Abstract
Arbuscular mycorrhizal (AM) fungi depend on a C supply from the plant host and simultaneously provide phosphorus to the colonized plant. We therefore evaluated the influence of external P on C allocation in monoxenic Daucus carota-Glomus intraradices cultures in an AM symbiosis. Fungal hyphae proliferated from a solid minimal medium containing colonized roots into a C-free liquid minimal medium with high or low P availability. Roots and hyphae were harvested periodically, and the flow of C from roots to fungus was measured by isotope labeling. We also measured induction of a G. intraradices high-affinity P transporter to estimate fungal P demand. The prevailing hypothesis is that high P availability reduces mycorrhizal fungal growth, but we found that C flow to the fungus was initially highest at the high P level. Only at later harvests, after 100 days of in vitro culture, were C flow and fungal growth limited at high P availability. Thus, AM fungi can benefit initially from P-enriched environments in terms of plant C allocation. As expected, the P transporter induction was significantly greater at low P availability and greatest in very young mycelia. We found no direct link between C flow to the fungus and the P transporter transcription level, which indicates that a good C supply is not essential for induction of the high-affinity P transporter. We describe a mechanism by which P regulates symbiotic C allocation, and we discuss how this mechanism may have evolved in a competitive environment.
Fungi in the phylum Glomeromycota proliferate as part of an arbuscular mycorrhizal (AM) association (5, 7, 32). Carbon is transferred from colonized plants to AM fungi (14), while the plants usually receive much of their P through hyphal uptake and fungal transfer to the host roots (26, 33). Colonization by AM fungi increases the size of the C sink in the roots (9) and may be a significant cost to the host plant that results in reduced growth at high P levels (1, 2, 15, 18, 19, 27). The adverse effect of high soil P levels on AM formation is primarily due to high P concentrations in the roots (31), which results in reduced C allocation to the AM fungus (24). Thus, there is an important connection between external P supply and C allocation to the fungal partner in the symbiosis. Moderate levels of P fertilization seem to be optimal for AM colonization (21). While the general effect of high P availability on C flow to the fungus has been well described, the effect of high P availability on C flow to the fungus within a few days is not known.
A monoxenic system with carrot root organ cultures in symbiosis with the AM fungus Glomus intraradices can be used to study fungal growth (3) and is a well-established model system for studying metabolism and transport in the AM symbiosis (5, 10). We used a system in which the level of P was varied in a compartment that was mainly available to extraradical hyphae (17), which delayed the time until the root P levels were affected by the P treatment. C allocation was measured by compound-specific isotope ratio mass spectrometry. Fungal P demand was inferred from the levels of G. intraradices P transporter (GiPT) transcription, which is induced by low orthophosphate (Pi) levels in the environment (17).
The objectives of this study were to investigate the influence of P availability on symbiotic C allocation to mycorrhizal fungi over time and to determine if the fungal P uptake system is regulated by the C allocated to the fungus. We tested the hypothesis that the effect of P on C allocation and efforts to acquire P from the environment is time dependent. If this hypothesis is correct, then there should be a significant statistical interaction between the P treatment factor and the time factor. Mycorrhizal fungal uptake of P is important for plant nutrition in most natural and agricultural ecosystems, and the regulation of the symbiosis is therefore critically important in the management of such ecosystems, as well as for understanding how the symbioses evolved.
MATERIALS AND METHODS
Monoxenic arbuscular mycorrhiza cultures.
The AM fungus G. intraradices Schenck & Smith (strain DAOM 197198; Biosystematics Research Center, Ottawa, Ontario, Canada) was grown monoxenically in mycorrhizal association with root organ cultures of carrot (Daucus carota L.). The root organ cultures were clones of carrot roots (line DC1) that were transformed with Agrobacterium rhizogenes carrying the T-DNA of the root-inducing plasmid, which was transferred to the plant genome (7). AM-colonized cultures were maintained at a constant temperature of 24°C on petri dishes with 0.3% Phytagel (Sigma Chemical Co., St. Louis, MO) as the gelling agent and with a minimal (M) nutrient medium (7) containing 10 g sucrose liter−1 as the C source and 35 μM P (as 4.8 mg KH2PO4 liter−1).
Experimental setup.
Plugs of solid medium containing carrot roots and mycelia and spores of G. intraradices were transferred from 3- to 4-month-old cultures to experimental two-compartment petri dishes with a diameter of 90 mm (17). In each plate one plug was placed in a hole in the solid minimal nutrient medium on the root side (about 20 ml medium). Two separate experiments were performed. In the first experiment, all of the plates contained complete M medium on the root side (complete-C treatment). In the second experiment, one half of the plates contained complete M medium, and the other half contained M medium with one-half (5 g liter−1) of the usual sucrose concentration (half-C treatment). The cultures were sealed with Parafilm “M” (American National Can, Chicago, IL). The second compartment of each of the petri dishes initially was empty. After 30 days the empty compartment was filled with liquid minimal medium (containing no Phytagel) that did not contain sucrose but contained either 25 μM P (low-P treatment) or 2.5 mM P (as KH2PO4) (high-P treatment). Roots passing over the barrier between the two compartments were removed at this time. The root side was supplied with 10 mg d-[13C]glucose (U-13C-6; 99% 13C; Cambridge Isotope Laboratories, Andover, Mass) in four replicate dishes that received each treatment 7 days before harvest. One dish that received the P-free treatment was not labeled and served as a control to measure the natural abundance of 13C in roots and AM fungal mycelium.
In the first experiment, four replicate plates each that received the low-P and high-P treatments were harvested 33, 47, and 75 days after the liquid medium was added.
In the second experiment, four replicate plates that received each of the four treatments (complete-C/low-P, complete-C/high-P, half-C/low-P, and half-C/high-P treatments) were harvested 21, 50, and 100 days after the liquid medium was added. For the half-C treatment there were no plates in which there was mycelium in the liquid compartment after 21 days. The four replicates that received each treatment which had the most mycelium were chosen as the experimental units. However, in a few cases there were not enough plates with hyphae growing into the liquid medium, and in these cases there were only three replicates for a treatment.
After 21 days spores were observed, but not all hyphae were spore bearing at this time. After 33 days (first experiment), all runner hyphae bore spores, and the absorbing hyphae had disappeared. The length of the sampling period was longer in the second experiment, and we collected hyphae that were both younger and older than the hyphae collected in the first experiment. Intraradical mycelium development was not recorded, but previous experiments have shown that vesicles form before lipid accumulation and thus do not reflect C metabolism very well (35). Previously we found that the entire root systems in these types of monoxenic cultures were viable for at least 180 days.
One half of the mycelium from each of the liquid media was collected and freeze-dried. The dry weight was determined, and the dried mycelia were stored at −20°C until 13C enrichment and lipid compositions were determined. The pH of the liquid growth medium was also determined. The solid medium in the root compartment was dissolved in 250 ml 10 mM sodium citrate by mixing with a magnetic stirrer for 1 h at low speed. The roots were separated from extraradical mycelium, collected, freeze-dried, weighed, and stored at −20°C for subsequent nutrient analyses, determination of 13C enrichment, and lipid analyses. The remaining half of the mycelia from the liquid compartments was collected for RNA extraction. The wet weights of these portions of the mycelia were determined and used to calculate the total dry weight of the mycelia from the liquid media. Material from three plates for each treatment at each time in both experiments was used for RNA extraction. Mycelia from each plate were collected from the liquid compartment, placed in sterile water at 4°C for <5 min, drained, frozen in liquid nitrogen, and stored at −80°C until RNA was extracted.
Lipid analysis.
Mycelia (1 to 4 mg) were milled with two iron balls (diameter, 7 mm) that were shaken in 50-ml Teflon tubes for 5 s. The roots (20 to 40 mg) were ball milled (15 s) in iron beakers. Lipids were extracted from mycelia and mycorrhizal roots by vortexing (1 min) in 10 ml of a one-phase mixture containing citrate buffer, methanol, and chloroform (0.8:2:1, vol/vol/vol; pH 4.0). The lipids were fractionated into neutral lipids, glycolipids, and phospholipids on prepacked silica columns (100-mg sorbent mass; Varian Medical Systems, Palo Alto, CA) by elution with 1.5 ml chloroform, 6 ml acetone, and 1.5 ml methanol, respectively (25). The fatty acid residues in neutral lipids and phospholipids were transformed into free fatty acid methyl esters and analyzed by gas chromatography by using a 50-m HP5 capillary fused silica column (Hewlett-Packard, Wilmington, DE) with H2 as the carrier gas (11). The fatty acids were identified on the basis of their retention times relative to those of the internal standard (fatty acid methyl ester 19:0) and were compared to fatty acids determined previously by gas chromatography-mass spectrometry.
Determination of 13C enrichment in solid samples and fatty acids.
Freeze-dried mycelium (approximately 20 μg) or ball-milled root material (approximately 100 μg) was enclosed in tin capsules, and the 13C atom% was determined with an isotope ratio mass spectrometer (20-20 stable isotope analyzer) interfaced with an ANCA-NT combustion module (PDZ Europa Scientific Instruments, Crewe, United Kingdom). Fatty acid methyl esters (prepared as described above) were analyzed with an isotope ratio mass spectrometer interfaced with a Hewlett-Packard gas chromatograph in order to determine the 13C atom% in neutral lipid fatty acids (NLFA) and phospholipid fatty acids (25). The gas chromatograph was equipped with a 50-m HP5 capillary column (Hewlett-Packard), and He was used as the carrier gas. The 13C enrichment (excess 13C atom%) was calculated by subtracting the natural abundance of 13C (1.14%).
Total C flow to AM fungi was calculated by using the extraradical mycelium in the liquid medium and the intraradical mycelium in the solid compartment. The amount of the AM signature NLFA 16:1ω5 in both fractions was estimated and multiplied by the enrichment of 13C in this signature. From this the total C flow to the AM fungal NLFA 16:1ω5 was calculated by assuming that little extraradical mycelium from the root compartment was lost during root collection. AM fungal excess 13C in NLFA 16:1ω5 (including both intraradical mycelium and extraradical mycelium) was calculated by multiplying the 13C enrichment in NLFA 16:1ω5 by the total amount of NLFA 16:1ω5-C.
Phosphorus content in liquid medium.
The orthophosphate (PO4-P) content in the liquid medium was analyzed spectrophotometrically by using the molybdate blue method of Murphy and Riley (20). One milliliter of a sample (diluted if necessary so that it contained less than 870 μg PO4-P liter−1) was mixed with 200 μl of a reaction mixture (1.25 M H2SO4, 4.9 mM ammonium molybdate, 30 mM ascorbic acid, 0.8 mM potassium antimony tartrate), and the extinction at A882 was determined after 1.5 h of incubation (the reaction was fully developed in 20 min and was stable for 24 h). A yellow complex was formed by phosphate and ammonium molybdate in the acid environment, and this complex was reduced by ascorbic acid to a blue compound.
Quantitative real-time PCR analyses.
Reverse transcription and quantitative real-time PCR analyses were carried out with total RNA from the fungal tissues to determine changes in the levels of transcription of the GiPT gene (accession number AF359112), which encodes a G. intraradices high-affinity phosphate transporter. The GiPT transcription levels are expressed below as relative values compared to the transcription levels of the G. intraradices β-tubulin gene (BE603903), a constitutively expressed housekeeping gene (30).
Total RNA was isolated from the fungal tissues using an RNeasy Plant Mini Kit (QIAGEN, Courtaboeuf, France) and was treated with DNase (Stratagene, La Jolla, CA) and reverse transcribed using Superscript II reverse transcriptase (Gibco BRL, Paisley, United Kingdom) by following the manufacturer's instructions. The 3′ primers 5′-CCGCCTTTACTCTTTCTTTCC-3′ (GiPT) and 5′-TCACCGCCATCCTCTTCTTC-3′ (β-tubulin) were used to prime the reactions. Quantitative PCR for GiPT and β-tubulin were carried out with 5′ primers 5′-TCCCAACACGTTACAGATCAAC-3′ (GiPT) and 5′-CAATGTCTGGCACTTTTGTCG-3′ (β-tubulin) and the 3′ primers described above. Real-time PCR was performed with the Mx3000P real-time PCR system (Stratagene) and SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA) to monitor double-stranded DNA synthesis. Each reaction mixture (final volume, 25 μl) contained 12.5 μl of 2× SYBR green PCR Master Mix (Applied Biosystems), 1 μl of the appropriate cDNA, 1 μl of the gene-specific forward primer, and 1 μl of the gene-specific reverse primer (final concentration of each primer, 0.1 μM). The PCR amplification protocol was 95°C for 10 min, followed by 60 cycles of 95°C for 30 s, 54°C for 30 s, and 72°C for 30 s. Product size and purity were confirmed by both melting-curve analysis (Mx3000P real-time PCR instrument software, version 2.0) and gel electrophoresis. The estimates of transcription levels for each treatment described below are averages of three independent PCR runs.
RESULTS
Root growth and C uptake.
Root growth increased significantly when the P level in the liquid medium was high (Table 1 and Fig. 1A). The difference between high and low P levels was greatest ∼50 days after medium addition. At the high P level, root biomass decreased slightly after 50 days. Reduced C levels in the solid medium decreased the root biomass. When the C level was low, the P level had little or no impact (Fig. 1A), indicating that root growth was C limited at the low C level and possibly P limited at the high C level.
TABLE 1.
Results of analysis of variance
| Parameter | Analysis of variance (P value)a
|
||||||
|---|---|---|---|---|---|---|---|
| Time effect
|
P availability effect
|
Time-P interaction
|
C effect (expt 2)b | ||||
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | Expt 1 | Expt 2 | ||
| Root dry mass (Fig. 1A) | 0.28 | <0.05 | 0.09 | <0.01 | 0.11 | 0.61 | <0.001 |
| Root C uptake (Fig. 1B) | <0.05 | 0.06 | 0.07 | <0.05 | <0.01 | <0.05 | 0.28 |
| C flow to the AM fungus (Fig. 2) | 0.22 | <0.01 | 0.25 | <0.05 | <0.05 | <0.001 | 0.23 |
| NLFA/phospholipid ratio, intraradical (Fig. 3A) | <0.01 | <0.001 | <0.05 | <0.05 | <0.01 | 0.84 | 0.16 |
| NLFA/phospholipid ratio, extraradical (Fig. 3B) | <0.01 | <0.01 | <0.05 | 0.18 | 0.77 | 0.69 | 0.38 |
| GiPT transcription levels (Fig. 4) | 0.31 | <0.01 | <0.01 | <0.05 | 0.76 | 0.61 | 0.82 |
For both experiments the results were analyzed using a two-way analysis of variance, including three harvests and two different P treatments.
The results of experiment 2 were also analyzed using a three-way analysis of variance, including the last two harvests, two P treatments, and two C treatments. P values from the two-way analysis of variance are shown for time and P effects, but for the C effect the P values are from a three-way analysis of variance.
FIG. 1.
Root dry mass (A) and C uptake (B) influenced by P availability and time in monoxenic D. carota cultures colonized with an AM fungus. The error bars indicate standard errors (n = 4). The different types of lines indicate the results of two separate experiments in which similar setups were used. Solid symbols indicate high P availability, and open symbols indicate low P availability. Circles indicate normal C availability, and triangles indicate reduced C availability.
The C uptake in roots (measured by determining the excess 13C content) was greatest for the high P level for the 21- to 33-day harvests and greatest at the low P level for the 75- to 100-day harvests (Fig. 1B). Thus, there were significant interactions between the time of harvest and the P level (Table 1). The availability of C did not significantly influence C uptake.
Hyphal growth and P depletion.
After 50 days, mycelial growth stopped if the P level was high. After this the amount of fungal biomass was constant or decreased slightly. Only in the second experiment was the amount of the hyphal biomass significantly greater for the high-P treatment (P < 0.001). At the ∼50-day harvests, the amounts of mycelial biomass were greatest for the high P level. The amounts of mycelial biomass were 5.6 and 7.7 mg (dry weight) for high P levels, compared to 3.5 and 2.1 mg for low P levels at this time. At the 75-day harvest, however, the amounts of biomass present for the low P level and for the high P level were not significantly different (4.4 mg at high P and 4.8 mg at low P).
For the high-P treatment after the 75-day harvest the Pi concentration was still >2 mM, compared with an initial level of 2.5 mM. In the low-P treatment, P was rapidly depleted. In the first experiment, the P concentration decreased from 25 μM to 0.54 μM by 33 days, and in the second experiment it decreased to 1.1 μM after 21 days. These levels remained constant for the remainder of each experiment.
Carbon flow to AM fungal lipids.
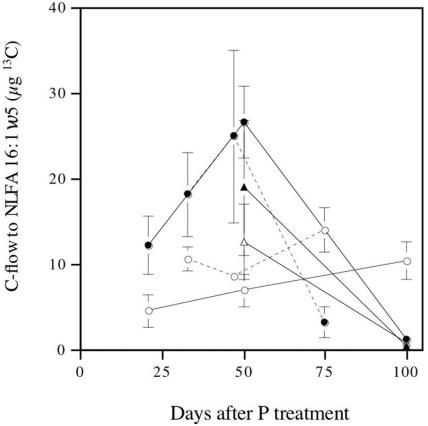
C flow to the AM fungus was calculated by determining C flow to 16:1ω5. This fatty acid is a signature for AM fungi, and the levels in noncolonized roots are very low. There were marked differences in the C allocation patterns for the different P levels. For the high P level, the C flow was high and continued to increase until ∼50 days after application (Fig. 2), at which time it decreased rapidly to a level lower than the level observed for the low P level at the 75- to 100-day harvests. The C level in the root compartment did not significantly influence C flow to the AM fungus.
FIG. 2.
C flow to the AM fungal mycelium as indicated by C flow to the signature NLFA 16:1ω5 estimated both in extraradical mycelium and in roots. The error bars indicate standard errors (n = 4). The different types of lines indicate the results of two separate experiments in which similar setups were used. Solid symbols indicate high P availability, and open symbols indicate low P availability. Circles indicate normal C availability, and triangles indicate reduced C availability.
Relative allocation to storage.
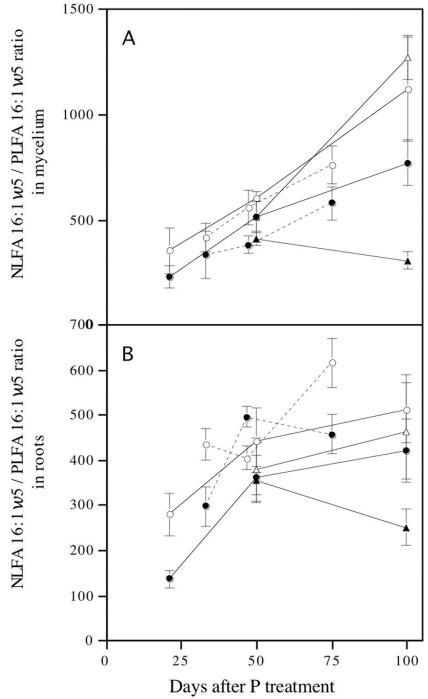
The hyphal neutral lipid-to-phospholipid ratio was determined from the 16:1ω5 fatty acid levels. In all cases the lower P levels resulted in the highest NLFA/PLFA ratio, indicating that there was more relative allocation to storage when P levels were low (Fig. 3). The results were similar for both intraradical and extraradical mycelia (measured in root samples). In contrast to the P level, a high C level resulted in the greatest relative allocation to storage, showing the significance of this parameter as a measure of C storage.
FIG. 3.
Neutral lipid-to-phospholipid ratios in intraradical (A) and extraradical (B) mycelia of G. intraradices in monoxenic cultures as estimated by use of the signature fatty acid 16:1ω5. The error bars indicate standard errors (n = 4). The different types of lines indicate the results of two separate experiments in which similar setups were used. Solid symbols indicate high P availability, and open symbols indicate low P availability. Circles indicate normal C availability, and triangles indicate reduced C availability.
GiPT transcription levels.
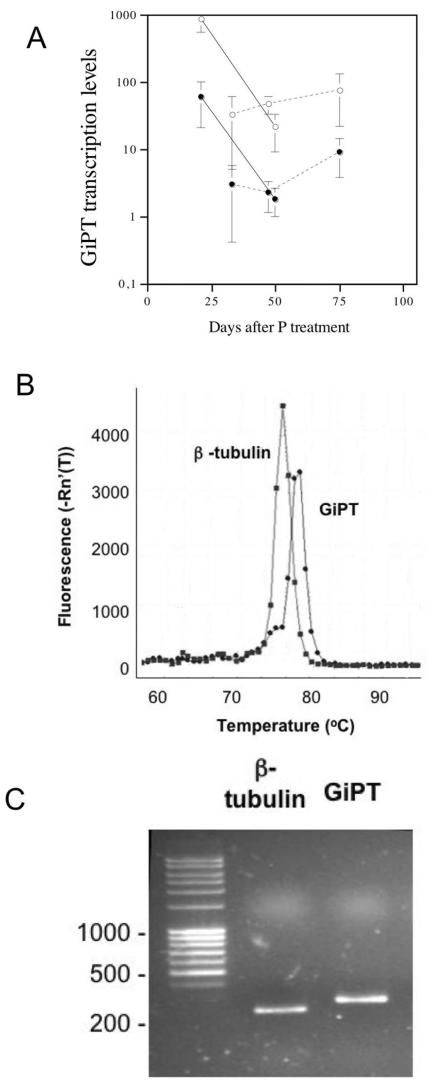
Transcript levels of GiPT were determined for extraradical hyphae collected from the liquid medium. A melting-curve analysis of the GiPT and β-tubulin sequences showed that single products were synthesized with each primer set and that there was no primer dimer contamination (Fig. 4B). Gel electrophoresis of the GiPT and β-tubulin gene sequences confirmed the sizes and purity of the amplified products (Fig. 4C).
FIG. 4.
(A) GiPT transcription levels as determined by quantitative PCR in the two experiments. The different types of lines indicate the results of two separate experiments in which similar setups were used. Solid symbols indicate high P availability, and open symbols indicate low P availability. The error bars indicate standard errors (n = 3). (B and C) GiPT gene induction expressed relative to induction of the β-tubulin gene, a constituently expressed gene. Dissociation curve profiles were generated (B) and gel electrophoresis of GiPT and β-tubulin PCR products (C) was performed using an Mx3000P real-time PCR system and SYBR green chemistry. The dissociation curves (temperature versus fluorescence) show that there is a single dissociation peak for each gene, which indicates that there was no primer dimer formation in the PCRs. Product quality and size were confirmed by gel electrophoresis.
Transcript levels were higher for the low P level than for the high P level (Table 1 and Fig. 4A). In the first experiment, transcript levels increased over time even though the Pi in the external medium was depleted by the day 75 harvest. In the second experiment transcript levels were measured at 21 days, when the fungus was sporulating less than it was sporulating at the later times. GiPT transcript levels were very high and decreased with time for both levels of P. A comparison of Fig. 2 and 4A shows that GiPT induction was not inhibited by reduced C flow.
DISCUSSION
AM fungal colonization of host plants and the associated growth of the soil hyphal network are inhibited at high soil P levels (21, 24). The mechanism responsible for this inhibition is that high plant P levels reduce C allocation to AM fungi (24, 31). By applying P to plant shoots, Sanders (31) demonstrated that plant P, and not soil P, inhibited AM formation. In monoxenic systems similar to the one evaluated in this study, there is an inverse correlation between root P levels and C allocation to the AM fungus (24). However, the relationship was not exact, and the variation between replicates was great even though the external P levels were quite different. We found that the long-term, indirect effect of the available high P levels on fungal growth is inhibitory, which is consistent with previous reports (21). However, the initial, short-term effect of increased levels of environmental P results in increased C flow to the fungus. Only after several weeks of symbiotic function does the high level of available P reduce the C flow to the fungus. These results help explain the variation observed in previous studies (24). In our study it was clear that the roots were P limited with the normal C treatment, while with reduced C levels the roots were C limited. Accordingly, the P treatment did not have any effect on root growth in this case, and root C uptake was also similar. The same was true for the C flow to the fungus, indicating that there is a very direct link between root C availability and C nutrition of the fungus. Monoxenic root cultures have been used in a large number of studies to evaluate the nutrition of AM fungi. At present, such cultures are the only way to study these fungi without interactions with other microorganisms, since AM fungi cannot be cultivated without the host roots (10). The experimental setup described here is similar to a situation in which unlimited C is not available to whole plants, which is common in areas where mineral nutrients are readily available (27). Therefore, by using monoxenic cultures, we can learn much about the growth of symbiotic fungi and how this growth depends on the interaction with the plant host. At the same time, the cultures reveal little about how the plants respond to symbiosis and nutrient treatments since root cultures do not have shoots.
The placement of energy-capturing structures in plants is critical, since roots allocate their resources to locations where they have the best opportunity to obtain a limiting nutrient (8). AM fungi may have higher C gain at locations in which roots are in direct contact with enriched medium. Thus, AM fungal proliferation could occur as a response to P-enriched patches (12) in a manner similar to the manner observed for organic nutrients (29, 34). Later, when P levels in plants become saturated, the overall C allocation to the AM fungus decreases. The direct responses of AM fungal mycelia seem to be limited unless roots are also present (24). The mycelia may not proliferate much in response to a P-enriched patch unless the plant allocates resources specifically for this patch. C supply is linked to P supply indirectly through the plant, which could explain why low levels of P fertilization may stimulate AM colonization (21).
The Pi transporter GiPT is expressed in external hyphae of G. intraradices and is not associated with P movement at the mycorrhizal interface, and its expression increases when mycorrhizal systems are grown with a Pi deficit (13, 17). Thus, this transporter may be responsible for P uptake by external hyphae from the environment (13). If P is not available in the nutrient medium, then the level of expression of GiPT is low (17). We found that GiPT expression increased when there was a P deficiency in the external hyphae of G. intraradices. However, when we harvested hyphae early (21 days), we found that expression was time dependent. The hyphae harvested at this time expressed GiPT at a higher level than hyphae at any other later time expressed GiPT. The higher proportion of absorbing hyphae at the day 21 harvest than of runner hyphae and spores indicates that these hyphae are particularly active in P uptake. These results stress the importance of hyphal age in studies of GiPT. We also found that GiPT transcription levels were relatively high even after the Pi in the nutrient medium was exhausted. Thus, in some cases the fungus remains capable of taking up Pi from the environment even in the absence of available P. This pattern might mean that the fungus needs to be able to quickly acquire transiently available Pi from the environment, e.g., when root or fungal phosphatase activity releases Pi from an organic P source. We found no correlation between C flow to the fungus and GiPT transcription level, which means that a good C supply is not essential for the induction of GiPT. Hence, while C nutrition and P nutrition were strongly linked between the plant and the fungus, in the fungus C nutrition and P nutrition had no detectable relationship in these experiments.
Carbon transferred from the host plant to the colonizing AM fungus is transformed from simple sugars into lipids in the intraradical mycelium (28). The neutral lipids are translocated in lipid bodies to the extraradical mycelium, and 16:1ω5 is a major component of these lipids (6, 24), although carbohydrates also are translocated in the mycelia (4). Lipids in the extraradical mycelia either are used for metabolism (e.g., through the glyoxylate cycle [16]) or are stored in spores formed by the mycelia. At the same time that the short-term C flow is studied with 13C labeling, long-term allocation strategies within the mycelia can be evaluated by estimating the ratio of the neutral lipids to the phospholipids. The phospholipids occur primarily in mycelial structures, while spores contain neutral lipids (21, 23). The neutral lipid-to-phospholipid ratio in AM fungi thus indicates the relative allocation to storage. An effect similar to the effect that we observed (i.e., lower relative allocation to storage with a high nutrient level) has been observed previously in response to P and to other nutrient sources (12, 29). Thus, the AM fungus has a strategy to increase the C allocated to reserves and resistant propagules in mycelia in environments with low nutrient levels. The fact that the neutral lipid-to-phospholipid ratio increased storage and not metabolically active strategies also is consistent with the increase in this ratio over time. As the mycelium ages, a larger proportion of the biomass is represented by persistent storage structures.
The AM fungal responses also can be viewed from the perspective of interactions between species of fungi or plants. Different AM fungi compete for the plant supply of C, and plant interactions and community formation are influenced by the AM fungal community (36). Reduced C flow should reduce AM fungal abundance, so it is to the fungus's advantage to provide P in a manner that leads the plant to allocate C to a particular mycelial patch, especially since different AM fungi colonize different parts of the root system under field conditions. A patch response benefits the fungus present in the patch, but plant-sufficient P conditions reduce the C supply to all fungi equally, not just those responsible for increased P availability. This could explain the apparent contradiction that a fungus provides a resource that, in the end, reduces the C supply. Overall community fertilization reduces the size of the AM fungal community and could result in a shift in species composition. In natural habitats nutrient sources occur as patches when an organism decomposes. In these situations, the fungi present benefit from a nutrient patch only if the plant allocates C in response to the nutrient (22).
The time-dependent outcome of symbiotic relationships that we observed can explain many variations in mycorrhizal interactions. Usually, mycorrhiza has a positive effect on plant growth; however, parasitic behavior of symbiotic microorganisms also may occur. The beneficial effects of the fungi must be viewed in a context in which several fungal individuals are competing for limiting resources. Our results are consistent with the hypothesis that fungi benefit from patches of nutrients. In general, P fertilization reduces fungal performance, and it is difficult to explain why fungi support plants with P if this support reduces fungal fitness. The fact that the plant allocates C to patches rich in P means that plants allocate C to parts of the root colonized by an efficient fungus. In the end, P enrichment reduces the fungal benefit, but this enrichment is unlikely to occur in most natural habitats. Thus, our results support the hypothesis that AM fungi benefit from supplying P to plants.
Acknowledgments
This work was supported by The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (Formas), as well as by the Crafoord Foundation.
REFERENCES
- 1.Abbott, L. K., A. D. Robson, and G. De Boer. 1984. The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol. 97:437-446. [Google Scholar]
- 2.Bååth, E., and J. Spokes. 1989. The effect of added nitrogen and phosphorus on mycorrhizal growth response and infection in Allium schoenoprasum. Can. J. Bot. 67:3227-3232. [Google Scholar]
- 3.Bago, B., C. Azcon-Aguilar, A. Goulet, and Y. Piché. 1998. Branched absorbing structures (BAS): a feature of the extraradical mycelium of symbiotic arbuscular mycorrhizal fungi. New Phytol. 139:375-388. [Google Scholar]
- 4.Bago, B., P. E. Pfeffer, J. Abubaker, J. Jun, J. W. Allen, J. Brouillette, D. D. Douds, P. J. Lammers, and Y. Shachar-Hill. 2003. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrates as well as lipid. Plant Physiol. 131:1496-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bago, B., P. E. Pfeffer, and Y. Shachar-Hill. 2000. Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol. 124:949-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bago, B., W. Zipfel, R. M. Williams, J. Jun, R. Arreola, P. J. Lammers, P. E. Pfeffer, and Y. Shachar-Hill. 2002. Translocation and utilization of fungal storage lipid in the arbuscular mycorrhizal symbiosis. Plant Physiol. 128:108-124. [PMC free article] [PubMed] [Google Scholar]
- 7.Bécard, G., and J. A. Fortin. 1988. Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 108:211-218. [DOI] [PubMed] [Google Scholar]
- 8.De Kroon, H., and M. J. Hutchings. 1995. Morphological plasticity in clonal plants: the foraging concept reconsidered. J. Ecol. 83:143-152. [Google Scholar]
- 9.Douds, D. D., Jr., C. R. Johnson, and K. E. Koch. 1988. Carbon cost of the fungal symbiont relative to net leaf P accumulation in a split-root VA mycorrhizal symbiosis. Plant Physiol. 86:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortin, J. A., G. Bécard, S. Declerck, Y. Dalpé, M. St.-Arnaud, A. P. Coughlan, and Y. Piché. 2002. Arbuscular mycorrhiza on root-organ cultures. Can. J. Bot. 80:1-20. [Google Scholar]
- 11.Frostegård, Å., A. Tunlid, and E. Bååth. 1993. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59:3605-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavito, M. E., and P. A. Olsson. 2003. Allocation of plant carbon to foraging and storage in arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 45:181-187. [DOI] [PubMed] [Google Scholar]
- 13.Harrison, M. J., and M. L. van Buuren. 1995. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378:626-629. [DOI] [PubMed] [Google Scholar]
- 14.Ho, I., and J. M. Trappe. 1973. Translocation of 14C from Festuca plants to their endomycorrhizal fungi. Nature 224:30-31. [DOI] [PubMed] [Google Scholar]
- 15.Jasper, D. A., A. D. Robson, and L. K. Abbott. 1979. Phosphorus and formation of vesicular-arbuscular mycorrhizas. Soil Biol. Biochem. 11:501-505. [Google Scholar]
- 16.Lammers, P. J., J. Jun, J. Abubaker, R. Arreola, A. Gopalan, B. Bago, C. Hernandez-Sebastia, J. W. Allen, D. D. Douds, P. E. Pfeffer, and Y. Shachar-Hill. 2001. The glyoxylate cycle in an arbuscular mycorrhizal fungus. Carbon flux and gene expression. Plant Physiol. 127:1287-1298. [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado-Mendoza, I. E., G. R. Dewbre, and M. J. Harrison. 2001. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus, Glomus intraradices, is regulated in response to phosphate in the environment. Mol. Plant-Microbe Interact. 14:1140-1148. [DOI] [PubMed] [Google Scholar]
- 18.Menge, J. A., D. Steirle, D. J. Bagyaraj, E. L. V. Johnson, and R. T. Leonard. 1978. Phosphorus concentrations in plants responsible for inhibition of mycorrhizal infection. New Phytol. 80:575-578. [Google Scholar]
- 19.Mosse, B. 1973. Plant growth responses to vesicular-arbuscular mycorrhiza. IV. In soil given additional phosphate. New Phytol. 72:127-136. [Google Scholar]
- 20.Murphy, J., and J. P. Riley. 1962. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27:31-36. [Google Scholar]
- 21.Olsson, P. A., E. Bååth, and I. Jakobsen. 1997. Phosphorus effects on the mycelium and storage structures of an arbuscular mycorrhizal fungus as studied in the soil and roots by analysis of fatty acid signatures. Appl. Environ. Microbiol. 63:3531-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsson, P. A., I. Jakobsen, and H. Wallander. 2002. Foraging and resource allocation strategies of mycorrhizal fungi in a patchy environment. Ecol. Stud. 157:93-115. [Google Scholar]
- 23.Olsson, P. A., and A. Johansen. 2000. Lipid and fatty acid composition of hyphae and spores of arbuscular mycorrhizal fungi at different growth stages. Mycol. Res. 104:429-434. [Google Scholar]
- 24.Olsson, P. A., I. M. van Aarle, W. G. Allaway, A. E. Ashford, and H. Rouhier. 2002. Phosphorus effects on metabolic processes in monoxenic arbuscular mycorrhiza cultures. Plant Physiol. 130:1162-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsson, P. A., I. M. van Aarle, M. E. Gavito, P. Bengtson, and G. Bengtsson. 2005. 13C incorporation into signature fatty acids as an assay for carbon allocation in arbuscular mycorrhiza. Appl. Environ. Microbiol. 71:2592-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson, J. N., and I. Jakobsen. 1993. Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol. 124:481-488. [Google Scholar]
- 27.Peng, S., D. M. Eissenstat, J. H. Graham, K. Williams, and N. C. Hodge. 1993. Growth depression in mycorrhizal citrus at high-phosphorus supply—analysis of carbon cost. Plant Physiol. 101:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeffer, P. E., D. D. Douds, Jr., G. Bécard, and Y. Shachar-Hill. 1999. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol. 120:587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravnskov, S., J. Larsen, P. A. Olsson, and I. Jakobsen. 1999. Effects of various organic compounds on growth and phosphorus uptake of an arbuscular mycorrhizal fungus. New Phytol. 141:517-524. [Google Scholar]
- 30.Ruiz-Lozano, J. M., C. Collados, R. Porcel, R. Azcon, and J. M. Barea. 2002. Identification of a cDNA from the arbuscular mycorrhizal fungus Glomus intraradices that is expressed during mycorrhizal symbiosis and up-regulated by N fertilization. Mol. Plant-Microbe Interact. 15:360-367. [DOI] [PubMed] [Google Scholar]
- 31.Sanders, F. E. 1975. The effect of foliar-applied phosphate on the mycorrhizal infections of onion roots, p. 261-276. In F. E. Sanders, B. Mosse, and P. B. Tinker (ed.), Endomycorrhizas. Academic Press, London, United Kingdom.
- 32.Smith, S. E., and D. J. Read. 1997. Mycorrhizal symbiosis, 2nd ed. Academic Press, San Diego, Calif.
- 33.Smith, S. E., F. A. Smith, and I. Jakobsen. 2003. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 133:16-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St. John, T. V., D. C. Coleman, and C. P. P. Reid. 1983. Association of vesicular-arbuscular mycorrhizal hyphae with soil organic particles. Ecology 64:957-959. [Google Scholar]
- 35.Van Aarle, I. M., and P. A. Olsson. 2003. Fungal lipid accumulation and development of mycelial structures by two arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 69:6762-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Heijden, M. G. A., J. N. Klironomos, M. Ursic, P. Moutoglis, R. Streitwolf-Engel, T. Boller, A. Wiemken, and I. R. Sanders. 1998. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69-72. [Google Scholar]