Abstract
Global gene expression was compared between the Nitrosomonas europaea wild type and a nitrite reductase-deficient mutant using a genomic microarray. Forty-one genes were differentially regulated between the wild type and the nirK mutant, including the nirK operon, genes for cytochrome c oxidase, and seven iron uptake genes. Relationships of differentially regulated genes to the nirK mutant phenotype are discussed.
Ammonia oxidizers are a widespread and ecologically important group of bacteria that catalyze the oxidation of ammonia to nitrite (NO2−) via hydroxylamine (NH2OH) in terrestrial, aquatic, and marine environments (5). Ammonia oxidizers also produce nitrous oxide (N2O) and nitric oxide (NO) either through the oxidation of NH2OH (13) or the reduction of NO2− (9, 18). The gene for a homologue to dissimilatory nitrite reductase, nirK, is present at the end of a four-gene cluster in Nitrosomonas europaea (7). The four genes in this cluster have been separately disrupted by integration of antibiotic resistance gene cassettes to determine their physiological role (1-3). The loss of NirK activity in N. europaea resulted in increased aerobic production of N2O and increased sensitivity of the bacteria to NO2− (1). Strains with mutations in each of the three genes preceding nirK were equally as sensitive as the nirK mutant to NO2− and became even more sensitive when NirK was introduced and expressed from a plasmid (3). In wild-type cells, expression of NirK increased with increasing NO2− concentration, but expression was not significantly changed by a decrease in O2 concentration (2). Lastly, disruption of the NsrR repressor divergently encoded upstream of the nirK gene cluster resulted in constitutive expression of NirK (2). Together, these results suggest that nirK and the three genes in its cluster are negatively regulated as an operon by NsrR to aerobically detoxify NO2− produced by N. europaea during ammonia oxidation rather than to anaerobically reduce NO2− as is typical for NirK-like enzymes in denitrifying bacteria (6).
The goal of this study was to find genes linked to the nirK mutant phenotype, i.e., increased N2O production and sensitivity to NO2−, by examining differences in global gene expression between wild-type and a nirK-deficient mutant of N. europaea.
N. europaea whole-genome microarray.
Construction of whole open reading frame (ORF) microarrays for N. europaea was done as described previously for Shewanella oneidensis MR-1 (11). Briefly, gene-specific fragments (<75% homology) were generated by PCR using individually designed primer sets (24) (see the supplemental material). All amplified products (8 to 16 reactions per primer set × 100 μl) were pooled together and purified using a robot (Biomek F/X Automated Workstation; Beckman). The range of product sizes was 100 to 1,200 bp, with most between 500 and 600 bp. Of the 2,603 total predicted genes, 2,318 ORFs were correctly amplified, and specific 50-mer oligonucleotide probes (74 ORFs) were synthesized for the remainder, representing 96.8% of the total predicted gene content of N. europaea. Diluted PCR products (50 ng μl−1) were spotted onto CMT-GAPS slides (Corning, Corning, NY) using a BioRobotics Microgrid II microarrayer (Genomic Solutions, Ann Arbor, MI). Control spots were as previously described (11).
Differential gene expression in wild-type versus NirK-deficient N. europaea.
The nirK mutant was generated from the wild type by integrating a kanamycin resistance gene cassette into the center of the nirK ORF via homologous recombination from an engineered plasmid introduced into N. europaea by conjugation (1). Batch cultures of wild-type and nirK mutant Nitrosomonas europaea (ATCC 19718) were grown in mineral medium to late exponential phase. Total cellular RNA was extracted from cells harvested from multiple cultures for each hybridization experiment (AquaPure RNA Isolation; Bio-Rad), treated with RNase-free DNase I (Ambion), and purified (RNeasy mini kit; QIAGEN). Fluorescently labeled cDNA libraries from total RNA were generated by incorporation of Cy3- or Cy5-dUTP by reverse transcriptase (SuperScript III; Invitrogen). Labeled cDNA was treated with NaOH (1 N, 65°C, 30 min), neutralized with Tris-Cl (1 M, pH 7.6), column purified (QIAGEN), and concentrated by vacuum centrifugation. Two sets of triplicate reactions were performed in which the fluorescent dyes were reversed during cDNA synthesis to minimize dye-specific effects that could influence analysis of hybridization signals to the microarrays. Labeled cDNA libraries (0.6 to 0.9 μg with 20 to 30% label incorporation frequency) were hybridized to N. europaea genomic microarrays in buffered solution (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% sodium dodecyl sulfate [SDS], 50% formamide, 0.1 mg · ml−1 salmon sperm DNA) in a chamber (Corning) under Hybrislips (Sigma-Aldrich) at 50°C for 12 to 16 h. The slides were washed with 2× SSC-0.1% SDS for 5 min at 42°C, 0.1× SSC-0.1% SDS for 10 min at 42°C, and four times with 0.1× SSC for 1 min at room temperature.
Scanned images (Scan Microarray Express, PerkinElmer) were analyzed using ImaGene version 5.6 (Biodiscovery). Spot grids were manually fitted to microarray images, and average signal intensity and local background were calculated for each microarray element. The quality of the hybridization signals was assessed for each microarray using the numbers of detected genes plus low and consistent background levels. Spots with poor signal quality, irregular shape, and/or high background were flagged and removed from each data set. Remaining data were transferred to Excel and Access 2001 (Microsoft Corp., Redmond, WA) and GeneSpring version 6.0 (Silicon Genetics). Normalization was done by scaling total fluorescence measured for both Cy3 and Cy5 to an equivalent averaged intensity across each microarray. Pearson correlation coefficients were calculated for 12 spots for each gene (duplicate spots across six microarrays), and genes showing statistically significant and greater-than-twofold differences in Cy3/Cy5 hybridizations from at least 5 spots were considered differentially regulated.
A total of 25 genes were up-regulated and 16 were down-regulated in nirK mutant relative to wild-type cells (Table 1). Twelve genes showed significant differences in expression between wild-type and nirK mutant N. europaea at a P value of <0.05 by Student's t test, and the remaining expression ratios were significant at a P value of <0.1. Genes were grouped into functional categories, i.e., energy production, signal transduction, biosynthesis, and transport, based on degrees of similarity to sequences and domains from multiple sequence databases (http://genome.ornl.gov/microbial/neur/embl/).
TABLE 1.
Differential gene expression in nirK mutant relative to wild-type N. europaea
| Gene IDa | Gene nameb | Putative functionb | Expression ratioc
|
|||
|---|---|---|---|---|---|---|
| Avg | SD | No. of replicates | Significance | |||
| Energy production | ||||||
| (NE0684) | coxB2 | Cytochrome c oxidase (sub. II) | 2.876 | 1.336 | 7 | |
| (NE0683) | coxA2 | Cytochrome c oxidase (sub. I) | 2.476 | 1.605 | 10 | |
| (NE0927) | pan1 | Multicopper oxidase | 3.610 | 0.542 | 12 | * |
| (NE0926) | Cytochrome c | 2.542 | 0.394 | 9 | * | |
| (NE0925) | cccA | Cytochrome c | 2.399 | 0.535 | 9 | * |
| (NE2064) | amoC2 | Ammonia monooxygenase (sub. C) | 0.630 | 0.180 | 6 | |
| (NE2063) | amoA2 | Ammonia monooxygenase (sub. A) | 0.730 | 0.187 | 5 | |
| (NE2062) | amoB2 | Ammonia monooxygenase (sub. B) | 0.560 | 0.141 | 5 | |
| (NE2303) | nirB | NAD(P)H nitrite reductase | 0.301 | 0.058 | 10 | * |
| (NE2304) | ycaC | Isochorismatase hydrolase | 0.392 | 0.057 | 11 | * |
| (NE2305) | Hypothetical | 0.380 | 0.085 | 9 | ||
| Signal transduction | ||||||
| NE1071 | fecI | Iron uptake sigma factor | 4.341 | 1.256 | 10 | |
| NE1099 | fecI | Iron uptake sigma factor | 4.542 | 2.737 | 9 | * |
| NE1101 | fecI | Iron uptake sigma factor | 3.352 | 1.118 | 10 | |
| NE1217 | fecI | Iron uptake sigma factor | 6.711 | 3.680 | 8 | |
| (NE2435) | fecI | Iron uptake sigma factor | 5.557 | 3.345 | 10 | |
| (NE2434) | fecR | Transmembrane receptor | 2.934 | 1.994 | 7 | |
| NE0534 | fecR | Transmembrane receptor | 0.515 | 0.070 | 8 | |
| Biosynthesis | ||||||
| NE1445 | nifU | Iron-sulfur cluster biosynthesis | 2.117 | 0.445 | 6 | |
| NE2150 | trpE | Anthranilate synthase | 2.407 | 0.333 | 8 | |
| (NE2300) | bioB | Biotin synthase | 3.172 | 0.910 | 11 | * |
| (NE2299) | bioF | Aminotransferase | 2.806 | 1.306 | 6 | |
| (NE2298) | bioH | Hydrolase | 3.232 | 0.348 | 12 | * |
| (NE2297) | bioC | SAM methylase | 2.972 | 0.239 | 11 | |
| (NE2296) | bioD | Dethiobiotin synthase | 2.423 | 0.439 | 10 | |
| NE1467 | Fatty acid desaturase | 0.610 | 0.050 | 8 | ||
| NE1826 | prsA | Phosphoribosyl transferase | 0.541 | 0.168 | 12 | |
| Transport | ||||||
| NE2124 | fiu | TonB-dependent receptor for Fe | 3.887 | 1.119 | 10 | * |
| NE0345 | czcA | Heavy metal efflux | 0.460 | 0.183 | 8 | |
| NE0577 | cysW | Sulfate transporter | 0.374 | 0.093 | 10 | |
| NE2184 | ptsH | Phosphocarrier HPr protein | 0.470 | 0.044 | 7 | |
| Miscellaneous | ||||||
| NE0200 | atpB | ATP synthase (subunit A) | 1.969 | 0.339 | 8 | |
| NE2143 | rpsD | Ribosomal protein S4:S4 | 2.764 | 0.595 | 10 | |
| NE2032 | amyA | Glycosyl hydrolase | 0.587 | 0.063 | 8 | |
| Unknown function | ||||||
| NE1542 | Hypothetical | 6.692 | 3.957 | 12 | * | |
| (NE2151) | Hypothetical | 2.925 | 1.124 | 9 | ||
| (NE2152) | Hypothetical | 3.266 | 1.312 | 10 | ||
| NE2154 | Hypothetical | 1.979 | 0.492 | 10 | * | |
| NE0314 | Hypothetical | 0.233 | 0.075 | 12 | ||
| NE2218 | Hypothetical | 0.379 | 0.099 | 12 | * | |
| NE2505 | Hypothetical | 0.554 | 0.096 | 10 | ||
Gene identification (ID) from N. europaea genome sequence (7). Genes grouped and in parentheses indicate operon structure based on presence of a single predicted sigma 70 promoter upstream of first ORF in each group (identified using BPROM; Softberry, Inc., Mt. Kisco, NY) and/or presence of overlapping ORFs.
Gene names and putative functions were derived from multiple searches of gene sequences against nucleotide and polypeptide domain databases (http://genome.ornl.gov/microbial/neur/embl/).
Average ratios of signal intensity are from hybridization of labeled nirK mutant/wild-type cDNA to N. europaea genomic microarrays. Standard deviations were calculated from number of indicated replicate spots out of a total of 12 hybridized positions. Spots with poor signal were removed from each data set. *, ratios that were significantly different at a P value of <0.05 across the indicated numbers of replicate spots. All other ratios were significantly different at a P value of <0.1.
Confirmation of hybridization ratios.
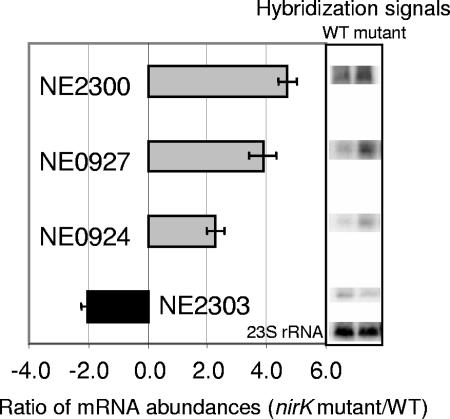
Ratios of nirK mutant/wild-type gene expression were confirmed by Northern blotting using three genes shown to be differentially expressed by the microarray analysis, NE2300, NE0927, and NE2303, and the nirK gene, NE0924. The same pools of RNA for making cDNA libraries were used for Northern hybridizations following standard protocols (17). Blots were probed with denatured, radiolabeled PCR products (Prime-a-Gene; Promega) generated with the same primer pairs for NE2300, NE0927, and NE2303 as those used for creating the microarrays and a primer pair targeting the 5′ end of NE0924 (nirK). Hybridized blots were exposed to a phosphor storage screen and hybridization signals were quantified (Typhoon phosphorimager; Amersham). Normalization of signal was accomplished by comparing levels of hybridization in three separate lanes to a probe specific for 23S rRNA of N. europaea, as described previously (20).
Calculated ratios of gene expression for nirK mutant/wild-type cells from the Northern blots were 4.7, 3.9, and −2.0 for NE2300, NE0927, and NE2303, respectively, which was comparable to the microarray hybridization ratios of 3.2, 3.6, and −3.3 for nirK mutant/wild-type cells for the same genes (Fig. 1 and Table 1). Although differential expression of the nirK gene could not be detected by microarray, expression of the nirK gene by Northern hybridization was about 2.4-fold higher in the nirK mutant relative to wild-type cells, a similar ratio for microarray hybridizations to NE0927, NE0926, and NE0925, which precede nirK in its cluster (i.e., 2.4 to 3.6). This result is consistent with a previous study suggesting that the four-gene nirK cluster is indeed expressed as an operon (3).
FIG. 1.
Northern hybridizations. Intensities of hybridization to specific DNA probes for each gene were normalized between lanes by comparing signals to hybridization intensity from 23S rRNA. The bar graph on the left is a pictorial representation of levels of hybridization signal quantified from blots shown on the right. Error bars indicate standard deviations of the means from three replicate blots. NE2300 = bioB; NE0927 = pan1, the first ORF in the nirK operon; NE0924 = nirK; NE2303 = nirB. WT, wild type.
Relationships of genes to the nirK mutant phenotype.
The main phenotypic characteristics of NirK-deficient N. europaea are elevated production of N2O and sensitivity to NO2− (1), suggesting that the absence of aerobic nitrite reductase activity leads to increased NO production and likely nitrosative stress. Below, we discuss the regulatory regions and potential activities of the differentially regulated genes between nirK mutant and wild-type N. europaea to explain the nirK-deficient mutant phenotype.
One of two operons encoding cytochrome c oxidase, coxBA2 (NE0683 and -0684), was up-regulated ∼2.6-fold in nirK mutant/wild-type N. europaea (Table 1). The CoxA subunit of cytochrome c oxidase has similar domains to NorB nitric oxide reductase, and mitochondrial cytochrome c oxidase can catalyze oxidation of NO to NO2− (12, 22). Intriguingly, a conserved FNR binding motif, TTGTT(TaacA)AACAA (lowercase letters are not included in the inverted repeat sequence for recognition by FNR protein), was found 17 bp upstream of the translational start and 7 bp downstream of the −10 consensus sequence of NE0684, suggesting repression of coxBA2 by Fnr (NE1719) (25). Besides its well-studied role in regulating O2-responsive genes (19), Fnr also regulates genes in response to NO, such as the NO-detoxifying Hmp (flavohemoglobin) gene (10). Based on its catalytic potential and presumed control by Fnr, CoxBA2 may be up-regulated by an excess of NO produced by NirK-deficient N. europaea and reduce it to N2O. Although the source of substrate NO would not be from NirK activity, it may be from the chemical oxidation of NH2OH, which has been shown to accumulate in continuous cultures of nirK mutant N. europaea (18). A separate transcriptome experiment using the same whole-genome microarray showed that both CoxBA2 and NorCB are up-regulated ca. 2.4-fold in growing versus NH3/carbonate-starved N. europaea cells, indicating equal importance of these enzymes to ammonia-oxidizing metabolism (23). Also, NorCB was previously found to be inessential for N2O production and tolerance to NO in batch cultures of N. europaea, and NorCB expression was not controlled by Fnr (4). Thus, CoxBA2 is an excellent candidate to examine as the additional aerobic NO reductase that protects N. europaea from NO produced from ammonia and hydroxylamine oxidation.
Up-regulation of genes related to iron uptake, including three fecI sigma factors (NE1071, -1099, and -1217), an fecIR sigma factor/response regulator pair (NE2435 and -2434), and the gene for a TonB-dependent receptor (NE2124), indicated an increased need for iron by NirK-deficient cells (7). None of these genes showed significant differences in expression in the microarray experiment of growing/starved N. europaea (23), suggesting their specific regulation in response to stress. FeS clusters are excellent targets for inactivation by NO (14), which may explain the need for iron by nirK mutant N. europaea and also why NifU (NE1445), an FeS cluster biosynthesis gene, was more highly expressed in nirK mutant versus wild-type cells.
nirB (NE2303), a gene with similarity to NAD(P)H-dependent nitrite reductase, and its two associated ORFs (NE2304 and NE2305) were down-regulated ca. 2.8-fold in nirK mutant/wild-type N. europaea. Aside from N assimilation, NirB also detoxifies NO2− produced by cytoplasmic nitrate reduction in enteric bacteria (15). Thus, down-regulation of NirB in nirK mutant/wild-type N. europaea is perplexing as up-regulation of NO2− detoxification mechanisms was expected in NirK-deficient cells. However, assuming that N. europaea NirB functions as an NADH-linked NO2− reductase, its decreased expression may partially explain the sensitivity of nirK mutant N. europaea to NO2− (1). It should be noted that unlike in enteric bacteria, nirB of N. europaea is not associated with a nirD homologue and the nirB promoter region lacks FNR binding motifs (8). Thus, the activity and regulation of NirB in relation to ammonia oxidation should be defined experimentally.
Of the other genes, up-regulation of the biosynthetic trpE (NE2150) gene in nirK mutant/wild-type N. europaea is weakly connected to nitrosative stress, as tryptophan residues are highly reactive with NO (21). Up-regulation of the entire biotin synthesis operon in NirK-deficient cells is intriguing, as the structure and regulation of the N. europaea operon is similar to that of other eubacterial biotin biosynthesis operons (16), and there are no reports of an increased need for biotin in response to nitrosative stress. The remaining differentially regulated genes, including those for ammonia monooxygenase, do not possess activities easily relatable to N2O production, NO2− sensitivity, or nitrosative stress, and none of the upstream regions of the remaining genes contained consensus sequences related to NO, such as Fnr or NsrR binding motifs. Of the hypothetical genes up-regulated in NirK-deficient N. europaea, NE2151 and NE2152 encode predicted membrane-spanning proteins and NE1542 is located immediately upstream of NE1543, which encodes another predicted multicopper oxidase.
Conclusion.
This study identified genes in N. europaea that were regulated as a consequence of losing aerobic nitrite reductase activity. Not surprisingly, similar levels of expression of the four-gene nirK cluster in the nirK-deficient mutant of N. europaea indicated their coregulation, likely in response to increased NO2− toxicity. Up-regulation of CoxBA2, iron uptake, and FeS cluster biosynthesis genes in nirK mutant/wild-type cells yielded clues for how N. europaea experiences and copes with nitrosative stress caused by lack of NirK activity. Further examination of specific physiological roles of differentially regulated genes in nirK mutant/wild-type N. europaea could further assist in revealing the significance of aerobic denitrification and nitrosative stress response in N. europaea. Microarray data analyzed in this study have been deposited in the Gene Expression Omnibus database with accession number GSE4517 (http://www.ncbi.nlm.nih.gov/projects/geo/query/acc.cgi?acc=GSE4517).
Supplementary Material
Acknowledgments
We thank H. J. E. Beaumont for generously providing the nirK mutant of N. europaea and, along with D. J. Arp, for critical reading of the manuscript.
This work was supported by initial complement funding, a grant from the UCR Academic Senate, a UC Regents' Faculty Fellowship to L. Y. Stein, and the U.S. Department of Energy Microbial Genome Program, Office of Biological and Environmental Research, Office of Science. Oak Ridge National Laboratory is managed by University of Tennessee-Battelle LLC for the DOE under contract DE-AC05-00OR22725.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Beaumont, H. J. E., N. G. Hommes, L. A. Sayavedra-Soto, D. J. Arp, D. M. Arciero, A. B. Hooper, H. V. Westerhoff, and R. J. M. van Spanning. 2002. Nitrite reductase of Nitrosomonas europaea is not essential for production of gaseous nitrogen oxides and confers tolerance to nitrite. J. Bacteriol. 184:2557-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaumont, H. J. E., S. I. Lens, W. N. M. Reijnders, H. V. Westerhoff, and R. J. M. van Spanning. 2004. Expression of nitrite reductase in Nitrosomonas europaea involves NsrR, a novel nitrite-sensitive transcription repressor. Mol. Microbiol. 54:148-158. [DOI] [PubMed] [Google Scholar]
- 3.Beaumont, H. J. E., S. I. Lens, H. V. Westerhoff, and R. J. M. van Spanning. 2005. Novel nirK cluster genes in Nitrosomonas europaea are required for NirK-dependent tolerance to nitrite. J. Bacteriol. 187:6849-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaumont, H. J. E., B. van Schooten, S. I. Lens, H. V. Westerhoff, and R. J. M. van Spanning. 2004. Nitrosomonas europaea expresses a nitric oxide reductase during nitrification. J. Bacteriol. 186:4417-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bothe, H., G. Jost, M. Schloter, B. B. Ward, and K.-P. Witzel. 2000. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol. Rev. 24:673-690. [DOI] [PubMed] [Google Scholar]
- 6.Brittain, T., R. Blackmore, C. Greenwood, and A. J. Thomson. 1992. Bacterial nitrite-reducing enzymes. Eur. J. Biochem. 209:793-802. [DOI] [PubMed] [Google Scholar]
- 7.Chain, P., J. Lamerdin, F. Larimer, W. Regala, V. Lao, M. Land, L. Hauser, A. B. Hooper, M. G. Klotz, J. M. Norton, L. A. Sayavedra-Soto, D. M. Arciero, N. G. Hommes, M. R. Whittaker, and D. J. Arp. 2003. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J. Bacteriol. 185:2759-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, J. 1996. Nitrate reduction to ammonia by enteric bacteria: redundancy, or a strategy for survival during oxygen starvation? FEMS Microbiol. Lett. 136:1-11. [DOI] [PubMed] [Google Scholar]
- 9.Colliver, B. B., and T. Stephenson. 2000. Production of nitrogen oxide and dinitrogen oxide by autotrophic nitrifiers. Biotechnol. Adv. 18:219-232. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Ramos, H., J. Crack, G. Wu, M. N. Hughes, C. Scott, A. J. Thomson, J. Green, and R. K. Poole. 2002. NO sensing by FNR: regulation of the Escherichia coli NO-detoxifying flavohaemoglobin, Hmp. EMBO J. 21:3235-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, H., Y. Wang, X. Liu, T. Yan, L. Wu, E. Alm, A. Arkin, D. K. Thompson, and J. Zhou. 2004. Global transcriptome analysis of the heat shock response of Shewanella oneidensis. J. Bacteriol. 186:7796-7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendriks, J., A. Warne, U. Gohlke, T. Haltia, C. Ludovici, M. Lubben, and M. Saraste. 1998. The active site of the bacterial nitric oxide reductase is a dinuclear iron center. Biochemistry 37:13102-13109. [DOI] [PubMed] [Google Scholar]
- 13.Jiang, Q. Q., and L. R. Bakken. 1999. Nitrous oxide production and methane oxidation by different ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 65:2679-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Justino, M. C., J. B. Vicente, M. Teixeira, and L. M. Saraiva. 2005. New genes implicated in the protection of anaerobically grown Escherichia coli against nitric oxide. J. Biol. Chem. 280:2636-2643. [DOI] [PubMed] [Google Scholar]
- 15.Page, L., L. Griffiths, and J. A. Cole. 1990. Different physiological roles of two independent pathways for nitrite reduction to ammonia by enteric bacteria. Arch. Microbiol. 154:349-354. [DOI] [PubMed] [Google Scholar]
- 16.Rodionov, D. A., A. A. Mironov, and M. S. Gelfand. 2002. Conservation of the biotin regulon and the BirA regulatory signal in eubacteria and archaea. Genome Res. 12:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Schmidt, I., R. J. M. van Spanning, and M. S. M. Jetten. 2004. Denitrification and ammonia oxidation by Nitrosomonas europaea wild-type, and NirK- and NorB-deficient mutants. Microbiology 150:4107-4114. [DOI] [PubMed] [Google Scholar]
- 19.Spiro, S., and J. R. Guest. 1990. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol. Rev. 75:399-428. [DOI] [PubMed] [Google Scholar]
- 20.Stein, L. Y., and D. J. Arp. 1998. Ammonium limitation results in the loss of ammonia-oxidizing activity in Nitrosomonas europaea. Appl. Environ. Microbiol. 64:1514-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki, T., H. F. Mower, M. D. Friesen, I. Gilibert, T. Sawa, and H. Ohshima. 2004. Nitration and nitrosation of N-acetyl-L-tryptophan and tryptophan residues in proteins by various reactive nitrogen species. Free Radic. Biol. Med. 37:671-681. [DOI] [PubMed] [Google Scholar]
- 22.Torres, J., M. A. Sharpe, A. Rosquist, C. E. Cooper, and M. T. Wilson. 2000. Cytochrome c oxidase rapidly metabolises nitric oxide to nitrite. FEBS Lett. 475:263-266. [DOI] [PubMed] [Google Scholar]
- 23.Wei, X., T. Yan, N. G. Hommes, X. Liu, L. Wu, C. McAlvin, M. G. Klotz, L. A. Sayavedra-Soto, J. Zhou, and D. J. Arp. 2006. Transcript profiles of Nitrosomonas europaea during growth and upon deprivation of ammonia and carbonate. FEMS Microbiol. Lett. 257:76-83. [DOI] [PubMed] [Google Scholar]
- 24.Xu, D., G. Li, L. Wu, J. Zhou, and Y. Xu. 2002. PRIMEGENS: robust and efficient design of gene-specific probes for microarray analysis. Bioinformatics 18:1432-1437. [DOI] [PubMed] [Google Scholar]
- 25.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.