Abstract
Phytochromes (phy) A and B provide higher plants the ability to perceive divergent light signals. phyB mediates red/far-red light reversible, low fluence responses (LFR). phyA mediates both very-low-fluence responses (VLFR), which saturate with single or infrequent light pulses of very low fluence, and high irradiance responses (HIR), which require sustained activation with far-red light. We investigated whether VLFR, LFR, and HIR are genetically coregulated. The Arabidopsis enhanced very-low-fluence response1 mutant, obtained in a novel screening under hourly far-red light pulses, showed enhanced VLFR of hypocotyl growth inhibition, cotyledon unfolding, blocking of greening, and anthocyanin synthesis. However, eve1 showed reduced LFR and HIR. eve1 was found allelic to the brassinosteroid biosynthesis mutant dim/dwarf1. The analysis of both the brassinosteroid mutant det2 in the Columbia background (where VLFR are repressed) and the phyA eve1 double mutant indicates that the negative effect of brassinosteroid mutations on LFR requires phyA signaling in the VLFR mode but not the expression of the VLFR. Under sunlight, hypocotyl growth of eve1 showed little difference with the wild type but failed to respond to canopy shadelight. We propose that the opposite regulation of VLFR versus LFR and HIR could be part of a context-dependent mechanism of adjustment of sensitivity to light signals.
Light perceived by phytochromes strongly affects growth and development throughout the life cycle of plants. The relevant light signals are widely divergent in different developmental contexts as illustrated by the following examples. First, whereas a brief exposure to light is often enough to promote the germination of weed seeds during soil tillage (Scopel et al., 1991), prolonged exposure to light is required to achieve full seedling de-etiolation. Second, stem growth inhibition is initiated by seedling emergence under high as well as under low red light (R) to far-red light (FR) ratios (Yanovsky et al., 1995; Smith et al., 1997). However, this R/FR ratio-compensated light control of axis growth (i.e. regulation buffered against changes in R/FR) is lost during the de-etiolation process itself and plants become competent to respond to reductions in R/FR ratio caused by vegetation canopies (Holmes et al., 1982). Third, de-etiolation is partially buffered against the different photoperiods that the seedling can face according to the date and place (latitude) of emergence from the soil (Mazzella and Casal, 2001). However, photoperiod is a key signal controlling the timing of flowering once the plant has surpassed the juvenile phase of development.
The wide array of light signals that phytochromes can perceive has been conceptualized as three modes of action (for review, see Casal et al., 1998). The very-low fluence response (VLFR) mediated by phytochrome A (phyA) is induced by radiation between 300 and 780 nm (Botto et al., l996; Shinomura et al., 1996). Brief light exposures are enough (although in some cases these exposures have to be periodically repeated to show a detectable effect; Casal et al., 2000). The low-fluence response (LFR) mediated by phytochrome B (phyB; and to a lesser extent phytochromes D, E, and probably C) is induced by R and not by FR (McCormac et al., 1993; Aukerman et al., 1997; Mazzella et al., 1997; Devlin et al., 1998). Actually, FR is able to revert the Pfr of phyB established by R to physiologically irrelevant levels. This results in the classical R/FR reversibility of LFR. The high-irradiance responses (HIR) mediated by phyA require sustained excitation with FR (Casal et al., 2000). Thus, light control of seed germination in many weeds is dominated by the VLFR component, de-etiolation under dense or open canopies is respectively dominated by the HIR or LFR components, the response to FR back-reflected by neighbors is dominated by the LFR, etc.
Adequate responses to the light environment require the correct hierarchy of these modes of action in each context, but we are relatively ignorant of the mechanisms that regulate such hierarchy. To identify elements of these mechanisms, we designed a protocol to search for mutants with enhanced VLFR during de-etiolation and investigated LFR and HIR in these genetic variants.
RESULTS
Isolation of the eve1 Mutant
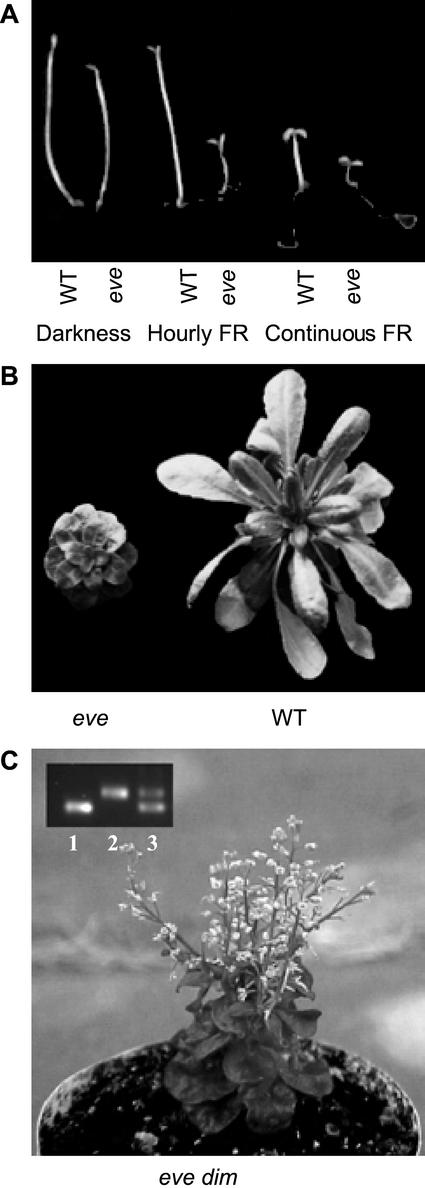
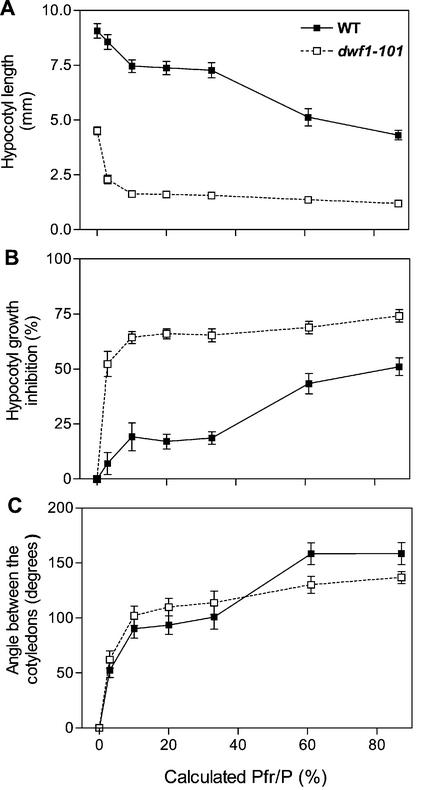
The eve1 (enhanced very-low-fluence responses 1) mutant was identified in a screening of M2 seed of Arabidopsis ecotype Landsberg erecta by its short hypocotyl and opened cotyledons under hourly pulses of FR (Fig. 1A). The hypocotyl was already shorter than the wild type (WT) in darkness (Fig. 2A) but the VLFR (i.e. the first phase of the response to light pulses providing different Pfr/P) was significantly enhanced in eve1. This enhanced VLFR of hypocotyl growth was obvious both when length was expressed relative to the dark controls (P < 0.001; Fig. 2B), and when length was expressed in absolute terms (length reduction caused by FR pulses providing a calculated Pfr/P = 10% compared with darkness: WT = 1.2 mm; eve1 = 3.1 mm; P < 0.05; Fig. 2A). The cotyledons of eve1 seedlings grown in darkness remained fully closed (Fig. 1A). Although the effect was not as dramatic as in the case of hypocotyl growth, the VLFR of cotyledon unfolding was also enhanced in eve1. The plateau reached by the VLFR (Pfr/P between 10% and 33%) was significantly higher in eve1 than in the WT (Fig. 2C, P < 0.05).
Figure 1.
Phenotype of eve1 seedlings (A) and adult plants (B) and of the F1 generation between eve1 and dim (C). In C, inset, a PCR marker for the dim (dwf1-2) allele was used in seedlings homozygous for eve1 (lane 1) or dim (lane 2). The presence of the dim allele in lane 3 demonstrates that the seedling in the photograph is product of a successful cross between eve1 (mother plant) and dim.
Figure 2.
dwf1-101 (previously designated eve1) shows enhanced VLFR and reduced LFR of hypocotyl growth (A and B) and cotyledon unfolding (C). The seedlings were exposed to hourly R/FR pulses predicted to establish the calculated Pfr/P displayed in abscissas. In B, the difference between hypocotyl length in darkness and a given light condition is expressed relative to the length in darkness. Data are means ± se of 18 replicate boxes.
The F1 generation of crosses between the WT Landsberg erecta and eve1 was similar to the WT in darkness and under hourly FR pulses (data not shown). Under pulsed FR the F2 generation showed a 3:1 segregation (22 seedlings with the eve1 phenotype in 87 F2 seedlings; χ2 = 3.6 10−5; P > 0.99). The adult phenotype of eve1 showed small rosettes and short stature (Fig. 1B). Flowering time under greenhouse conditions was normal (leaves at flowering ± se: WT = 13.1 ± 0.5; eve1 = 13.3 ± 0.9). The short hypocotyl in darkness and the dwarf phenotype of the adult plant cosegregated in F2 populations. The angle of the cotyledons under pulsed FR was significantly higher in F2 plants that subsequently showed the eve1 compared with the WT adult phenotype (cotyledon angle, degrees: WT adult phenotype = 57 ± 8; eve1 adult phenotype = 123 ± 7; P < 0.0005). Thus, the adult phenotype cosegregates with the enhanced VLFR (as the cotyledons do not unfold in darkness, all the difference under pulsed FR is because of the VLFR). This indicates that all the observed features were caused by the same locus.
eve1 Is Allelic to dwf1/dim Mutants
The F2 of eve1 Landsberg erecta × WT Columbia was used to map the mutant to the upper arm of chromosome 3, 20.4 cM apart from the marker nga172. The dwf1/dim mutants map in the vicinity of this location and also show a dwarf adult phenotype and reduced hypocotyl growth (Takahashi et al., 1995; Klahre et al., 1998; Choe et al., 1999). The eve1 mutant failed to complement dim/dfw1-2 (Fig. 1C), whereas the F1 generation of crosses between the WT and eve1 or dim showed a WT phenotype. Thus, eve1 is allelic to dwf1/dim and was renamed dwf1-101.
Reduced LFR in dwf1-101
The slope of the LFR of hypocotyl growth was reduced in dwf1-101 (percent inhibition/percent calculated Pfr/P, between 33% and 87%: WT = 0.60 ± 0.1; dwf1-101 = 0.16 ± 0.07; P < 0.001; Fig. 2B). In dwf1-101, hypocotyl growth inhibition under continuous FR (83% ± 1%) was even stronger than the maximum reached under LFR conditions (74% ± 1%; P < 0.001), indicating there was room for a significantly stronger hypocotyl response. The dwf1-101 mutation elevated the plateau of the VLFR of cotyledon unfolding but decreased the plateau of the LFR (P < 0.05, Fig. 2C). Thus, dwf1-101 showed enhanced VLFR but reduced LFR.
Reduced HIR in dwf1-101
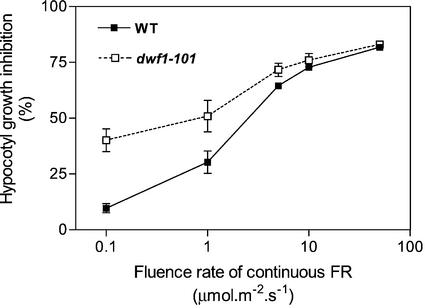
One of the distinctive features of the HIR of phyA is its strong fluence-rate dependency (Fig. 3). The largest difference between dwf1-101 and the WT was observed at the lowest fluence rate of continuous FR tested here and gradually decreased at higher fluence rates. A mutant enhancing HIR should present a steeper fluence rate-response relationship, and a mutant without effects on HIR should produce parallel curves. The reduced slope observed for hypocotyl growth inhibition suggests a negative effect of the dwf1-101 mutation on HIR. The angle between cotyledons was higher in dwf1-101 only for the lowest fluence rate tested (degrees, WT = 8 ± 3; dwf1-101= 42 ± 10; P < 0.01).
Figure 3.
Reduced slope of the hypocotyl growth inhibition response to continuous FR in dwf1-101. Hypocotyl length in dark controls: WT = 11.4 ± 0.3; dwf1-101 = 4.6 ± 0.3. Data are means ± se of nine replicate boxes.
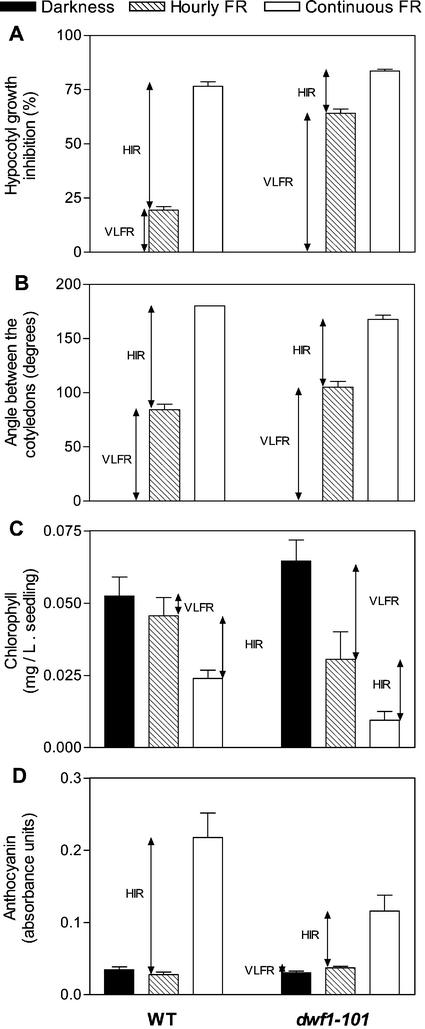
The HIR is the portion of the effect of continuous FR that cannot be mimicked by hourly pulses of the same spectral composition providing the same total fluence. Thus, to measure the HIR we compared several responses in seedlings exposed to pulsed or continuous FR (Fig. 4). The dwf1-101 mutant showed enhanced VLFR of hypocotyl growth inhibition (P < 0.005), cotyledon unfolding (P < 0.05), blocking of greening after transfer to white light (P < 0.005), and anthocyanin levels (P < 0.07). The HIR was significantly reduced in each case (P < 0.01), except for blocking of greening (P > 0.5). Compared with the WT, anthocyanin levels were reduced in dwf1-101 seedlings grown under continuous FR (P < 0.05). Thus, dwf1-101 showed enhanced VLFR but reduced HIR.
Figure 4.
dwf1-101 shows enhanced VLFR and reduced HIR of hypocotyl growth inhibition (A), cotyledon unfolding (B), blocking of greening (C), and anthocyanin synthesis (D). The seedlings were grown in darkness, hourly pulses of FR or continuous FR (at equal total fluence) before measurements or transfer to white light (chlorophyll experiments). Hypocotyl length in dark controls: WT = 8.4 ± 0.2; dwf1-101 = 4.3 ± 0.2. Data are means ± se of 12 (A and B), nine (C), or six (D) replicate boxes.
Reduced LFR and HIR in det2
To investigate whether the enhanced VLFR is a feature common to other mutants affecting brassinosteroid levels, we analyzed the response to hourly FR in a previously isolated dwf1/dim allele (Takahashi et al., 1995; Klahre et al., 1998) and in det2 (Li et al., 1996) both in the Columbia background. WT seedlings showed no significant cotyledon unfolding under hourly FR and this is consistent with the deficient VLFR observed in the presence of Columbia alleles of the VLF1 and VLF2 loci (Yanovsky et al., 1997). det2 also failed to unfold the cotyledons under hourly FR (Fig. 5A), but dim did show enhanced unfolding (angle between the cotyledons, degrees: WT = 0 ±0; dim = 70 ± 10; P < 0.01). This suggests that for the VLFR phenotype, Columbia alleles of VLF1 and VLF2 loci are epistatic to a putative effect of det2 but not to the effect of dim. In our hands dim had a stronger dark phenotype (shorter hypocotyls) than det2 and this correlates with the relative impacts of these mutants on VLFR.
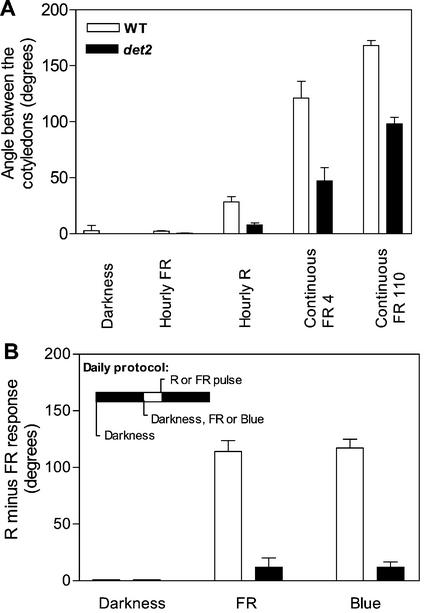
Figure 5.
Reduced HIR and LFR in the brassinosteroid mutant det2. In A, the seedlings were grown in darkness or under hourly FR, hourly R, or continuous FR (4 or 100 μmol m−2 s−1) In B, the seedlings were daily exposed to a R versus a FR pulse given in factorial combination with 3 h of FR, 3 h of blue light, or darkness. The LFR (angle between the cotyledons for seedlings receiving a R pulse minus angle between the cotyledons for the seedlings receiving a FR pulse) is indicated for the 3 h of FR, 3 h of blue light, or no previous light conditions. Data are means ± se of 21 replicate boxes.
The lack of VLFR in det2 Columbia offered the possibility to test whether the negative effects of dwf1-101 on LFR and HIR can be observed in a genetic background where VLFR are not expressed. Cotyledon unfolding of seedlings exposed to hourly pulses of R or to continuous FR was reduced by the det2 mutation (P < 0.01; Fig. 5A). A similar pattern was observed for hypocotyl growth inhibition (data not shown). This indicates that both LFR and HIR were negatively affected in det2.
The LFR was further characterized by analyzing seedlings exposed daily to a R or a FR pulse (5 min) preceded by a blue light, FR or no photoperiod (3 h) because blue or FR pretreatments enhance the LFR mediated by phyB (Casal and Boccalandro, 1995). The difference in angle between cotyledons caused by terminal R versus FR pulses (i.e. the LFR) was negligible in the absence of the 3-h blue or FR exposure and was amplified by blue or FR photoperiods. The LFR response was reduced by the det2 mutation (P < 0.001; Fig. 5B).
The Enhanced VLFR and the Reduced LFR of dwf1-101 Depend on phyA
The dwf1-101 mutant was crossed by the phyA-201 null allele. The F2 generation showed approximately a quarter of seedlings (23 of 97; χ2 = 0.05; P > 0.8) with fully folded cotyledons (angle below 10 degrees) under continuous FR. This is similar to the proportion observed in F2 of crosses between WT and phyA. Approximately a quarter of the seedlings with closed cotyledons (5 of 23; χ2 = 0.15; P > 0.7) showed a hypocotyl shorter than expected for a phyA mutant but longer than expected for a seedling bearing phyA. Because the dwf1-101 mutant shows intermediate hypocotyl length even in darkness, these seedlings were selected as homozygous phyA dwf1-101 double mutants.
In the WT background, the VLFR of hypocotyl growth depends on the activity of phyA (Yanovsky et al., 1997; Fig. 6A). The dwf1-101 mutant showed enhanced VLFR but this effect was abolished in the phyA background (note reduced hypocotyl growth inhibition in the phyA dwf1-101 mutant for calculated Pfr/P at or below 10%; Fig. 6A). Noteworthy, even in the phyA background the dwf1-101 mutation enhanced the response for Pfr/P = 20%. The photoreceptor mediating the latter residual effect remains to be elucidated. The slope of the LFR (Pfr/P higher than 30%) was reduced by the dwf1-101 mutation in the WT background but not in the phyA background (Fig. 6A).
Figure 6.
Seedling phenotype of the phyA dwf1-101 double mutant (A) and adult phenotype of the phyA dwf1-101 and phyB dwf1-101 double mutants (B). In A, data are means ± se of six replicates. Hypocotyl length in dark controls: WT = 9.7 ± 0.6; dwf1-101 = 4.8 ± 0.1; phyA = 11.8 ± 0.5; phyA dwf1-101 = 5.0 ± 0.3.
Cotyledon unfolding showed a similar pattern. Enhanced VLFR in the WT but not in the phyA background (angle between the cotyledons, degrees, for Pfr/P = 3%: WT = 5 ± 2; dwf1-101 = 46 ± 5; P < 0.0001; phyA = 0 ± 0; phyA dwf1-101 = 0 ± 0). Reduced LFR in the WT but not in the phyA background (Δ angle between the cotyledons between 33% and 61%, degrees: WT = 77 ± 7; dwf1-101 = 39 ± 11; P < 0.01; phyA = 160 ± 6; phyA dwf1-101 = 159 ± 8; P > 0.9).
The adult phenotype of the phyA dwf1-101 and phyB dwf1-101 double mutants was similar to the single dwf1-101 mutant (Fig. 6B), indicating that neither phyA nor phyB are necessary for the dwf1-101 effect at this stage.
Inmunologically Detectable Levels of phyA Are Normal in dwf1-101
Because both the enhanced VLFR and the reduced LFR observed in dwf1-101 depend on phyA (Fig. 6A), we investigated whether these effects were the result of alterations in phyA levels. A monoclonal antibody specific for phyA was used for this purpose. No significant differences were observed between WT and dwf1-101 etiolated seedlings (Fig. 7).
Figure 7.
Normal levels of inmunochemically detectable phyA in dwf1-101. The seedlings were grown in darkness for 4 d after the R pulse given for the induction of seed germination.
The dwf1-101 Mutant Fails to Respond to Canopy Shadelight
Under laboratory conditions, the dwf1-101 mutant showed enhanced VLFR and reduced LFR and HIR. To investigate the consequences of this altered photobiological behavior in terms of perception of natural light signals, the seedlings were grown under sunlight or canopy shadelight. The R/FR ratio beneath the canopy was 0.8 compared with 1.1 outside the canopy, and radiation within the visible range was reduced to a 14%. Despite the enhanced VLFR of the dwf1-101 mutant, hypocotyl length was only slightly shorter than the WT under full sunlight conditions (Fig. 8). However, whereas the WT was taller under canopy shadelight than under full sunlight (a typical “shade-avoidance” response) the dwf1-101 mutant failed to respond to the presence of a dense canopy (Fig. 8).
Figure 8.
The dwf1-101 mutant fails to respond to the presence of a shading canopy. The seedlings were grown in pots under sunlight or canopy shade light. The length of the hypocotyl (mm) in dark controls (grown near the other seedlings inside dark boxes) was: WT = 11.4 ± 0.7; dwf1-101 = 8.1 ± 0.4. Data are means ± se of 12 replicate plants.
DISCUSSION
In systems like Drosophila melanogaster, re-isolation of mutants previously identified by means of a different protocol has provided useful insight into the complexity of regulatory interactions between pathways (e.g. Price et al., 1997). This is also beginning to be the case in Arabidopsis (e.g. Beaudoin et al., 2000). Here we have isolated eve1, a new allele of the dim/dwf1 mutants that are deficient in brassinosteroid biosynthesis (Klahre et al., 1998; Choe et al., 1999). dwf1-101 showed reduced hypocotyl length, increased cotyledon unfolding, increased anthocyanin levels, and blocking of greening under hourly FR. In darkness, hypocotyl length was reduced by the dwf1-101 mutation but to a lesser extent than under pulsed FR. Cotyledon unfolding, anthocyanin synthesis and greening defects were not observed in dark controls. The phyA dwf1-101 mutant failed to respond to pulsed FR indicating that in the WT, DWF1 is involved in the repression of VLFR mediated by phyA (Fig. 9). Therefore, we propose a role of DWF1 in down-regulation of VLFR.
Figure 9.
Model based on genetic and physiological data showing the proposed role of DWF1 in the phytochrome signaling network.
The bas1-D mutant (Neff et al., 1999), which shows reduced brassinosteroid levels presumably because of enhanced steroid hormone inactivation by hydroxylation, exhibits hypersensitivity of hypocotyl growth inhibition to R, FR, and blue light. This effect of bas1-D is not reduced by a phyB mutation under R or by a cry1 mutation under blue light, but (as observed here for dwf1-101) it is abolished by the phyA mutation under FR (Neff et al., 1999). Here we show that hypersensitivity is not restricted to hypocotyl growth inhibition but is specific to the VLFR mode of phyA signaling. The VLFR is predicted to operate under continuous R, FR, or blue light, because any of these light conditions exceeds the minimum requirements of VLFR. Thus, the behavior of bas1-D (Neff et al., 1999) and dwf1-101 is consistent with a role of brassinosteroids in the repression of VLFR.
It is surprising that whereas VLFR were enhanced, LFR and HIR were partially repressed in dwf1-101 and det2 mutants. This uncovers a new role of brassinosteroids as positive regulators in the phytochrome signaling network. The latter conclusion is based on three complementary approaches to quantify LFR and HIR. After a photobiological approach, LFR and HIR were respectively calculated as the difference between either hourly R pulses or continuous FR and hourly pulses of FR. Hourly pulses of FR induce VLFR but provide neither enough Pfr to activate the LFR of phyB, nor the sustained activation required to elicit the HIR of phyA. After a genetic approach, LFR and HIR were analyzed without the interference of VLFR in the det2 mutant, where VLFR were not observed because of the Columbia background (Yanovsky et al., 1997). Physiologically, HIR could be analyzed by using a response like anthocyanin synthesis where VLFR are negligible. The three approaches consistently showed reduced LFR and/or HIR in brassinosteroid mutants.
We had previously observed that phyA and fhy1 mutants have enhanced phyB-mediated responses to R (Mazzella et al., 1997; Cerdán et al., 1999) whereas Columbia alleles of the VLF loci reduce VLFR but do not enhance phyB-mediated responses (Yanovsky et al., 1997). These observations have been interpreted as a negative regulation of phyB signaling exerted by elements of the phyA-FHY1 VLFR pathway upstream the point of action of VLF1 and VLF2 (Fig. 9). The positive effect of DWF1 and DET2 on phyB signaling required phyA signaling in the VLFR, as indicated by the similar LFR in phyA and phyA dwf1-101 mutants (Fig. 6A). The positive effect of DET2 was not abolished even in the Columbia background (Fig. 5). Thus, we propose that brassinosteroids down-regulate early steps of the VLFR signaling pathway upstream the action of VLF loci and this results in amplification of phyB-mediated signaling (Fig. 9).
Although brassinosteroid mutants also have reduced HIR, the dependence of this regulation on VLFR signaling cannot be tested by using a phyA mutant to eliminate VLFR because these mutants also lack HIR. However, we have observed that transgenic plants that overexpress phyA have enhanced VLFR and reduced HIR (J.J. Casal, S.J. Davis, M.J. Yanovsky, R.C. Clough, E.T. Jordan-Beebe, and R.D. Vierstra, unpublished data). Thus, we have tentatively included a negative link between VLFR and HIR (Fig. 9) to account for the reduced HIR in dwf1-101 and det2.
Present results indicate a role of brassinosteroids in fine tuning of phytochrome-mediated responses. Brassinosteroids would shift the sensitivity from the range of weak light signals versus darkness (experienced by seeds during soil tillage or etiolated seedlings close to the surface of the soil) to the range of modifications in R/FR ratio and irradiance caused by neighbor plants. The significance of this regulation is highlighted by the impaired responses to canopy shadelight in dwf1-101 (Fig. 8). Thus, changes in brassinosteroid levels would help to adjust plant sensitivity to different light signals according to the developmental and environmental context. Light down-regulates a small G protein, which in turn acts positively on a variant P450 that catalyzes C-2 hydroxylation in brassinosteroid biosynthesis (Kang et al., 2001). This mutual influence, where brassinosteroids regulate light responses and light regulates brassinosteroid levels, could play a key role in the dialog between environmental and endogenous cues controlling plant development.
MATERIALS AND METHODS
Plant Material
Mutagenized seed of Arabidopsis of the ecotype Landsberg erecta was purchased from Lehle Seeds (Round Rock, TX). For the mutant screening the seeds were incubated in boxes (175 × 225 mm2 and 45-mm height) containing 0.8% (w/v) agar for 3 d at 7°C before transfer to the specific protocol conditions. The WT was Landsberg erecta. The dim/dwf1-2 mutant (Takahashi et al., 1995; Klahre et al., 1998; Choe et al., 1999) was used for complementation analysis and in unreported physiological experiments (compared with the WT Columbia). The det2 mutant (Li et al., 1996) was compared with the WT Columbia in physiological experiments. Seed samples of dim and det2 were provided by the Arabidopsis Biological Resource Center (Ohio State University, Columbus). The phyA-201 (Nagatani et al., 1993) and phyB-5 (Reed et al., 1993) were used to obtain double mutants.
For laboratory experiments with eve1 (dwf1-101), det2, or dim, seeds of Arabidopsis were sown in clear plastic boxes (40 × 33 mm2 × 15-mm height) containing 3 mL of 0.8% (w/v) agar. The number of seeds per box was 15, 50, or 200 in morphological, chlorophyll, and anthocyanin experiments, respectively. The seeds were incubated in darkness at 7°C for 3 d, given a R pulse to promote seed germination, and incubated in darkness (25°C) for 24 h before light treatments. For greenhouse experiments, the seedlings were sown in pots (35-mm diameter, 7.5-mm height) containing soil. The seeds were chilled and induced to germinate as described for laboratory experiments.
Hypocotyl Growth and Cotyledon Unfolding
The seedlings were exposed either to hourly pulses of R, FR, or R plus FR mixtures (3 min, 15–40 μmol m−2 s−1; these fluence rates saturate the response to the pulses) that provided a series of calculated Pfr/P (for details of light sources, spectral distribution and Pfr/P calculations, see Casal et al., 1991; Yanovsky et al., 2000), or to continuous FR (calculated Pfr/P = 10%, fluence rates between 0.1 and 100 μmol m−2 s−1), whereas control seedlings remained in darkness. In some experiments, hourly and continuous FR were compared at equal total fluence (36 mmol m−2 h−1). To amplify the LFR mediated by phyB, in some experiments with the det2 mutant the seedlings were daily exposed to 3 h FR or blue light (40 μmol m−2 s−1) provided by fluorescent lamps in combination with a 2-mm-thick blue acrylic filter. Hypocotyl length was measured to the nearest 0.5 mm with a ruler in the 10 longest seedlings (this eliminates defective seedlings). The angle between the cotyledons was measured in the same seedlings with a protractor. Seedling data were averaged per box (one replicate) and used for statistics.
In greenhouse experiments, the pots were placed under sunlight (photoperiod 14 h), under a dense canopy of tomato plants or in complete darkness (inside a box wrapped in aluminum foil). The R/FR ratio was measured with a Skye SKR 110 sensor (Skye Instruments Ltd, Llandrindod Wells, Powys, UK). Four days after transfer to the greenhouse, the seedlings were removed from the soil and hypocotyl length was measured to the nearest 0.5 mm with a ruler.
Chlorophyll and Anthocyanin Levels
For blocking of greening experiments, 24 h after the R pulse to induce germination, the seedlings were transferred to hourly pulses (3 min, 40 μmol m−2 s−1) or continuous (2 μmol m−2 s−1) long wavelength FR (Pfr/P = 3%) provided by an incandescent lamp in combination with a water filter and an RG9 filter (Schott, Maintz, Germany), or remained in darkness. Three days later, the seedlings were transferred to continuous fluorescent white light (100 μmol m−2 s−1) for 2 d (note that in previous experiments we used 1 d and this results in different chlorophyll background levels; Yanovsky et al., 2000). The seedlings were harvested in N,N′-dimethylformamide and incubated in darkness at −20°C for at least 3 d. Absorbance was measured at 647 and 664 nm, and chlorophyll levels were calculated according to Moran (1982).
For anthocyanin experiments, the seedlings were exposed for 3 d to hourly pulses (3 min) or continuous FR (calculated Pfr/P = 10%; 36 mmol m−2 h−1) and subsequently extracted with 1 mL of 1% (w/v) HCl methanol. Measurements of A530 were corrected for chlorophyll absorption (657 nm) according to Mancinelli et al. (1991).
Immunochemical Detection of phyA and phyB
Extracts were prepared from samples harvested on ice according to Martinez-García et al. (1999). The extracts were subjected to SDS-PAGE in 1.5-mm thick, 4.5%/7.5% stacking/resolving gel (Mini Protean II, Bio-Rad, Richmond, CA). Proteins were electroblotted to nitrocellulose (0.45-μm pore size, Sigma, St Louis) following manufacturer's indications. The remaining protein-binding capacity was blocked with 5% (w/v) skim milk, 50 mm Tris-Cl, and 200 mm NaCl, pH 7.4 for 30 min at 37°C. The anti phyA monoclonal antibody 073D raised in mouse against purified phytochrome from etiolated oats was kindly provided by Dr. Peter H. Quail (University of California, Berkeley, and U.S. Department of Agriculture Plant Gene Expression Center, Albany, CA). The blots were incubated overnight at 4°C with this primary antibody at a dilution of 1:1,000. After washing, the membrane was incubated with 1:500 affinity isolated alkaline-phosphatase-conjugated antibody to mouse IgG developed in goat (Sigma). The bands were visualized by incubating the blots in 0.1 m Tris (pH 9.5), 100 mm NaCl, and 5 mm MgCl2 containing 0.165 mg ml−1 5-bromo4-chloro-3-indoyl phosphate, p-toluidine salt, and 0.33 mg ml−1 nitroblue tetrazolium (Sigma).
Mapping
A mapping population was generated by crossing the eve1 mutant in Landsberg erecta with the Columbia ecotype. DNA was isolated from 85 plants showing compact rosettes and reduced stature following the protocol described by Rogers and Bendich (1988). Markers for simple sequence length polymorphisms (Arabidopsis database Stanford University, Palo Alto, CA; http://www.Arabidopsis.org) were used to map the position of eve1.
ACKNOWLEDGMENTS
We thank Peter H. Quail for antiserum against phyA and the Arabidopsis Biological Resource Center (Ohio State University, Columbus) for seed stocks. We also thank Matías Quinn for conducting the experiments with dim, María Crepi and Pedro Gundel for technical support, and Dr R. Staneloni for helpful technical advice.
Footnotes
This work was supported by the Fondo Nacional de Ciencia y Técnica (grant no. BID 1201/OC–AR–PICT 06739), the University of Buenos Aires (grant no. TG59), and the Fundación Antorchas (grant no. A–13622/1–40).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010668.
LITERATURE CITED
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 2000;12:1103–1116. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto JF, Sánchez RA, Whitelam GC, Casal JJ. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA, Benedetto D, De Miguel LC. Light promotion of seed germination in Datura ferox is mediated by a highly stable pool of phytochrome. Photochem Photobiol. 1991;53:249–254. [Google Scholar]
- Casal JJ, Sánchez RA, Botto JF. Modes of action of phytochromes. J Exp Bot. 1998;49:127–138. [Google Scholar]
- Casal JJ, Yanovsky MJ, Luppi JP. Two photobiological pathways of phytochrome A activity, only one of which shows dominant negative suppression by phytochrome B. Photochem Photobiol. 2000;71:481–486. doi: 10.1562/0031-8655(2000)071<0481:tppopa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cerdán PD, Yanovsky MJ, Reymundo FC, Nagatani A, Staneloni RJ, Whitelam GC, Casal JJ. Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J. 1999;18:499–507. doi: 10.1046/j.1365-313x.1999.00475.x. [DOI] [PubMed] [Google Scholar]
- Choe S, Dilkes BD, Gregory BP, Ross AS, Yuan H, Noguchi T, Fujioka S, Takatsuto S, Tanaka A, Yoshida S, Tax FE, Feldmann KA. The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999;119:897–907. doi: 10.1104/pp.119.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin P, Patel SR, Whitelam GC. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1488. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MG, Beggs CJ, Jabben M, Schäfer E. Hypocotyl growth in Sinapis alba L.: the roles of light quality and quantity. Plant Cell Environ. 1982;5:45–51. [Google Scholar]
- Kang J-G, Yun J, Kim D-H, Chung K-S, Fujioka S, Kim J-I, Dae H-W, Yoshida S, Takatsuto S, Song P-S, Park C-M. Light and brassinosteroid signals are integrated via a dark-induced small G protein in etiolated seedling growth. Cell. 2001;105:625–636. doi: 10.1016/s0092-8674(01)00370-1. [DOI] [PubMed] [Google Scholar]
- Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua N-H. The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell. 1998;10:1677–1690. doi: 10.1105/tpc.10.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Mancinelli AL, Rossi F, Moroni A. Cryptochrome, phytochrome and anthocyanin production. Plant Physiol. 1991;96:1079–1085. doi: 10.1104/pp.96.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-García JF, Monte E, Quail PH. A simple, rapid and quantitative method for preparing Arabidopsis protein extracts for immunoblot analysis. Plant J. 1999;20:251–257. doi: 10.1046/j.1365-313x.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- Mazzella MA, Alconada Magliano TM, Casal JJ. Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ. 1997;20:261–267. [Google Scholar]
- Mazzella MA, Casal JJ. Interactive signalling by phytochromes and cryptochromes generates de-etiolation homeostasis in Arabidopsis thaliana. Plant Cell Environ. 2001;24:155–162. [Google Scholar]
- McCormac AC, Wagner D, Boylan MT, Quail PH, Smith H, Whitelam GC. Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome B-encoding cDNAs: evidence that phytochrome A and phytochrome B have distinct photoregulatory functions. Plant J. 1993;4:19–27. [Google Scholar]
- Moran R. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S, Chory J. BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:15316–15323. doi: 10.1073/pnas.96.26.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JV, Savenye ED, Lum D, Breitkreutz A. Dominant enhancers of Egfr in Drosophila melanogaster: genetic links between the Notch and Egfr signaling pathways. Genetics. 1997;147:1139–1153. doi: 10.1093/genetics/147.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SO, Bendich AJ. Extraction of DNA from plant tissue. In: Gelvin S, Schilperrot RA, editors. Plant Molecular Biology Manual. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–10. [Google Scholar]
- Scopel AL, Ballaré CL, Sánchez RA. Induction of extreme light sensitivity in buried weed seeds and its role in the perception of soil cultivations. Plant Cell Environ. 1991;14:501–508. [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectra for phytochrome A- and phytochrome B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H, Xu Y, Quail PH. Antagonistic but complementary actions of phytochromes A and B allow optimum seedling de-etiolation. Plant Physiol. 1997;114:637–641. doi: 10.1104/pp.114.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Gasch A, Nishizawa N, Chua N-H. The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes Dev. 1995;9:97–107. doi: 10.1101/gad.9.1.97. [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Luppi JP. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia dissect two branches of phytochrome A signalling pathways that correspond to the very-low fluence and high-irradiance responses of phytochrome. Plant J. 1997;12:659–667. doi: 10.1046/j.1365-313x.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Whitelam GC. Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 1995;18:788–794. [Google Scholar]
- Yanovsky MJ, Whitelam GC, Casal JJ. fhy3-1 retains inductive responses of phytochrome A. Plant Physiol. 2000;123:235–242. doi: 10.1104/pp.123.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]