Abstract
The biosynthetic gene cluster for the aromatic polyketide steffimycin of the anthracycline family has been cloned and characterized from “Streptomyces steffisburgensis” NRRL 3193. Sequence analysis of a 42.8-kbp DNA region revealed the presence of 36 open reading frames (ORFs) (one of them incomplete), 24 of which, spanning 26.5 kb, are probably involved in steffimycin biosynthesis. They code for all the activities required for polyketide biosynthesis, tailoring, regulation, and resistance but show no evidence of genes involved in l-rhamnose biosynthesis. The involvement of the cluster in steffimycin biosynthesis was confirmed by expression of a region of about 15 kb containing 15 ORFS, 11 of them forming part of the cluster, in the heterologous host Streptomyces albus, allowing the isolation of a biosynthetic intermediate. In addition, the expression in S. albus of the entire cluster, contained in a region of 34.8 kb, combined with the expression of plasmid pRHAM, directing the biosynthesis of l-rhamnose, led to the production of steffimycin. Inactivation of the stfX gene, coding for a putative cyclase, revealed that this enzymatic activity participates in the cyclization of the fourth ring, making the final steps in the biosynthesis of the steffimycin aglycon similar to those in the biosynthesis of jadomycin or rabelomycin. Inactivation of the stfG gene, coding for a putative glycosyltransferase involved in the attachment of l-rhamnose, allowed the production of a new compound corresponding to the steffimycin aglycon compound also observed in S. albus upon expression of the entire cluster.
Anthracycline antibiotics are aromatic polyketide compounds widely used as therapeutics for the treatment of a variety of cancers (37). Daunorubicin and doxorubicin were the first identified anthracyclines, isolated from Streptomyces spp. in the early 1960s, and remain clinically widely used today as single agents or in adjuvant therapy in the treatment of hematological and solid tumors, such as acute leukemia, high-grade lymphoma, breast cancer, and bladder cancer. This has prompted the study of this family of natural products to the level of their biosynthesis in order to develop new bioactive compounds by recombinant DNA technology (33). In particular, three anthracycline compounds have been studied so far: daunorubicin-doxorubicin (18), nogalamycin (59, 56), and aclacinomycin (42, 43). These studies have revealed the assembly of this type of compound by type II polyketide synthases (PKS) that consist of several discrete proteins carrying a set of iteratively used enzyme activities.
Steffimycin (Fig. 1A) belongs to the anthracycline family of compounds and contains in its structure several distinctive characteristics not present in other anthracyclines: (i) the C-10 keto group, to which there is usually a carboxymethyl group (nogalamycin and aclacinomycin) attached or neither substitution nor a functional group (daunorubicin); (ii) two methoxy groups at C-2 and C-8 not present in nogalamycin, aclacinomycin, or daunorubicin; and (iii) a neutral deoxysugar instead of the aminosugar normally attached to C-7 in other anthracyclines. Steffimycin was isolated in 1967 as antibiotic U-20,661 (2) from “Streptomyces steffisburgensis,” which also produces 8-demethoxy steffimycins (23). Later, other members of this subfamily of compounds were isolated: aranciamycin from Streptomyces echinatus (19, 49); steffimycin B from Streptomyces elgreteus (6), which produces in addition steffimycin and steffimycin C (7); and finally steffimycin D from a Streptomyces sp. (52).
FIG. 1.
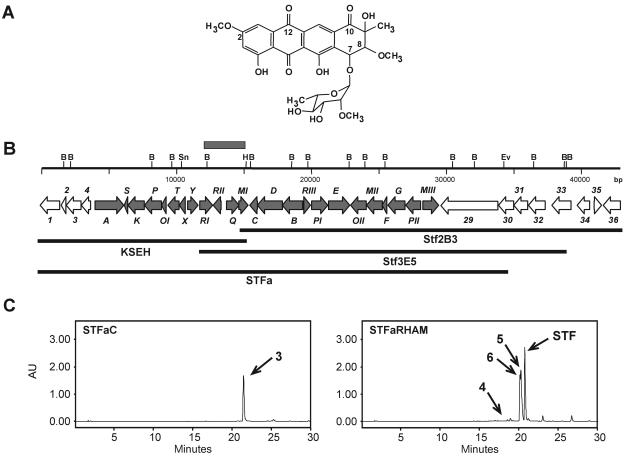
(A) Structure of steffimycin. (B) Genetic organization of the steffimycin biosynthesis gene cluster. White arrows, genes not involved in steffimycin biosynthesis; gray arrows, genes involved in steffimycin biosynthesis. The gray box represents the 2.9-kb HindIII-BamHI fragment used as a probe for cosmid library screening. KSEH, STFa, Stf2B3, and Stf3E5 represent DNA regions present in plasmids pEM4KSEH and pEM4STFa and in cosmids Stf2B3 and Stf3E5, respectively. (C) HPLC analysis of S. albus strains STFaC (containing pEM4STFa and pEM4) and STFaRHAM (containing pEM4STFa and pRHAM). Peaks 3, 4, 5, 6, and STF correspond to compounds in Fig. 4. AU, arbitrary units; B, BamHI; H, HindIII; Sn, SnaBI; Ev, EcoRV; STF, steffimycin.
Steffimycin was shown to be nontoxic in mice but highly cytotoxic in mammalian cell cultures (44). However, it showed a low inhibitory effect on the growth of mouse leukemia L1210 (32) and P388 cells (57). Nevertheless, steffimycin was recently shown to induce a high apoptotic response in HCT116 colon carcinoma cells expressing p53 by inducing DNA damage (12). In addition, steffimycin D derivatives inhibit the growth of ras oncogene-expressed cells (52), steffimycin B analogues containing chemical modification in C-3 are highly active against mouse leukemia P388 cells (57), and aranciamycin derivatives were found to inhibit DNA synthesis of Yoshida sarcoma tumor cells (4).
Here, we report the cloning and characterization of the gene cluster for steffimycin biosynthesis from “Streptomyces steffisburgensis” NRRL 3193. Heterologous expression in Streptomyces albus and disruption experiments showed the involvement of the gene cluster in the biosynthesis of steffimycin, leading to the isolation and characterization of three biosynthetic intermediates. One of them allowed the identification of a mode for the cyclization of the fourth ring different than that previously described for the biosynthesis of other anthracyclines, such as nogalamycin or daunorubicin, where an intermediate containing a carboxymethyl group at C-10 is required, and similar to that described for the biosynthesis of the angucyclines jadomycin and rabelomycin. In addition, the steffimycin gene cluster provides new enzymatic tools for the modification of other anthracyclines for the generation of novel anticancer compounds.
MATERIALS AND METHODS
Strains, culture conditions, and plasmids.
“Streptomyces steffisburgensis” NRRL 3193, the steffimycin producer, and mutants were routinely grown on tryptone soy broth. For protoplast formation, they were grown on R5 liquid medium (21). Regeneration of protoplasts after transformation was carried out on solid R5 agar plates using standard procedures (21); after regeneration, clones were grown on agar plates containing A medium for sporulation (14). Streptomyces albus J1074 (8) was used as a host for expression of steffimycin genes. Escherichia coli DH10B (Invitrogen) and ET12567 (28) were used for subcloning. Escherichia coli ED8767 (38) was used to propagate a cosmid library.
Plasmids pOJ260 (3) and pEM4 (41) were used for gene replacement and gene expression, respectively. pBLUNT (Invitrogen) was used for cloning of PCR products. pFBA and pLHyg were used as donors of the apramycin resistance gene aac(3)IV and the hygromycin resistance gene hyg, respectively (26, 39). pKC505 (45) was used to construct a cosmid library. pRHAM was used for expression in S. albus as a source of genes coding for enzymes involved in l-rhamnose biosynthesis (46). When antibiotic selection of transformants was needed, 100 μg/ml ampicillin, 20 μg/ml tobramycin, 25 μg/ml apramycin, 50 μg/ml thiostrepton, or 50 μg/ml hygromycin was used.
DNA manipulation.
DNA manipulations were performed according to standard procedures for E. coli (47) and for Streptomyces (21). A cosmid library of “S. steffisburgensis” genomic DNA was constructed in pKC505 according to procedures published in the literature (45). Ligations were in vitro packaged using the Gigapack III Gold packaging extract kit according to the manufacturer's handbook (Stratagene). A number of the resulting E. coli transductants were picked and transferred to 96-well microtiter plates containing Luria broth medium and 100 μg/ml ampicillin. Clones were replica plated onto Luria agar plates containing ampicillin. After overnight growth at 37°C, colonies were transferred to nylon membrane filters for in situ colony hybridization analysis according to published methods (47) and screened using a labeled probe that was generated using the DIG DNA labeling and detection kit (Roche).
Isolation of the steffimycin gene cluster.
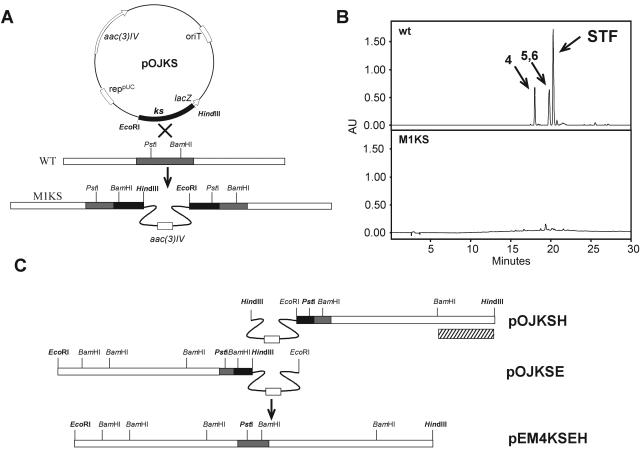
In order to generate a mutant defective in steffimycin production, we used a sequence coding for a ketoacyl synthase (KS) from “S. steffisburgensis” present in the databases under accession no. ABO24979. An internal fragment encompassing nucleotides 352 to 995 was obtained by PCR from “S. steffisburgensis” total DNA using the oligoprimers KSSTEFI (5′-TTTAAGCTTACCTGGCAGGTCTCCGAGG-3′; the HindIII site is underlined) and KSSTEFII (5′-AGAATTCATGGAGCTGACCGGTGTCCG-3′; the EcoRI site is underlined). The PCR conditions used were 97°C for 5 min; 30 cycles of 95°C for 30 s, 65°C for 45 s, and 72°C for 1 min; and a final extension cycle at 72°C for 10 min. Pfx DNA polymerase (Invitrogen) and 2.5% dimethylsulfoxide (DMSO) were used for all amplifications. A PCR product of 643 bp was cloned into HindIII-EcoRI-digested pOJ260 to obtain plasmid pOJKS, which was introduced into “S. steffisburgensis” by protoplast transformation, and transformants were selected with apramycin. Southern analysis of the mutant strain was performed using pOJKS as a probe.
Isolation of flanking DNA regions surrounding the KS was achieved by HindIII and EcoRI digestions (in two separate reactions) of total DNA recovered from the mutant strain. Religation of each digestion and transformation into E. coli DH10B rendered two pOJ260 derivatives containing approximately 7.5 kb each, pOJKSH (from HindIII digestion and religation) and pOJKSE (from EcoRI digestion and religation). To recover the inserts, pOJKSH was digested with PstI-HindIII and pOJKSE was digested with PstI-EcoRI, and both fragments were cloned together into pEM4 digested with EcoRI-HindIII. The resultant plasmid, pEM4KSEH, was used for expression in S. albus and for sequencing. A 2.9-kb HindIII-BamHI fragment isolated from pOJKSH was used as a probe for cosmid library screening (Fig. 1B and 2C).
FIG. 2.
(A) Strategy to generate mutant M1KS in “S. steffisburgensis.” The black and gray boxes represent the PCR-amplified fragments used for disruption and the same region in the “S. steffisburgensis” chromosome, respectively. (B) HPLC analysis of “S. steffisburgensis” wild type (wt) and strain M1KS. Peaks 4, 5, 6, and STF correspond to compounds in Fig. 4. (C) Strategy for recovery of the KS DNA flanking region. The dashed box represents the 2.9-kb HindIII-BamHI fragment used as a probe for cosmid library screening. aac(3)IV, apramycin resistance gene; AU, arbitrary units; STF, steffimycin.
DNA sequencing and analysis.
DNA sequencing was performed on double-stranded DNA templates by the dideoxynucleotide chain termination method (48) with the Cy5 Autocycle Sequencing Kit (Pharmacia Biotech); an Alf-express automatic DNA sequencer (Pharmacia) was used. Computer-aided database searching and sequence analysis were carried out with the University of Wisconsin Genetics Computer Group software (9) and the BLAST program (1).
Cloning of the steffimycin gene cluster.
In order to clone the entire steffimycin gene cluster in a single construct, pEM4KSEH was digested with HindIII and EcoRV that cut within stfMIII and within the thiostrepton resistance gene in pEM4, respectively. Then, a 19.7-kb HindIII-EcoRV fragment from cosmid Stf2B3, containing from the StfMIII C-terminal coding region to orf30, was subcloned into pEM4KSEH digested with HindIII-EcoRV, generating pEM4STF. In this construct, all genes from orf1 to orf30 were present, with orf30 incomplete. pEM4STF lacks a portion of the thiostrepton resistance gene, making selection in Streptomyces impossible. To solve this, a 1.6-kb EcoRV-SmaI fragment from pFBA (26) containing the apramycin resistance gene aac(3)IV was subcloned into EcoRV-digested pEM4STF, leading to pEM4STFa. This final construct, pEM4STFa, was used for heterologous expression in S. albus, together with pEM4 (41) or pRHAM (46).
Inactivation of stfX and stfG.
The stfX gene was inactivated by SnaBI digestion of pEM4KSEH and cloning of the hyg gene (in the same direction as stfX) from EcoRV-digested pLHyg. The resultant construct, pEM4KSEHX-Hyg, was used for expression in S. albus to verify metabolite production.
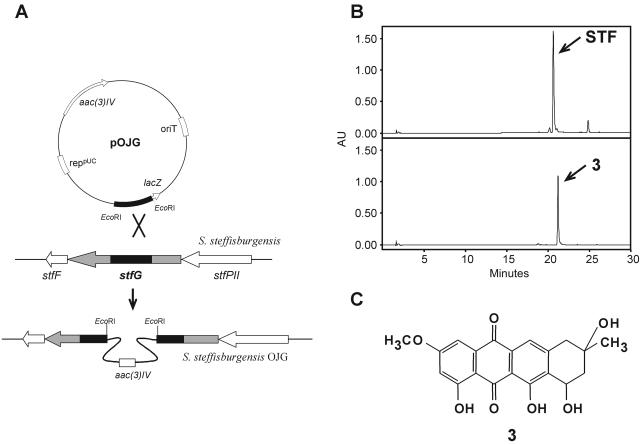
To inactivate the stfG gene, an internal fragment of 442 bp was amplified by PCR from “S. steffisburgensis” total DNA using oligoprimers G3 (5′-CTGCTGTGGGGGCCGGATCTCTTC-3′) and G4 (5′-CGCCGAGGGAGATGCGATCCGGAT-3′). The PCR conditions used were 97°C for 5 min; 15 cycles of 95°C for 30 s, 70°C to 55°C for 45 s, and 72°C for 1 min; 15 cycles of 95°C for 30 s, 63°C for 45 s, and 72°C for 1 min; and a final extension cycle at 72°C for 10 min. The PCR product was cloned into pBLUNT. The resultant plasmid was digested with EcoRI, and the fragment was cloned into pOJ260 to obtain plasmid pOJG, which was introduced into “S. steffisburgensis” by protoplast transformation, and transformants were selected with apramycin. Southern analysis of the mutant strain (strain OJG) was performed using pOJG as a probe.
Analysis of steffimycin production by HPLC and liquid chromatography (LC)-mass spectrometry.
Steffimycin production was assessed qualitatively by growing the “S. steffisburgensis” wild-type strain, mutants, or S. albus expressing pEM4 derivatives on solid R5A medium. After 5 days at 30°C, agar plugs containing 1.5 ml of agar media were extracted with 1 ml ethyl acetate, and the presence of steffimycin in the extract was analyzed by high-performance liquid chromatrography (HPLC) as described below. In liquid cultures, strains were grown as a seed culture in tryptone soy broth (30 ml in a 250-ml Erlenmeyer flask). After 2 days of incubation in a rotary incubator (30°C; 250 rpm), 2.5% (vol/vol) of the cultures were used to inoculate 30 ml of R5A liquid medium. After 5 additional days of incubation with shaking, the cultures were harvested for analysis; 1-ml aliquots of fermentation broth were removed and adjusted to pH ∼3 by the addition of 90% formic acid. Ethyl acetate (300 μl) was added, and the sample was then mixed vigorously for 30 min. The phases were separated by centrifugation in a microcentrifuge, and then the ethyl acetate was removed by evaporation using a Speed-Vac. Residues were resuspended in methanol and clarified by centrifugation. Analysis of steffimycin production was performed by HPLC in a reversed-phase column (Symmetry C18; 4.6 by 250 mm; Waters) with acetonitrile and 0.1% trifluoroacetic acid (TFA) in water as solvents. A linear gradient from 10 to 100% acetonitrile in 30 min, at a flow rate of 1 ml/min, was used. Detection and spectral characterization of peaks were performed with a photodiode array detector and Millennium software (Waters), and bidimensional chromatograms were extracted at 433 nm.
LC-mass spectrometry analysis was performed in a Symmetry C18 column (2.1 by 150 mm; Waters) using an Alliance chromatographic module coupled with a photodiode array detector 2996 and mass spectrometer ZQ4000 (Waters-Micromass). The chromatographic conditions were as previously described with a flow rate of 0.25 ml/min. Mass analysis was performed by electrospray ionization (ESI) in positive mode, with a capillary voltage of 3 kV and cone voltage of 20 V.
Isolation and physicochemical properties of the new intermediates of steffimycin biosynthesis.
Compounds 1 and 2 were purified from cultures of S. albus carrying pEM4KSEHX-Hyg. The culture conditions were as described above. Cultures were harvested by centrifugation (12,000 rpm; 30 min), and the supernatant was filtered using a 1-μm Mini Profile cartridge (Pall). The filtrated broth was solid phase extracted (SepPak C18; 10 g; Waters) and then eluted by a linear gradient of methanol and 0.1% TFA in water (0 to 100% in 30 min at a flow rate of 10 ml/min), taking fractions every 5 min. Fractions containing compound 1 or 2 were concentrated by evaporation using a rotavapor after the addition of 10 ml 0.1 M phosphate buffer (pH 7.0). The extracts were dissolved in 5 ml DMSO-methanol (50:50) and then chromatographed in a μBondapak C18 cartridge (PrepPak; 25 by 100 mm; Waters) via isocratic elution with acetonitrile and 0.1% TFA in water (55:45) at 10 ml/min. Pure compounds were solid phase extracted to remove the acid; 20.8 mg of compound 1 and 32 mg of compound 2 were recovered from 5-liter cultures.
The structures of the new compounds 1 and 2 were elucidated by nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry. The ESI mass spectra (ESI-MS) were acquired using a Finnigan LCQ mass spectrometer. The electron impact (EI) ionization mass spectra were measured using a Finnigan PolarisQ mass spectrometer. The high-resolution EI ionization mass spectra were recorded at 25 eV on a JEOL JMS-700T M station (magnetic-sector instrument) at a resolution greater than 10,000. The UV spectra were recorded on a Varian model CARY50 spectrophotometer. All NMR data were recorded in (d5-pyridine using either a Varian Mercury 300 or a Varian Inova 400 MHz spectrometer. All NMR assignments were confirmed by heteronuclear single quantum correlation and heteronuclear multiple bond correlation spectra, allowing an unambiguous assignment of all signals.
Nucleotide sequence accession number.
The sequence of the insert in pEM4KSEH and cosmid Stf2B3 (700-bp overlapping region; 42,877 bp) was deposited in the EMBL database under accession number AM156932.
RESULTS AND DISCUSSION
Isolation and characterization of the steffimycin gene cluster.
For the isolation of the steffimycin gene cluster, we took advantage of a DNA sequence from “S. steffisburgensis” available from the databases (accession no. ABO24979) coding for part of a KS. BLAST analysis of the deduced partial protein coded by this DNA sequence showed high similarities to KSs involved in the biosynthesis of different type II aromatic polyketides. In particular, the best match corresponded to Snoa1 (76% identity, 86% similarity), involved in the biosynthesis of nogalamycin (59). With this information, a PCR fragment was amplified from “S. steffisburgensis” total DNA, verified by DNA sequencing to contain KS-like amino acid sequences, and cloned into pOJ260 generating pOJKS (Fig. 2A). This construct in a Streptomyces suicide vector was used for gene disruption experiments in order to generate a nonproducing mutant. After transformation of “S. steffisburgensis” protoplasts, apramycin-resistant colonies were selected and analyzed for steffimycin production by LC-mass spectrometry. All isolated colonies were found to correspond to nonproducing mutants, and one of them (M1KS) (Fig. 2B) was selected for further studies. The correct insertion in the “S. steffisburgensis” chromosome was verified by Southern hybridization using the PCR product as a probe (data not shown).
Once the region containing the steffimycin gene cluster was identified, digestions and religations of total DNA from the mutant strain (as described in Materials and Methods) allowed the recovery of plasmid pOJ260 containing chromosomal-DNA fragments adjacent to the point of insertion and covering 15 kb in two fragments of approximately 7.5 kb each (pOJKSE and pOJKSH). The inserts in these constructs were cloned together, leading to plasmid pEM4KSEH (Fig. 2C).
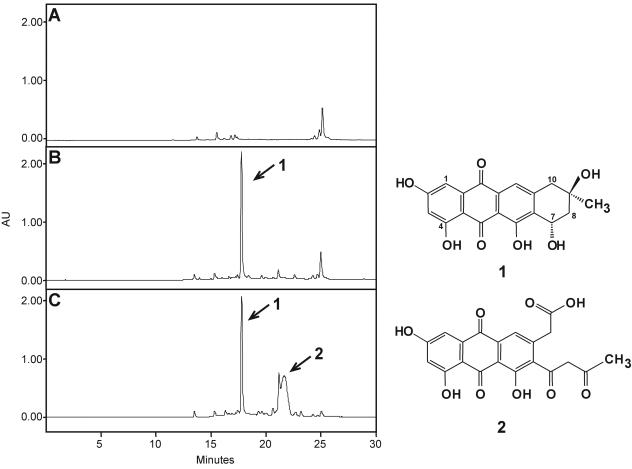
Expression of pEM4KSEH in S. albus led to the production of a red compound with an HPLC retention time of 17.9 min and a mass of 357 m/z ([M+H]+), which might correspond to 2-O-demethyl-8-demethoxy-10-deoxysteffimycinone (compound 1) (Fig. 3B). Compound 1 was converted to steffimycin when fed to the “S. steffisburgensis” M1KS mutant (data not shown), confirming this compound as an intermediate of steffimycin biosynthesis.
FIG. 3.
HPLC analysis of S. albus expressing pEM4 (A), pEM4KSEH (B), and pEM4KSEHX-Hyg (C) and the products accumulated (1 and 2). AU, arbitrary units.
Preliminary partial sequencing of the insert pEM4KSEH showed a region of approximately 4 kb presumably not involved in steffimycin biosynthesis at the EcoRI side of the insert. The remaining 11 kb of the insert contained genes possibly involved in the biosynthesis of steffimycin.
The insert in pOJKSH was used as a probe to screen a cosmid library of “S. steffisburgensis” chromosomal DNA. Three overlapping positive cosmid clones (Stf2B3, Stf2D7, and Stf3E5) were isolated. The insert in pEM4KSEH and cosmid Stf2B3 (700-bp overlapping region) were sequenced.
Analysis of the sequence for open reading frames (ORFs) and alignment with related sequences in databases revealed the presence of 36 ORFs (one of them incomplete). Of these, 24 ORFs (covering a region of 26.5 kb) are probably involved in steffimycin biosynthesis as structural, regulatory, and resistance genes and are flanked by 12 ORFs containing genes involved in primary metabolism and housekeeping genes (Fig. 1B and Table 1).
TABLE 1.
Proposed functions of deduced steffimycin gene cluster products
| ORF | No. of aaa | Proposed function in steffimycin biosynthesis | Closest similar protein, origin, (% identity/similarity), accession no. |
|---|---|---|---|
| orf1 | 483 | Putative IMP dehydrogenase SCL6.18c, S. coelicolor A3(2), (91/96), CAB76883 | |
| orf2 | 117 | Hypothetical protein SCL6.19c, S. coelicolor A3(2), (78/89), CAB76884 | |
| orf3 | 347 | Putative transcriptional regulator SCL6.20c, S. coelicolor A3(2), (96/98), CAB76885 | |
| orf4 | 228 | Ribulose-phosphate 3-epimerase SCL6.21c, S. coelicolor A3(2), (95/98), CAB76886 | |
| sfrA | 712 | Transmembrane efflux protein | Putative membrane protein similar to ActII-3 SAV6879, Streptomyces avermitilis MA-4680, (76/86), BAC74590 |
| stfS | 86 | Acyl carrier protein | Acyl carrier protein NcnC, Streptomyces arenae DSM40737, (64/80), AAD20269 |
| stfK | 406 | Chain length factor | Ketosynthase β Snoa2, Streptomyces nogalater ATCC 27451, (72/81), CAA12018 |
| stfP | 422 | Ketoacyl synthase | Ketosynthase, “Streptomyces steffisburgensis” JCM 4833T, (98/98), BAA92281 |
| stfOI | 111 | Monooxygenase | Oxygenase SnoaB, Streptomyces nogalater ATCC 27451, (47,65), CAA12015 |
| stfT | 274 | C-7 ketoreductase | Ketoreductase ChaL, Streptomyces chartreuses HKI-249, (46/72), CAH10174 |
| stfX | 146 | Fourth-ring cyclase | SnoO, Streptomyces nogalater ATCC 27451, (52/62), AAF01807 |
| stfY | 259 | Second- and third-ring cyclase | Putative polyketide cyclase SnoaM, Streptomyces nogalater ATCC 27451, (74/83), AAF01818 |
| stfRI | 290 | SARP family regulator | Putative SARP family pathway-specific regulatory protein ORF71, Streptomyces rochei 7434AN4, (55/73), BAC76529 |
| stfRII | 196 | Putative transcriptional regulator | Putative two-component response regulator Gra-orf10, Streptomyces violaceoruber Tu22, (49/69), CAA09631 |
| stfQ | 309 | Aromatase | Putative aromatase SAV2383, Streptomyces avermitilis MA-4680, (51/63), BAC70094 |
| stfMI | 225 | O-Methyltransferase | Putative O-methyltransferase EncK, Streptomyces maritimus, (40/59), AAF81726 |
| stfC | 187 | Unknown | SWIM Zn finger Franean1DRAFT_6984, Frankia sp. strain EAN1pec, (56/70), ZP_00567311 |
| stfD | 618 | Unknown | SNF2-related domain:helicase, C-terminal Franean1DRAFT_6983, Frankia sp. strain EAN1pec, (72/81), ZP_00567310 |
| sfrB | 502 | Transmembrane efflux protein | Putative transmembrane efflux protein SCE22.16c, Streptomyces coelicolor A3(2), (41/61), CAB90983 |
| stfRIII | 116 | Putative transcriptional regulator | Hypothetical DNA-binding protein YcgE, Bacillus licheniformis ATCC 14580, (31/59), AAU23698 |
| stfPI | 402 | Monooxygenase | Cytochrome P450 Franean1DRAFT_4258, Frankia sp. strain EAN1pec, (46/59), ZP_00570217 |
| stfE | 526 | Dehydrogenase | BusJ, Saccharopolyspora pogona NRRL 30141, (55/68), AAY88927 |
| stfOII | 387 | Monooxygenase | Possible dioxygenase Mb3186c, Mycobacterium bovis AF2122/97, (51/64), NP_856831 |
| stfMII | 391 | O-Methyltransferase | Putative O-methylase SnogY, Streptomyces nogalater ATCC 27451, (54/68), CAA12021 |
| stfF | 113 | Unknown | 5-Chloromuconolactone dehalogenase ClcF, Rhodococcus opacus 1CP, (38/66), CAD28145 |
| stfG | 420 | Glycosyltransferase | Putative glycosyltransferase SnogE, Streptomyces nogalater ATCC 27451, (62/74), AAF01809 |
| stfPII | 366 | Cytochrome P450-like | Protein similar to DnmQ AknT, Streptomyces galilaeus ATCC 31615, (36/47), AAF73456 |
| stfMIII | 392 | O-Methyltransferase | Methyltransferase MycE, Micromonospora griseorubida, (42/58), BAC57026 |
| orf29 | 1,404 | Hypothetical protein SAV4434, S. avermitilis MA-4680, (37/49), NP_825611 | |
| orf30 | 374 | Hypothetical protein FG11565.1, Gibberella zeae PH-1, (41/57), EAA78433 | |
| orf31 | 344 | Trypsin-like serine proteases Npun02003978, Nostoc punctiforme PCC 73102, (35/51), ZP_00110193 | |
| orf32 | 415 | Uncharacterized protein conserved in bacteria PphaDRAFT_1502, Pelodictyon phaeoclathratiforme BU-1, (41/57), ZP_00590365 | |
| orf33 | 475 | Conserved hypothetical Sun-family protein SCL6.29c, S. coelicolor A3(2), (90/95), CAB76894 | |
| orf34 | 310 | Methionyl-tRNA formyltransferase SCL6.30c, S. coelicolor A3(2), (89/95), CAB76895 | |
| orf35 | 183 | Hypothetical protein SAV6876, S. avermitilis MA-4680, (88/91), BAC74587 | |
| orf36 | 422b | Putative primosomal protein N′ SCL6.32c, S. coelicolor A3(2), (86/90), CAB76897 |
Number of amino acids of the deduced protein.
Incomplete.
Several ORFs (orf1 to orf4 and orf33 to orf36) showed by BLAST analysis significant similarities to related proteins from Streptomyces coelicolor A3(2) and Streptomyces avermitilis MA-4680. Interestingly, they also share the same genetic organization. Other ORFs (orf29 to orf32) did not show similarities to proteins in databases that would suggest a role for these proteins in steffimycin biosynthesis (Table 1 and Fig. 1B).
Genes encoding a type II polyketide synthase and related genes.
Biosynthesis of anthracyclines begins with the condensation of small carboxylic acids by the iterative action of a type II PKS composed of two ketosynthases (KSα and KSβ, also known as chain length factor [CLF]) and an acyl carrier protein. Once the polyketide backbone is formed, different ketoreductases (KR), cyclases (CYC), aromatases, and oxygenases take actions to fold and decorate the polyketide into an aromatic compound.
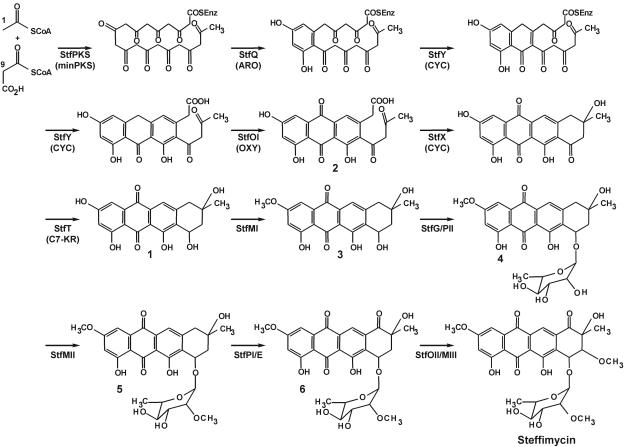
Biosynthesis of steffimycin resembles in the initial steps that of other anthracycline antibiotics previously described. In particular, steffimycin biosynthesis mimics nogalamycin biosynthesis from 10 acetates up until the formation of 2-hydroxyl-nogalonic acid (compound 2) (Fig. 4). However, they differ in the absence of a polyketide reductase, a homologue of SnoaD, responsible for the lack of a C-2 hydroxyl group in nogalamycin (23, 17), a residue present in steffimycin (Fig. 1A).
FIG. 4.
Proposed pathway for the biosynthesis of steffimycin.
In the steffimycin cluster, stfP, stfK, and stfS code for the minimal PKS. Then, aromatization of the first ring would likely be performed by the stfQ product and cyclization of the second and third rings by the stfY product, and the stfOI gene would code for an oxygenase involved in anthrone oxygenation at C-12. These functions are proposed based on the high degree of similarity of these products to similar enzymes present in databases and involved in the biosynthesis of different anthracyclines (aclacinomycin, daunorrubicin, nogalamycin, and rhodomycin) and related compounds (chartreusin, chromomycin A3, mithramycin, and simocyclinone) (Table 1).
At this point, cyclization to the fourth ring takes place, apparently by a different mechanism. In the biosynthesis of nogalamycin and daunorubicin, at least three activities are required to transform nogalonic acid to nogalaviketone or aklanonic acid to aklaviketone. The C-10 carboxyl-methyl transferases SnoaC/DnrC (17, 29), the nogalonic-aklanonic acid methyl ester cyclases SnoaL/DnrD (51, 20), and homologues of ActVI-ORFA, SnoO/DpsH, with an unclear function (17, 16), are required for cyclization (Fig. 5). However, in the equivalent steps in steffimycin biosynthesis (to transform compound 2 into compound 1), only one activity is probably required (Fig. 4 and 5). StfX, due to its homology to SnoO (Table 1), DnrH, MtmX, and CmmX, and also based on disruption experiments (see below), corresponds to a CYC likely involved in the closure of the fourth ring.
FIG. 5.
Comparison of cyclization of the fourth ring in the biosyntheses of different aromatic polyketides. (A) Nogalamycin (56) and daunorubicin (18). (B) Steffimycin. (C) UWM6 in jadomycin and rabelomycin biosynthesis (22, 35).
Finally, a C-7 KR, coded by stfT, similar to SnoaF/DnrE (17, 18), reduces the C-7 keto group (Fig. 4 and 5). In daunorubicin biosynthesis, an additional step catalyzed by the DnrP esterase is required to remove the carboxymethyl group at C-10 after glycosylation (29, 10).
Since 2-O-demethyl-8-demethoxy-10-deoxysteffimycinone (compound 1) was isolated from S. albus expressing pEM4KSEH, which contains the stfPKS (minPKS), stfQ (aromatase), stfY (CYC), stfOI (oxygenase), stfX, and stfT (C7 KR) genes (Fig. 3 and 4), this implies that activities encoded by those genes are sufficient for biosynthesis of compound 1, with StfX as the unique candidate for cyclization of the fourth ring. In addition, it demonstrates that methylesterification of the carboxyl group is not required to activate the cyclization reaction, as in daunorubicin biosynthesis (20).
Post-PKS genes.
The next step in the biosynthesis of steffimycin is a C-2-O methylation accomplished by an O-methyltransferase, leading to 8-demethoxy-10-deoxysteffimycinone (compound 3), which might be the substrate for the glycosyltranferase (Fig. 4). There are three genes in the cluster coding for O-methyltransferases, stfMI, stfMII, and stfMIII. Based on the similarities shown in Table 1, StfMII could be the methyltransferase responsible for methylation of the C-2′ hydroxyl group in l-rhamnose, since its close homologue, SnogY, is involved in the methylation of the deoxysugar l-nogalose (56). StfMI and StfMIII might be involved in O methylation of the 2- and 8-hydroxyl groups, respectively, since coexpression of pEM4KSEH (stfMI incomplete) and cosmid Stf3E5 (Fig. 1B) in S. albus leads to production of compound 3 (Fig. 4), while coexpression of pEM4KSEH and cosmid Stf2B3 (stfMI incomplete) (Fig. 1B) leads to production of compound 1 (Fig. 4 and data not shown).
Two genes are involved in the attachment of the sugar moiety l-rhamnose, stfG and stfPII. StfG homologues are well-known glycosyltransferases involved in the biosynthesis of other anthracyclines. StfPII resembles cytochromes P450 that lack the conserved Cys necessary to bind the heme prosthetic group. The roles of several enzymes belonging to that class of cytochromes, AknT (27), EryCII (60), and DesVIII (5), have been recently unraveled as proteins needed to activate their counterpart glycosyltransferases (AknS, EryCI, and DesVII) during the process of glycosylation.
Another aspect of the glycosylation process is the total absence of sugar biosynthesis genes in the cluster. This seems to be a general feature of all gene clusters containing l-rhamnose so far characterized, such as elloramycin (A. Ramos, unpublished results) and spinosyn (58). Whether those genes are shared for steffimycin and cell wall biosyntheses, as in S. spinosa (30), remains to be addressed.
After glycosylation of compound 3, late steps in steffimycin biosynthesis involve 2′-O methylation of l-rhamnose (probably by StfMII) and oxidation of C-10 to a keto group (probably by cytochrome P450 StfPI and dehydrogenase StfE), followed by introduction of the 8-methoxy group (likely by StfOII and StfMIII) (Fig. 4). Although there is no experimental evidence of those steps being catalyzed by the enzymes described, the sequence of events might be as shown, since compounds 8-demethoxy-2′-demethyl-steffimycin D (compound 4), 8-demethoxy-steffimycin D (compound 5), and 8-demethoxy-steffimycin (compound 6) (Fig. 2B and 4) have been previously characterized (24). Those compounds were also detected by LC-mass spectrometry from cultures of “S. steffisburgensis” during the development of this work (data not shown).
Regulatory and resistance genes.
Three putative regulatory genes are located in the steffimycin gene cluster (stfRI, stfRII, and stfRIII).
StfRI is similar to a variety of pathway-specific Streptomyces antibiotic regulatory proteins (SARP) (Table 1), including DnrI from Streptomyces peucetius (54). StfRII resembles several putative regulatory proteins similar to two-component response regulators (Table 1), such as DnrN from S. peucetius. StfRII might be essential for transcription of StfRI, as DnrN controls the expression of the pathway-specific transcriptional factor DnrI involved in daunorubicin biosynthesis (15). As in S. peucetius (40, 15), no putative sensor kinase gene (usually located near the response regulator gene) was found in the steffimycin gene cluster. However, an upper level of regulation by the pleiotropic regulatory gene bldA that encodes tRNA-Leu (24) might be involved in steffimycin production, since there is one TTA codon in stfRI and two in stfRII.
A third regulatory protein, StfRIII, with similarity to DNA-binding proteins (Table 1) and to putative regulatory proteins of the MarR family (pfam01047) from S. coelicolor and S. avermitilis, is also present in the cluster. Whether StfRIII is involved in regulation of steffimycin biosynthesis or resistance remains to be determined, since MarR proteins are usually involved in antibiotic resistance as repressors and are also multidrug-binding proteins whose repressor activities are affected by their interactions with different compounds (50).
Two genes in the cluster (sfrA and sfrB) are candidates to be involved in steffimycin resistance. SfrA and SfrB are similar to integral membrane proteins of the MMPL family (pfam03176) and permeases of the major facilitator superfamily (MFS; COG0477). They contain 12 and 14 putative membrane-spanning helices, respectively, and are probably involved in the export of and self-resistance to steffimycin.
“S. steffisburgensis” shows resistance to at least 50 μg/ml steffimycin. S. albus expressing Stf2B3 (sfrB is present) is resistant to 25 μg/ml steffimycin, while S. albus expressing pEM4KSEH (sfrA is present) shows no resistance to steffimycin at all (data not shown). This result points to SfrB as the real resistance determinant. However, cooperation between SfrB and SfrA to confer the level of resistance observed in “S. steffisburgensis” cannot be excluded.
Genes of unknown function.
Three genes in the cluster code for proteins whose alignment with proteins from databases does not allow a suggestion of their roles in steffimycin biosynthesis. The stfF gene product belongs to muconolactone delta-isomerases (pfam02426), and its close homologue ClcF (Table 1) is a 5-chloromuconolactone dehalogenase that converts 5-chloromuconolactone to cis-dienelactone (36).
The stfC gene product shows similarity to hypothetical proteins containing SWIM-like zinc-chelating domains (31). StfD homologues are proteins of the SNF2 family defined by the presence of a conserved motif called the SNF2 domain (pfam00176). This domain is found in ATP-dependent proteins involved in a variety of processes, including transcription, regulation, DNA repair, DNA recombination, and chromatin unwinding. It has been mentioned that in prokaryotes lacking a SWIM domain in SWI2/SNF2 ATPases, a gene encoding a SWIM domain-containing protein is located directly upstream of the gene for a SWI2/SNF2 ATPase (31), as is the case for stfD-stfC (SNF2 and SWIM proteins, respectively) in the steffimycin cluster (Fig. 1B).
The involvement of StfD and StfC in resistance to steffimycin, contributing as some kind of repair system analogous to the UV repair system found in daunorubicin (25), mithramycin (13), and chromomycin (34) gene clusters, might be possible and remains to be addressed.
Heterologous expression of the steffimycin gene cluster in S. albus.
To definitively verify that the isolated cluster led to the production of steffimycin, a new construct, pEM4STFa, was generated in which all genes proposed to be involved in steffimycin biosynthesis were included. Since no l-rhamnose biosynthesis genes were identified in the sequenced region, pEM4STFa was introduced in S. albus, together with a second plasmid, pRHAM, which is known to lead to the production of l-rhamnose (46), thus generating strain STFaRHAM. In a control experiment, pEM4STFa and pEM4 were simultaneously introduced in S. albus, generating strain STFaC.
Analysis of products accumulated by S. albus STFaC (Fig. 1C) showed the production of a red compound with an HPLC retention time of 21.25 min and a mass of 371 m/z ([M+H]+), which might correspond to 8-demethoxy-10-deoxysteffimycinone, compound 3 (Fig. 4), probably steffimycin aglycon. No glycosylated compounds were detected.
Cultures of S. albus STFaRHAM (Fig. 1C) showed the production of a mixture of red compounds, the major one of which corresponded to steffimycin, showing an HPLC mobility of 20.7 min and a mass of 575 m/z ([M+H]+). In addition, other minor compounds were identified by LC-mass spectrometry as compounds 4, 5, and 6 (Fig. 1C and 4) with mobilities of 18.7, 20.3, and 20.1 min and masses of 517, 531, and 545 m/z ([M+H]+), respectively. Compounds 4, 5, and 6 were previously detected by LC-mass spectrometry, as mentioned above, in cultures of “S. steffisburgensis” (Fig. 2B). These expression experiments clearly show the presence in the sequenced steffimycin cluster of all the genes required for the biosynthesis of the polyketide, the tailoring steps, and decoration of the sugar moiety, with the exception of genes coding for the enzymes involved in the biosynthesis of l-rhamnose. In addition, plasmid pEM4STFa represents a useful tool for the future development of steffimycin derivatives containing different sugar moieties.
Inactivation of stfX and stfG.
Two gene inactivation experiments were performed, first, to determine if stfX is involved in cyclization of the fourth ring of 2-hydroxy-nogalonic acid (compound 2) (Fig. 4), and second, to isolate the substrate for StfG-mediated glycosylation and to verify its correspondence to the previously identified compound 3 in cultures of S. albus strain STFaC (Fig. 1C).
stfX was inactivated in pEM4KSEH by insertion of a copy of the hyg gene into a unique SnaBI site. pEM4KSEHX-Hyg was expressed in S. albus, and the production of red compounds was monitored in both liquid and solid media by LC-mass spectrometry. In both media, the formation of compound 1 previously observed by expression of pEM4KSEH in S. albus was detected. In addition, a new compound 2 with an HPLC retention of 21.59 min and a mass of 399 m/z ([M+H]+), which might correspond to 2-hydroxy-nogalonic acid (Fig. 3C), was also identified. Compound 2 was also detected as a minor compound in cultures of S. albus pEM4KSEH and of “S. steffisburgensis,” but in S. albus pEM4KSEHX-Hyg, the production rose 30-fold while that of compound 1 remained constant.
These results are in agreement with previous results observed by disruption of actVI-ORFA, involved in actinorhodin biosynthesis by S. coelicolor (53), where it leads to the accumulation of possible biosynthetic intermediates with a gross reduction in actinorhodin biosynthesis. The authors suggested that ActVI-ORFA played a general role in stabilizing the multicomponent type II PKS complex, rather than in catalyzing the ring formation itself (53). Our results point to a spontaneous cyclization of the fourth ring, with the StfX protein facilitating the process.
In the biosynthesis of the angucycline antibiotics jadomycin and rabelomycin, cyclases JadI and PgaF catalyze an intramolecular aldol condensation to form the fourth angular ring concomitant with the removal of a carboxyl group (22, 35), which leads to the common intermediate UWM6 (Fig. 5). Apart from the similarities between the cyclization mechanisms of steffimycin biosynthesis and UWM6 formation, there is no significant similarity between StfX and JadI or PgaF, probably due to the structures of the products of the enzymatic reactions (anthracycline versus angucycline) and because JadI and PgaF are also involved in third-ring cyclizations.
stfG was inactivated by gene disruption using plasmid pOJG (Fig. 6). Cultures of “S. steffisburgensis” OJG revealed the production, as expected, of compound 3 with a mobility of 21.25 min and a mass of 371 m/z ([M+H]+), which might correspond to 8-demethoxy-10-deoxysteffimycinone (Fig. 4 and 6C). This compound was previously observed in cultures of S. albus strain STFaC, in which no genes coding for enzymes involved in l-rhamnose biosynthesis are present (Fig. 1C). No glycosylated compounds were detected by LC-mass spectrometry analysis. For further confirmation of compound 3 as an intermediate in the biosynthesis of steffimycin, it was fed to “S. steffisburgensis” strain M1KS, which was able to convert it to steffimycin (data not shown). In addition, disruption of stfG was complemented in trans by stfG expressed in pEM4, restoring steffimycin production.
FIG. 6.
(A) Strategy to generate a mutant in stfG. (B) HPLC analysis of “S. steffisburgensis” (top) and “S. steffisburgensis” OJG (bottom). (C) Product accumulated by the mutant. aac(3)IV, apramycin resistance gene; AU, arbitrary units.
Structure elucidation of 2-O-demethyl-8-demethoxy-10-deoxysteffimycinone (compound 1) and 2-hydroxy-nogalonic acid (compound 2).
Compound 1 showed a molecular ion peak in low-resolution EI-MS at m/z 356 [M]+. The ESI-MS of compound 1 displayed in the negative ion mode a molecular ion of m/z 355 [M-H]−, suggesting a molecular mass of 356 g/mol. The molecular formula was determined from high-resolution EI-MS to be C19H16O7. The presence of the peri-hydroxyanthraquinone chromophore in compound 1 is in agreement with the observed UV maximum at a λmax of 435 nm in the UV/visible-light spectrum. The 1H NMR spectrum of compound 1 showed two typical signals of chelated OH groups at δ 13.11 and 12.49, one singlet at δ 7.77, and two meta-coupled aromatic protons at δ 7.69 and 6.98. Furthermore, one proton-coupled oxygen-bound carbon at δ 5.80, two pairs of methylene protons at δ 3.18 and 3.12 and δ 2.71 and 2.42, and one methyl group at δ 1.71 were identified. The 13C NMR of compound 1 revealed the presence of 19 carbon atoms. The quaternary carbon signals at δ 191.2 and 182.3 could be assigned to two carbonyl groups of a quinone system. The spectra showed three aromatic methines at δ 121.6, 110.1, and 109.3 and nine aromatic or olefinic quaternary carbon atoms, three of which, at δ 167.8, 166.2, and 162.1, could be assigned to aromatic carbons connected to oxygen atoms. Furthermore, two signals at δ 69.1 and 64.5 for aliphatic quaternary carbons connected to oxygen atoms, two methylenes at δ 46.1 and 45.8, and one methyl group at δ 30.8 were delivered. Searches in the Chemical Abstract database produced no results, pointing to a new metabolite. The structure of compound 1 was established, taking into consideration 1JC-H and 3JC-H long-range couplings observed in the heteronuclear single quantum correlation and the constant time inverse-detected gradient accordion resolved-heteronuclear multiple bond correlation spectra, respectively, and by comparison of the spectral data with the structurally related 8-demethoxy-2′-demethyl-steffimycin D (compound 4) (Fig. 4) (23). The stereochemistry of ring A was deduced to be (7S, 9S), because of a missing nuclear Overhauser effect correlation between 7-H and 9-CH3, while 9-CH3 showed nuclear Overhauser effects with each of the H atoms of the 8- and the 10-methylene groups (Fig. 7A). The absolute stereochemistry was established through comparison with compound 4. Schmidt-Bäse et al. determined the absolute configuration of steffimycin B by X ray (49). In this study, the configurations of the stereocenters in ring A were established to be 7R, 8S, and 9S. The ring adopted a half-chair conformation without an extra stabilizing intramolecular H bond from 9-OH to 7-O (see the supplemental material).
FIG. 7.
(A) Nuclear Overhauser enhancement effects observed in ring A of structure 1 confirm the relative stereochemistry of the centers in ring A, as shown. (B) Chemical structures of the closest relatives of 2-hydroxynogalonic acid (compound 2), 58B (55) and aklanonic acid (11). (C) Tautomeric mixture of 2-hydroxynogalonic acid (compound 2) as evident from the 1H NMR spectrum (data for the minor tautomers are not listed). Compounds 1 and 2 correspond to compounds in Fig. 4.
Compound 2 accommodates the similarity of aklanonic acid's UV spectrum (λmax in methanol, 435, 287, and 258 nm.). It showed in the negative ion mode of ESI a mass spectrum of an [M-H]− peak at m/z 397, corresponding to a molecular mass of 398 g/mol. The 1H NMR spectrum of compound 2 revealed one sharp singlet at δ 8.21, two meta-coupled aromatic protons at δ 7.69 (2.1 Hz) and 6.99 (2.1 Hz), and one olefinic proton at δ 6.31. Furthermore, one methylene group at δ 4.25 and CH3 protons at δ 2.07 were observed. The 13C NMR spectrum of compound 2 exhibited five carbonyl carbons at δ 192.8, 190.7, 186.1, 182.0, and 173.0, with the chemical shift being consistent with a COOH group. Furthermore, quaternary carbon atoms attached to oxygen were observed at δ 168.3, 168.2, and 166.4, with four sp2 methine carbons at δ 122.0, 110.9, 109.4, and 105.0, respectively, and six sp2 quaternary carbon atoms. Additionally, one methylene group at δ 40.6 and one methyl signal at δ 25.0 were observed.
Searching the resulting structure 2 gave no hits in Chemical Abstracts. Therefore, compound 2 is new. Structure 2 was also confirmed by comparison of the spectral data with the structurally related aklanonic acid and compound 58B (Fig. 7B) (11, 55). Compound 2 (2-hydroxy-nogalonic acid) differs from aklanonic acid and from compound 58B in being a free acid versus a methylcarboxylate and through its acetyl starter group instead of a propionyl starter group (lower side chain). The structure elucidation was complicated by the fact that compound 2 exists in an equilibrium of three tautomeric forms (Fig. 7C). However, as is well known from simple 1,3-diketones, as well as from aklanonic acid, the keto-enol equilibrium lies far on the enol side. This is also evident for compound 2 from its 1H NMR data.
In conclusion, the isolation and characterization of the steffimycin gene cluster and its heterologous expression in S. albus provide a new tool for the generation of novel anthracycline derivatives by combinatorial biosynthesis. The characterization of this cluster has also provided new insights into the enzymatic mechanisms for polyketide ring cyclization. In contrast to other anthracyclines, for which two enzymes are required for cyclization of the fourth ring, in the case of steffimycin, this cyclization event can probably occur spontaneously, with the presence of the stfX product facilitating the process.
Supplementary Material
Acknowledgments
This research was supported by the Spanish Ministry of Education and Science (BMC2003-00478), the Plan Regional de I+D+I del Principado de Asturias (grant GE-MED01-05), the U.S. National Institutes of Health (grant CA 102102), and Red Temática de Investigación Cooperativa de Centros de Cáncer (Ministy of Health, Spain). We thank Obra Social Cajastur for financial support to C.O. and the Spanish Ministry of Education and Science for a Ph.D. student fellowship for S.G.
The NMR core center of the University of Kentucky is acknowledged for the use of their spectrometers. We also thank Jack Goodmann (University of Kentucky Mass Spectrometry Facility) for the high-resolution mass spectra.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Bergy, M. E., and F. Reusser. 1967. A new antibacterial agent (U-20,661) isolated from a Streptomycete strain. Experientia 23:254-255. [DOI] [PubMed] [Google Scholar]
- 3.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 4.Bols, M., L. Binderup, J. Hansen, and P. Rasmussen. 1992. Inhibition of collagenase by aranciamycin and aranciamycin derivatives. J. Med. Chem. 35:2768-2771. [DOI] [PubMed] [Google Scholar]
- 5.Borisova, S. A., L. Zhao, C. E. Melancon III, C. L. Kao, and H. W. Lium. 2004. Characterization of the glycosyltransferase activity of desVII: analysis of and implications for the biosynthesis of macrolide antibiotics. J. Am. Chem. Soc. 126:6534-6535. [DOI] [PubMed] [Google Scholar]
- 6.Brodasky, T. F., and F. Reusser. 1974. Steffimycin B, a new member of the steffimycin family: isolation and characterization. J. Antibiot. 27:809-813. [DOI] [PubMed] [Google Scholar]
- 7.Brodasky, T. F., S. Mizsak, and J. R. Hoffstetter. 1985. Steffimycin C, a new member of the steffimycin anthracyclines. Isolation and structural characterization. J. Antibiot. 38:849-855. [DOI] [PubMed] [Google Scholar]
- 8.Chater, K. F., and L. C. Wilde. 1980. Streptomyces albus G mutants defective in the SalGI restriction-modification system. J. Gen. Microbiol. 116:323-334. [DOI] [PubMed] [Google Scholar]
- 9.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickens, M. L., N. D. Priestley, and W. R. Strohl. 1997. In vivo and in vitro bioconversion of epsilon-rhodomycinone glycoside to doxorubicin: functions of DauP, DauK, and DoxA. J. Bacteriol. 179:2641-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckardt, K., D. Tresselt, G. Schumann, W. Ihn, and C. Wagner. 1985. Isolation and chemical structure of aklanonic acid, an early intermediate in the biosynthesis of anthracyclines. J. Antibiot. 38:1034-1039. [DOI] [PubMed] [Google Scholar]
- 12.Erdal, H., M. Berndtsson, J. Castro, U. Brunk, M. C. Shoshan, and S. Linder. 2005. Induction of lysosomal membrane permeabilization by compounds that activate p53-independent apoptosis. Proc. Natl. Acad. Sci. USA 102:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández, E., F. Lombo, C. Méndez, and J. A. Salas. 1996. An ABC transporter is essential for resistance to the antitumor agent mithramycin in the producer Streptomyces argillaceus. Mol. Gen. Genet. 251:692-698. [DOI] [PubMed] [Google Scholar]
- 14.Fernández, E., U. Weissbach, C. Sánchez Reillo, A. F. Braña, C. Méndez, J. Rohr, and J. A. Salas. 1998. Identification of two genes from Streptomyces argillaceus encoding glycosyltransferases involved in transfer of a disaccharide during biosynthesis of the antitumor drug mithramycin. J. Bacteriol. 180:4929-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuya, K., and C. R. Hutchinson. 1996. The DnrN protein of Streptomyces peucetius, a pseudo-response regulator, is a DNA-binding protein involved in the regulation of daunorubicin biosynthesis. J. Bacteriol. 178:6310-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlitz, M., G. Meurer, E. Wendt-Pienkowski, K. Madduri, and C. R. Hutchinson. 1997. The effect of the daunorubicin dpsH gene on the choice of starter unit and cyclization pattern reveals that type II polyketide synthases can be unfaithful yet intriguing. J. Am. Chem. Soc. 119:7392-7393. [Google Scholar]
- 17.Hautala, A., S. Torkkell, K. Raty, T. Kunnari, J. Kantola, P. Mantsala, J. Hakala, and K. Ylihonko. 2003. Studies on a second and third ring cyclization in anthracycline biosynthesis. J. Antibiot. 56:143-153. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson, C. R. 1997. Biosynthetic studies of daunorubicin and tetracenomycin C. Chem. Rev. 97:2525-2536. [DOI] [PubMed] [Google Scholar]
- 19.Keller-Schierlein, W., J. Sauerbier, U. Vogler, and H. Zahner. 1970. Aranciamycin. Helv. Chim. Acta 53:779-789. [DOI] [PubMed] [Google Scholar]
- 20.Kendrew, S. G., K. Katayama, E. Deutsch, K. Madduri, and C. R. Hutchinson. 1999. DnrD cyclase involved in the biosynthesis of doxorubicin: purification and characterization of the recombinant enzyme. Biochemistry 38:4794-4799. [DOI] [PubMed] [Google Scholar]
- 21.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation. Norwich, United Kingdom.
- 22.Kulowski, K., E. Wendt-Pienkowski, L. Han, K. Yang, L. C. Vining, and C. R. Hutchinson. 1999. Functional characterization of the jadI gene as a cyclase forming angucyclinones. J. Am. Chem. Soc. 121:1786-1794. [Google Scholar]
- 23.Kunnari, T., J. Tuikkanen, A. Hautala, J. Hakala, K. Ylihonko, and P. Mantsala. 1997. Isolation and characterization of 8-demethoxy steffimycins and generation of 2,8-demethoxy steffimycins in Streptomyces steffisburgensis by the nogalamycin biosynthesis genes. J. Antibiot. 50:496-501. [DOI] [PubMed] [Google Scholar]
- 24.Leskiw, B. K., E. J. Lawlor, J. M. Fernández-Abalos, and K. F. Chater. 1991. TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative, Streptomyces mutants. Proc. Natl. Acad. Sci. USA 88:2461-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomovskaya, N., S. K. Hong, S. U. Kim, L. Fonstein, K. Furuya, and C. R. Hutchinson. 1996. The Streptomyces peucetius drrC gene encodes a UvrA-like protein involved in daunorubicin resistance and production. J. Bacteriol. 178:3238-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozano, M. J., L. L. Remsing, L. M. Quirós, A. F. Braña, E. Fernández, C. Sánchez, C. Méndez, J. Rohr, and J. A. Salas. 2000. Characterization of two polyketide methyltransferases involved in the biosynthesis of the antitumor drug mithramycin by Streptomyces argillaceus. J. Biol. Chem. 275:3065-3074. [DOI] [PubMed] [Google Scholar]
- 27.Lu, W., C. Leimkuhler, G. J. Gatto, Jr., R. G. Kruger, M. Oberthur, D. Kahne, and C. T. Walsh. 2005. AknT is an activating protein for the glycosyltransferase AknS in l-aminodeoxysugar transfer to the aglycone of aclacinomycin A. Chem. Biol. 12:527-534. [DOI] [PubMed] [Google Scholar]
- 28.MacNeil, D. J., K. M. Gewain, C. L. Ruby, G. Dezeny, P. H. Gibbons, and T. MacNeil. 1992. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61-68. [DOI] [PubMed] [Google Scholar]
- 29.Madduri, K., and C. R. Hutchinson. 1995. Functional characterization and transcriptional analysis of a gene cluster governing early and late steps in daunorubicin biosynthesis in Streptomyces peucetius. J. Bacteriol. 177:3879-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madduri, K., C. Waldron, and D. J. Merlo. 2001. Rhamnose biosynthesis pathway supplies precursors for primary and secondary metabolism in Saccharopolyspora spinosa. J. Bacteriol. 183:5632-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makarova, K. S., L. Aravind, and E. V. Koonin. 2002. SWIM, a novel Zn-chelating domain present in bacteria, archaea and eukaryotes. Trends Biochem. Sci. 27:384-386. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzawa, Y., T. Oki, T. Takeuchi, and H. Umezawa. 1981. Structure-activity relationships of anthracyclines relative to cytotoxicity and effects on macromolecular synthesis in L1210 leukemia cells. J. Antibiot. 34:1596-1607. [DOI] [PubMed] [Google Scholar]
- 33.Méndez, C., and J. A. Salas. 2003. On the generation of novel anticancer drugs by recombinant DNA technology: the use of combinatorial biosynthesis to produce novel drugs. Comb. Chem. High Throughput Screen. 6:513-526. [DOI] [PubMed] [Google Scholar]
- 34.Menéndez, N., M. Nur-E-Alam, A. F. Braña, J. Rohr, J. A. Salas, and C. Méndez. 2004. Tailoring modification of deoxysugars during biosynthesis of the antitumour drug chromomycin A by Streptomyces griseus ssp. griseus. Mol. Microbiol. 53:903-915. [DOI] [PubMed] [Google Scholar]
- 35.Metsa-Ketela, M., K. Palmu, T. Kunnari, K. Ylihonko, and P. Mantsala. 2003. Engineering anthracycline biosynthesis toward angucyclines. Antimicrob. Agents Chemother. 47:1291-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moiseeva, O. V., I. P. Solyanikova, S. R. Kaschabek, J. Groning, M. Thiel, L. A. Golovleva, and M. Schlomann. 2002. A new modified ortho cleavage pathway of 3-chlorocatechol degradation by Rhodococcus opacus 1CP: genetic and biochemical evidence. J. Bacteriol. 184:5282-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monneret, C. 2001. Recent developments in the field of antitumour anthracyclines. Eur. J. Med. Chem. 36:483-493. [DOI] [PubMed] [Google Scholar]
- 38.Murray, N. E., W. J. Brammar, and K. Murray. 1977. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol. Gen. Genet. 150:53-61. [DOI] [PubMed] [Google Scholar]
- 39.Olano, C., B. Wilkinson, C. Sánchez, S. J. Moss, R. Sheridan, V. Math, A. J. Weston, A. F. Braña, C. J. Martin, M. Oliynyk, C. Méndez, P. F. Leadlay, and J. A. Salas. 2004. Biosynthesis of the angiogenesis inhibitor borrelidin by Streptomyces parvulus Tu4055: cluster analysis and assignment of functions. Chem. Biol. 11:87-97. [DOI] [PubMed] [Google Scholar]
- 40.Otten, S. L., J. Ferguson, and C. R. Hutchinson. 1995. Regulation of daunorubicin production in Streptomyces peucetius by the dnrR2 locus. J. Bacteriol. 177:1216-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quirós, L. M., I. Aguirrezabalaga, C. Olano, C. Méndez, and J. A. Salas. 1998. Two glycosyltransferases and a glycosidase are involved in oleandomycin modification during its biosynthesis by Streptomyces antibioticus. Mol. Microbiol. 28:1177-1185. [DOI] [PubMed] [Google Scholar]
- 42.Raty, K., T. Kunnari, J. Hakala, P. Mantsala, and K. Ylihonko. 2000. A gene cluster from Streptomyces galilaeus involved in glycosylation of aclarubicin. Mol. Gen. Genet. 264:164-172. [DOI] [PubMed] [Google Scholar]
- 43.Raty, K., J. Kantola, A. Hautala, J. Hakala, K. Ylihonko, and P. Mantsala. 2003. Cloning and characterization of Streptomyces galilaeus aclacinomycins polyketide synthase (PKS) cluster. Gene 293:115-122. [DOI] [PubMed] [Google Scholar]
- 44.Reusser, F. 1967. Mode of action of antibiotic U-20,661. J. Bacteriol. 93:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson, M. A., S. Kuhstoss, P. Solenberg, N. A. Schaus, and R. N. Rao. 1987. A new shuttle cosmid vector, pKC505, for streptomycetes: its use in the cloning of three different spiramycin-resistance genes from a Streptomyces ambofaciens library. Gene 61:231-241. [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez, L., C. Oelkers, I. Aguirrezabalaga, A. F. Braña, J. Rohr, C. Méndez, and J. A. Salas. 2000. Generation of hybrid elloramycin analogs by combinatorial biosynthesis using genes from anthracycline-type and macrolide biosynthetic pathways. J. Mol. Microbiol. Biotechnol. 2:271-276. [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 48.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt-Bäse, K., M. Noltemeyer, E. Egert, E. Eigelt, and A. Zeeck. 1993. Structure of the anthracycline antibiotic aranciamycin. Acta Crystallogr. C 49:250-253. [Google Scholar]
- 50.Schumacher, M. A., and R. G. Brennan. 2002. Structural mechanisms of multidrug recognition and regulation by bacterial multidrug transcription factors. Mol. Microbiol. 45:885-893. [DOI] [PubMed] [Google Scholar]
- 51.Sultana, A., P. Kallio, A. Jansson, J. S. Wang, J. Niemi, P. Mantsala, and G. Schneider. 2004. Structure of the polyketide cyclase SnoaL reveals a novel mechanism for enzymatic aldol condensation. EMBO J. 23:1911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzukake-Tsuchiya, K., Y. Moriya, K. Yamazaki, M. Hori, N. Hosokawa, T. Sawa, H. Iinuma, H. Naganawa, C. Imada, and M. Hamada. 1990. Screening of antibiotics preferentially active against ras oncogene-expressed cells. J. Antibiot. 43:1489-1496. [DOI] [PubMed] [Google Scholar]
- 53.Taguchi, T., K. Itou, Y. Ebizuka, F. Malpartida, D. A. Hopwood, C. M. Surti, K. I. Booker-Milburn, G. R. Stephenson, and K. Ichinose. 2000. Chemical characterisation of disruptants of the Streptomyces coelicolor A3(2) actVI genes involved in actinorhodin biosynthesis. J. Antibiot. 53:144-152. [DOI] [PubMed] [Google Scholar]
- 54.Tang, L., A. Grimm, Y. X. Zhang, and C. R. Hutchinson. 1996. Purification and characterization of the DNA-binding protein DnrI, a transcriptional factor of daunorubicin biosynthesis in Streptomyces peucetius. Mol. Microbiol. 22:801-813. [DOI] [PubMed] [Google Scholar]
- 55.Tobe, H., A. Yoshimoto, T. Ishikura, H. Naganawa, T. Takeuchi, and H. Umezawa. 1982. New anthracyclinone metabolites from two blocked mutants of Streptomyces galilaeus MA144-M1. J. Antibiot. 35:1641-1645. [DOI] [PubMed] [Google Scholar]
- 56.Torkkell, S., T. Kunnari, K. Palmu, P. Mantsala, J. Hakala, and K. Ylihonko. 2001. The entire nogalamycin biosynthetic gene cluster of Streptomyces nogalater: characterization of a 20-kb DNA region and generation of hybrid structures. Mol. Genet. Genom. 266:276-288. [DOI] [PubMed] [Google Scholar]
- 57.Wiley, P. F., D. W. Elrod, and D. E. Harper. 1988. Chemical modification of steffimycin B. J. Antibiot. 41:343-351. [DOI] [PubMed] [Google Scholar]
- 58.Waldron, C., P. Matsushima, P. R. Rosteck, Jr., M. C. Broughton, J. Turner, K. Madduri, K. P. Crawford, D. J. Merlo, and R. H. Baltz. 2001. Cloning and analysis of the spinosad biosynthetic gene cluster of Saccharopolyspora spinosa. Chem. Biol. 8:487-499. [DOI] [PubMed] [Google Scholar]
- 59.Ylihonko, K., J. Tuikkanen, S. Jussila, L. Cong, and P. Mantsala. 1996. A gene cluster involved in nogalamycin biosynthesis from Streptomyces nogalater: sequence analysis and complementation of early-block mutations in the anthracycline pathway. Mol. Gen. Genet. 251:113-120. [DOI] [PubMed] [Google Scholar]
- 60.Yuan, Y., H. S. Chung, C. Leimkuhler, C. T. Walsh, D. Kahne, and S. Walker. 2005. In vitro reconstitution of EryCIII activity for the preparation of unnatural macrolides. J. Am. Chem. Soc. 127:14128-14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.