticin 3147. Proc. Natl. Acad. Sci. USA 102:18584-18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado, A., D. Brito, P. Fevereiro, R. Tenreiro, and C. Peres. 2005. Bioactivity quantification of crude bacteriocin solutions. J. Microbiol. Methods 62:121-124. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty, B. A., C. Hill, J. F. Weidman, D. R. Richardson, J. C. Venter, and R. P. Ross. 1998. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactis DPC3147. Mol. Microbiol. 29:1029-1038. [DOI] [PubMed] [Google Scholar]
- 13.Galvin, M., C. Hill, and R. P. Ross. 1999. Lacticin 3147 displays activity in buffer against gram-positive bacterial pathogens which appear insensitive in standard plate assays. Lett. Appl. Microbiol. 28:355-358. [DOI] [PubMed] [Google Scholar]
- 14.Hayes, F., C. Daly, and G. F. Fitzgerald. 1990. Identification of the minimal replicon of Lactococcus lactis subsp. lactis UC317 plasmid pCI305. Appl. Environ. Microbiol. 56:202-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinzmann, S., K. D. Entian, and T. Stein. 2006. Engineering Bacillus subtilis ATCC 6633 for improved production of the lantibiotic subtilin. Appl. Microbiol. Biotechnol. 69:532-536. [DOI] [PubMed] [Google Scholar]
- 16.Holo, H., Z. Jeknic, M. Daeschel, S. Stevanovic, and I. F. Nes. 2001. Plantaricin W from Lactobacillus plantarum belongs to a new family of two-peptide lantibiotics. Microbiology 147:643-651. [DOI] [PubMed] [Google Scholar]
- 17.Holo, H., and I. F. Nes. 1995. Transformation of Lactococcus by electroporation. Methods Mol. Biol. 47:195-199. [DOI] [PubMed] [Google Scholar]
- 18.Kruszewska, D., H. G. Sahl, G. Bierbaum, U. Pag, S. O. Hynes, and A. Ljungh. 2004. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J. Antimicrob. Chemother. 54:648-653. [DOI] [PubMed] [Google Scholar]
- 19.Kuipers, O. P., M. M. Beerthuyzen, R. J. Siezen, and W. M. De Vos. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281-291. [DOI] [PubMed] [Google Scholar]
- 20.McAuliffe, O., C. Hill, and R. P. Ross. 2000. Each peptide of the two-component lantibiotic lacticin 3147 requires a separate modification enzyme for activity. Microbiology 146:2147-2154. [DOI] [PubMed] [Google Scholar]
- 21.McAuliffe, O., C. Hill, and R. P. Ross. 2000. Identification and overexpression of ltnl, a novel gene which confers immunity to the two-component lantibiotic lacticin 3147. Microbiology 146:129-138. [DOI] [PubMed] [Google Scholar]
- 22.McAuliffe, O., T. O'Keeffe, C. Hill, and R. P. Ross. 2001. Regulation of immunity to the two-component lantibiotic, lacticin 3147, by the transcriptional repressor LtnR. Mol. Microbiol. 39:982-993. [DOI] [PubMed] [Google Scholar]
- 23.Morgan, S. M., P. M. O'Connor, P. D. Cotter, R. P. Ross, and C. Hill. 2005. Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrob. Agents Chemother. 49:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navaratna, M. A., H. G. Sahl, and J. R. Tagg. 1998. Two-component anti-Staphylococcus aureus lantibiotic activity produced by Staphylococcus aureus C55. Appl. Environ. Microbiol. 64:4803-4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pag, U., C. Heidrich, G. Bierbaum, and H. G. Sahl. 1999. Molecular analysis of expression of the lantibiotic Pep5 immunity phenotype. Appl. Environ. Microbiol. 65:591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rince, A., A. Dufour, S. Le Pogam, D. Thuault, C. M. Bourgeois, and J. P. Le Pennec. 1994. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 60:1652-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan, M. P., R. W. Jack, M. Josten, H. G. Sahl, G. Jung, R. P. Ross, and C. Hill. 1999. Extensive post-translational modification, including serine to d-alanine conversion, in the two-component lantibiotic, lacticin 3147. J. Biol. Chem. 274:37544-37550. [DOI] [PubMed] [Google Scholar]
- 28.Ryan, M. P., M. C. Rea, C. Hill, and R. P. Ross. 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uguen, P., T. Hindré, S. Didelot, C. Marty, D. Haras, J.-P. Le Pennec, K. Vallée-Réhel, and A. Dufour. 2005. Maturation by LctT is required for biosynthesis of full-length lantibiotic lacticin 481. Appl. Environ. Microbiol. 71:562-565. [DOI] [PMC free article] [PubMed] [Google Scholar]