Abstract
The solvent-tolerant strain Pseudomonas putida DOT-T1E was grown in batch fermentations in a 5-liter bioreactor in the presence and absence of 10% (vol/vol) of the organic solvent 1-decanol. The growth behavior and cellular energetics, such as the cellular ATP content and the energy charge, as well as the cell surface hydrophobicity and charge, were measured in cells growing in the presence and absence of 1-decanol. Although the cells growing in the presence of 1-decanol showed an about 10% reduced growth rate and a 48% reduced growth yield, no significant differences were measured either in the ATP and potassium contents or in the energy charge, indicating that the cells adapted completely at the levels of membrane permeability and energetics. Although the bacteria needed additional energy for adaptation to the presence of the solvent, they were able to maintain or activate electron transport phosphorylation, allowing homeostasis of the ATP level and energy charge in the presence of the solvent, at the price of a reduced growth yield. On the other hand, significantly enhanced cell hydrophobicities and more negative cell surface charges were observed in cells grown in the presence of 1-decanol. Both reactions occurred within about 10 min after the addition of the solvent and were significantly different after killing of the cells with toxic concentrations of HgCl2. This adaptation of the surface properties of the bacterium to the presence of solvents seems to be very similar to previously observed reactions on the level of lipopolysaccharides, with which bacteria adapt to environmental stresses, such as heat shock, antibiotics, or low oxygen content. The results give clear physiological indications that the process with P. putida DOT-T1E as the biocatalyst and 1-decanol as the solvent is a stable system for two-phase biotransformations that will allow the production of fine chemicals in economically sound amounts.
Solvent-tolerant bacteria such as Pseudomonas putida DOT-T1E are potential biocatalysts for use in two-phase fermentation systems for the synthesis of high-yield, economically interesting amounts of fine chemicals, as they allow the application of solvents exhibiting chemical properties needed for successful implementation of whole-cell two-phase biotransformations. In such a biotechnological process, an organic solvent phase functions as both a source and sink for toxic organic substrates and products, respectively (45), allowing continuous and easy removal of the products (20, 22, 45, 48). A second solvent phase is beneficial because it keeps the concentrations of both the substrates and the products in the aqueous phase below a level that would lead to growth inhibition or even to the death of the bacteria.
One of the highly solvent-tolerant bacteria is P. putida DOT-T1E. This bacterium has been the subject of many investigations concerning the mechanisms responsible for solvent tolerance properties (33, 37). Very recently, an extended proteomic survey was performed to identify all processes responsible for the strain's adaptation to toxic solvents such as toluene, showing that a whole cascade of mechanisms is necessary to allow the bacterium to survive in the presence of such hazardous solvents (38). Additionally, a detailed description of the selection of the ideal solvent for two-phase fermentations with this bacterium was carried out (29). The major result of this investigation was that 1-decanol seems to be the ideal solvent for this bacterium in such biotransformation processes (29, 35).
For successful application of this system—in competition with traditional chemical synthesis—the stability of the biocatalyst and high growth yields are necessary in order to guarantee high production rates. Therefore, a detailed characterization of the growth behavior (growth rates and yields) and cellular energetics of the cells when grown in the presence of a solvent is necessary. Next to the well-described changes in the membrane composition (7), surface properties (6) are also thought to be important to allow complete adaptation to solvents.
The aim of this study was to investigate the exact growth parameters, energetics, and cell surface properties of P. putida DOT-T1E in a two-liquid-phase system with 1-decanol to assess the stability and activity of this biocatalytic system for future biotechnological applications.
MATERIALS AND METHODS
Strain and chemicals.
Pseudomonas putida DOT-T1E was described previously (32). All chemicals used were reagent grade and were obtained from commercial sources.
Culture conditions.
P. putida DOT-T1E was cultivated in a mineral medium as described by Hartmans et al. (8), with sodium succinate as the carbon and energy source. Adaptation of the bacterium to the solvent was achieved by growing the cells in the presence of 10% (vol/vol) 1-decanol in overnight cultures. Cells were grown in 50-ml shaking cultures at 30°C in a horizontally shaking water bath at 180 rpm.
Preparation of energized resting cells.
Exponentially growing cells (50 ml) were harvested by centrifugation and suspended in an equal volume of potassium phosphate buffer (50 mM, pH 7.0), with 4 g/liter sodium succinate as the energy source. Experiments were started 45 min after the suspension of cells, by which time growth had stopped completely.
Fermentations.
Fermentations were carried out in a 5-liter fermenter (ISF-205; INFORS GmbH, Einsbach, Germany). Parameters such as temperature (°C), pH, CO2 output (%), O2 content (%), pO2 (%), airflow (liters/min), stirrer speed (rpm), and total weight (kg) of the fermenter were monitored online. IRIS software (INFORS Control AG, Bottmingen, Switzerland) allowed control of the measured parameters. Data were obtained and recorded with a Servomex Analyser series 1400 instrument (East Sussex, England). Standard parameters for fermentations were as follows: 30°C, pH 7.1, and 1,500 rpm. The fermenter was inoculated with an overnight culture so that a starting optical density of 0.08 at 560 nm was reached. Due to difficulties in measuring the optical density in a dispersed two-phase system, growth data were obtained via measuring the protein content of the cells (4). In the computer-controlled fermentation unit, growth was determined continuously as CO2 production. For the fermentations carried out in this study, a direct correlation between CO2 production and protein content was observed.
Cellular K+ content.
Five-milliliter samples were harvested at regular intervals before and after addition of the solvent. Separation of cells from the supernatant was carried out by rapid centrifugation (Heraeus centrifuge; 10,000 rpm, 10 min). The cell pellet was disrupted by incubation in 5% trichloroacetic acid at 90°C for 15 min, and debris was removed by centrifugation. The K+ content of the supernatant was measured by inductively coupled plasma-optical emission spectroscopy using a Spectroflame P/M spectrometer (SPECTRO Analytical Instruments, Kleve, Germany). All experiments were carried out three times, with a standard deviation of <10%.
ATP concentration.
One milliliter of cell suspension was added to 0.5 ml ice-cold 1.3 M perchloric acid (23 mM EDTA) in 2-ml sterile Eppendorf tubes. After being mixed, the cell extract was incubated for 30 min at 4°C and subsequently centrifuged at 10,000 rpm for 15 min (4°C). One milliliter of the supernatant was transferred to sterile Eppendorf tubes, and the pH was set to 7.5 by using a 0.72 M KOH (0.16 M KHCO3) solution. Again, the sample was centrifuged at 10,000 rpm for 15 min (4°C), and 0.5 ml of the supernatant was stored at −20°C for later analysis. The ATP concentration in the cells was determined by using a luciferin-luciferase bioluminescence reaction (ATP kit SL; BioThema AB, Sweden) (25). In this reaction, light is formed from free ATP and luciferin via the enzyme luciferase from fireflies and is measured in a Victor2 Wallac 1420 multilabel counter spectrophotometer (Perkin-Elmer Life Sciences GmbH, Germany). Data analysis was performed using the Wallac 1420 workstation software Wallac 1420 Manager, version 2.00 (release 8; Perkin-Elmer Life Sciences GmbH). The photometer was equipped with two dispensers that were able to pump adjustable amounts of Tris buffer and luciferin-luciferase solution into the wells of the microtiter plate just before measurement of the samples.
Energy charge.
The adenylate energy charge was measured by using an energy charge kit from BioThema AB, Sweden. Besides ATP measurement by the luciferin-luciferase reaction, the conversion of ADP to ATP by pyruvate kinase and the conversion of AMP to ADP by myokinase were necessary to determine the energy charge (24). The adenylate energy charge (EC) is given by the following equation (3):
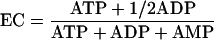 |
Characterization of bacterial cell surface properties.
Physicochemical cell surface properties of bacteria were investigated by using standard methods, as described by others (43). The electrophoretic mobility (μ) of bacterial suspensions in 10 mM KNO3 at pH 6.2 was determined in a Doppler electrophoretic light scattering analyzer (Zetasizer Nano ZS; Malvern Instruments Ltd., Malvern, Worcestershire, United Kingdom) at 100 V. The zeta potential (ζ) was approximated from the electrophoretic mobility as an indirect measure of cell surface charge according to the method of Helmholtz-von Smoluchowski (12). The isoelectric points (IEPs) of bacteria were determined from ζ-pH plots obtained by measuring μ in 10 mM HNO3-KNO3 solutions, with pHs varying between 2 and 6.5, using an MPT-2 autotitrator (Malvern Instruments Ltd., Malvern, Worcestershire, United Kingdom).
Bacterial lawns needed for contact angle (θw) measurements were prepared by collecting cell suspensions in 10 mM KNO3 on 0.45-μm-pore-size Micropore filters (Schleicher & Schuell, Dassel, Germany), mounting the filters on glass slides, and drying them for 2 h at room temperature. Cells exposed to 1-decanol were washed six times with 10 mM KNO3. Cell surface hydrophobicities were derived from θw values for water drops on the bacterial lawns, using a DSA 100 drop-shape analysis system (Krüss GmbH, Hamburg, Germany) (43). According to an earlier classification, cells exhibiting contact angles of <20°, 20° to 50°, and >50° are hydrophilic, intermediately hydrophilic, and hydrophobic, respectively (34).
RESULTS AND DISCUSSION
Detailed growth behavior of P. putida DOT-T1E in batch fermentations in the presence and absence of 1-decanol.
Five-liter batch fermentations with a mineral medium and with sodium succinate as the energy and carbon source were performed in the presence or absence of a 10% (vol/vol) phase of 1-decanol. The cells of P. putida DOT-T1E showed similar growth behaviors in the presence and absence of the solvent (Fig. 1A). The results for all growth parameters are summarized in Table 1. For comparison of the results, the data of Isken et al. (14) for P. putida S12, another highly tolerant strain, with toluene as the solvent, were added to the table. The growth rates obtained in the presence of 1-decanol were about 90% as high as those for cells growing in the absence of the solvent, which contrasts with the growth rate of P. putida S12, which was reduced to 76% in the presence of toluene. In the presence of decanol, the amount of released CO2 in the course of the fermentation was 85% of the value obtained without the solvent (Fig. 1A). However, the cell yield was only about 52% in the presence of 1-decanol, which corresponds to the value of 59% measured for P. putida S12 in the presence of toluene. The reductions in growth parameters can be explained by the higher energy consumption that is used to run all adaptive mechanisms that are needed in order to maintain cell physiology in the presence of a solvent.
FIG. 1.
Growth and energetics of P. putida DOT-T1E in a 5-liter batch fermenter in the presence and absence of 10% (vol/vol) 1-decanol. (A) CO2 production in the presence (dashed line) and absence (solid black line) of 1-decanol. Protein concentrations in the presence (▪) and absence (□) of 1-decanol are also given. (B) Cellular ATP concentrations in the presence (•) and absence (○) of 1-decanol. DW, dry weight. (C) Cellular adenylate energy charges in the presence (▴) and absence (▵) of 1-decanol.
TABLE 1.
Calculated values for growth rates, doubling times, and relative yields for batch fermentations with P. putida DOT-T1E in the presence and absence of a supersaturated concentration of 1-decanol and literature values for chemostat cultures of highly solvent-tolerant P. putida S12 in the presence and absence of a saturating concentration (6.2 mM) of toluene (14)
| Parameter | Value for indicated fermentation
|
|||
|---|---|---|---|---|
|
P. putida DOT-T1E, with sodium succinate as the C source
|
P. putida S12, with glucose as the C source
|
|||
| Control | + 1-Decanol | Control | + Toluene | |
| Growth rate (h−1) | 0.70 | 0.63 | 0.71a | 0.54a |
| Doubling time (min) | 59 | 66 | 58b | 76b |
| Yield (g protein/g C source) | 0.21 | 0.11 | 0.34 | 0.20 |
| Relative cell yield (%) | 100 | 52 | 100 | 59 |
Maximum dilution rate (growth rate) possible in chemostat cultures not leading to washout of the cells.
Maximum doubling time possible in chemostat cultures not leading to washout of the cells.
Effect of 1-decanol on energetics.
Organic solvents such as 1-decanol are known to be toxic to cells, mainly due to their permeabilizing effect on membranes (11, 41). This leads to a loss of important cellular components and ions (9, 21), including a decrease in the proton gradient (40). Additionally, the loss of cellular ATP after the addition of phenols was described (10). Next to this loss of energetic potential caused by chemical effects of the solvents, the adaptive mechanisms, especially the activities of at least three energy-dependent efflux pumps (39), are consuming energy that cannot be used for growth. This is reflected in the lower growth yields observed in this and former investigations (14). Taking into consideration all these possible negative effects of solvents on bacteria, one would assume a quite disturbed energetic level of the cells, which stands in strong contradiction to the growth rate of the bacteria in the presence of 1-decanol. In our first experiments, the ATP content of the cells was measured in shaking cultures. In this case, nonadapted cells of P. putida DOT-T1E showed a dramatic loss of ATP (89 to 92%) when exposed to the organic solvent 1-decanol in shake-flask cultures as energized resting cells (data not shown).
Astonishingly, the ATP contents in 5-liter batch fermentations, in contrast to the case in the experiment with energized resting cells, were even higher in the presence of 10% (vol/vol) 1-decanol. Figure 1B shows the development of the ATP contents of cells grown in 5-liter batch fermentations in the presence and absence of 1-decanol. At time zero, similar ATP concentrations were measured, showing ATP contents of 8 to 9 nmol/mg dry weight, which is similar to results found previously for aerobic bacteria (42).
These lower values for cells grown in fermentations without 1-decanol can be explained by decreasing specific ATP production at higher growth rates, indicating a higher energetic efficiency of carbon substrate utilization during fermentations in the absence of 1-decanol (17).
The time-dependent pattern of the ATP content of the cells was very similar for both experiments. In the first 2 h, the ATP concentration increased as the bacteria entered the exponential growth phase because of the abundance of sodium succinate and the relatively low demand for ATP for anabolic processes at this stage. In the exponential growth phase, upon limitation of the energy source for ATP formation through catabolic activities, the ATP concentration declined rapidly. The time point at which the minimum ATP concentration was reached coincided with the time point at which the cells entered stationary phase due to the exhaustion of the energy source (Fig. 1B). A similar curve was described by Muller et al. for the membrane potential (Δψ) of cells of Acinetobacter calcoaceticus during growth on acetate as the carbon and energy source. The membrane potential, as part of the proton motive force which drives ATP synthesis, can be used as an indicator for the energetic state of living systems as well (28).
Since the ATP content by itself does not always reflect the actual energy status, the concentrations of the other adenine nucleotides and the adenylate energy charge, which allows an exact expression of the energetic level of the cells under different growth conditions, were measured and calculated, respectively (Fig. 1C). The energy charge of the cells showed no difference, regardless of the presence or absence of 1-decanol. Apparently, the complete adaptation of the cells of P. putida DOT-T1E to 1-decanol is reflected in the absence of differences in the bioenergetics of this microorganism during fermentations with and without 1-decanol.
A steep decline in the energy charge for fermentations both with and without 1-decanol was observed after 2 h. The reasons for this drop are the same as those for the decrease in the ATP concentration. The decline to ECs of ≈0.3 to 0.45 is comparable to the values described in the literature at which viability of the microorganisms is still maintained before the cells die (5, 23, 24).
When the energy source became limiting, the ATP concentration decreased, whereas the ADP and AMP concentrations kept increasing (data not shown). It could be concluded that ADP and AMP were formed from ATP because the sum of nucleotides was more or less constant. Chapman et al. described a stabilization of the energy charge at this growth stage by a reduction in the sum of nucleotides in a short period of continued synthesis of RNA in Escherichia coli and a late drop in the energy charge when this process is not possible anymore (5). However, this stabilization of the energy charge was not observed in P. putida DOT-T1E, resulting in an earlier drop in the energy charge (from an EC of ≈0.8 down to an EC of ≈0.3), when the cells were in the middle of their exponential growth phase, irrespective of the presence or absence of 1-decanol.
The results on the energetics of the cells were additionally supported by measurements of the cellular potassium concentration, where no significant differences could be observed between fermentations with and without 1-decanol (data not shown). Since leakage of potassium ions is an important parameter for determining membrane damage in the presence of toxins (21, 29), this indicated that the cell membranes also completely adapted to the presence of a second phase of 1-decanol. The cells were obviously able to maintain the appropriate ion gradients across the membrane that are necessary for effective ATP synthesis. From these results, it can be concluded that although the bacteria need additional energy for their adaptation to the presence of the solvent, they are able to maintain or activate their electron transport phosphorylation, allowing homeostasis of the ATP level and energy charge in the presence of the solvent, at the price of a reduced growth yield.
Physicochemical surface properties of cells adapted to the presence of 1-decanol.
Already in 1998 (6), modifications of surface properties by changing the compositions of the very outer layers of gram-negative bacteria were suggested as an adaptive response in solvent-tolerant bacteria to a second phase of a toxic organic compound. In order to evaluate the influence of a second phase of 1-decanol on physicochemical cell surface properties, the water contact angles (θw) (44) and zeta potentials (ζ) (43) were measured to describe the cell surface charge and hydrophobicity of P. putida DOT-T1E. To guarantee the measurement of the cell properties and concomitantly to avoid measurement interferences by the physicochemical effect of 1-decanol adhering to or accumulating in the cells, six washing steps with the harvested cell pellets and 10 ml of 10 mM KNO3 were carried out. After six or more washing steps, no changes in either θw or ζ were observed, indicating that all reversibly bound 1-decanol was washed out from the cells (data not shown).
The results of the surface property measurements for 5-liter fermentations are summarized in Fig. 2. Cells exposed to 1-decanol immediately showed significantly increased water contact angles (θw = 85°) about 50° above θw of cells growing in the absence of the solvent (θw = 37°) (Fig. 2A). Contact angles of the cells in the lag and early exponential phases of both types of cultures increased to 110° and 72° for cells grown in the presence and absence of 1-decanol, respectively. Cells grown in the presence and absence of 1-decanol were negatively charged, with the former (ζ = −30 mV) exhibiting (Fig. 2B) 15 mV more negative ζ potentials than the latter (ζ = −15 mV). ζ values decreased during the fermentations, to −50 mV (in the presence of 1-decanol) and −25 mV (in the absence of 1-decanol), correlating with corresponding changes of the water contact angle and supporting earlier observations by others describing a negative correlation between cell hydrophobicity and surface charge (26, 43, 44). Previous findings have demonstrated that the whole-cell IEP (i.e., the pH at which ζ becomes zero) is a suitable indicator for predicting the biochemical surface compositions of bacteria (34). Literature data demonstrate that IEPs of ≤2.8 indicate the presence of significant amounts of cell surface polysaccharides inhibiting adhesion to both hydrophobic and hydrophilic surfaces (34). Continuously more negative ζ values between pH 2 and pH 6.5 and no significant changes of the IEP were found in the absence and presence of the solvent (data not shown). The observed IEP of about 2.5 is lower than published IEPs for other Pseudomonas strains (34), possibly indicating the presence of significant amounts of cell surface polymers, such as lipopolysaccharides (LPS), in Pseudomonas putida DOT-T1E.
FIG. 2.
Surface properties of P. putida DOT-T1E cells grown in a 5-liter batch fermenter in the presence and absence of 10% (vol/vol) 1-decanol. (A) Contact angles in the presence (•) and absence (○) of 1-decanol. (B) Zeta potentials of cells in the presence (▴) and absence (▵) of 1-decanol.
The steep increase in cell hydrophobicity (contact angle from about 30° to about 85°) (Fig. 2A) and the increase in ζ potential (from about −15 mV to about −30 mV) (Fig. 2B) of cells grown in the presence of the solvent already at the beginning of the fermentations were surprising. It should be noted that in the fermenter experiments, the first samples (t = 0) were taken after filling of the fermenter with medium, which took about 30 min, during which the cells were already in contact with 1-decanol. This led to the question of whether the measured changes reflect physiological changes or if they were caused by (abiotic) physicochemical interactions of 1-decanol with the cell wall. Therefore, a series of batch experiments with cells that had previously been treated with lethal concentrations of HgCl2 (0.1 mM) were carried out. HgCl2 is known to lead to cell death without having an effect on the physical and chemical properties of cell surfaces. A HgCl2 concentration of 0.1 mM led to a complete loss of viability of the cells (data not shown). 1-Decanol was added to the cells after 30 min of incubation with HgCl2 (Fig. 3). After the addition of 1-decanol to living cells, the contact angle of the cells increased drastically, from 27° to 85°, after an initial delay of several minutes (Fig. 3A). In contrast, dead cells showed only a moderate but much more rapid increase, to 48°. This is a clear indication that the observed increase in the hydrophobicity of cells grown in the presence of 1-decanol was caused by physiological changes that could only be carried out by living cells, as opposed to the much faster physicochemical effect which is seen and expected for dead cells. Nearly the same effect was measured for the ζ potential (Fig. 3B). In this case, the living cells also showed a slower response than the dead cells, but with a greater final decrease in the ζ potential.
FIG. 3.
Effect of preincubation with a toxic concentration (0.1 mM) of HgCl2 on surface properties of P. putida DOT-T1E cells in the presence of 10% (vol/vol) 1-decanol. (A) Contact angles of living (•) and dead (○) cells. (B) Zeta potentials of living (▴) and dead (▵) cells. The arrows indicate the addition of 0.1 mM HgCl2, and the dashed lines mark the presence of 10% (vol/vol) 1-decanol.
The contact angle and the zeta potential showed slight changes as a function of growth both in the presence and in the absence of 1-decanol (Fig. 2). Until the middle of the exponential phase, the contact angle increased and then stayed rather steady. The values for the zeta potential became constantly more negative in the course of growth until the beginning of the stationary phase.
In this study, it was proven for the first time that changes in surface properties as a cellular response to stress also occur as an adaptive mechanism to the presence of toxic organic solvents. The very fast physiological response can be explained by the formation of membrane vesicles, mainly consisting of B-band LPS, that lead to a fast and drastic increase in the hydrophobicity of the cells (15, 36).
The major component in gram-negative cells that affects surface properties such as charge and hydrophobicity is the composition of the LPS layer of the outer membrane. The so-called O-specific region on the very outer cell surface especially has an effect on surface properties. In the LPS of Pseudomonas aeruginosa, the O-specific region contains two major components. The A band, a low-molecular-mass LPS, consists of a homopolymer of d-rhamnose, with only minor amounts of 2-keto-3-deoxyoctonic acid. The B band, a high-molecular-mass LPS, consists of a heteropolymer of mainly uronic acid derivatives and N-acetylfucosamine. The rapidly occurring complete loss of B-band LPS upon stress compared to the amounts of A-band LPS present on the surface has been shown to affect surface charge, surface hydrophobicity, adhesion to hydrophobic surfaces, biofilm formation, and susceptibility to antimicrobial agents and host defense (18). In addition, one investigated solvent-tolerant strain, P. putida Idaho, changed its LPS composition when grown in the presence of o-xylene. A higher-molecular-weight LPS band disappeared and was replaced by a lower-molecular-weight band in the presence of the aromatic compound (31).
In Pseudomonas aeruginosa, the quantity of LPS was reported to be high in the initial phases of growth but then to decrease significantly to constant levels in the stationary phase. A strong increase in the yield of LPS in the mid- and late exponential growth phases was observed (46).
P. aeruginosa is known for being able to alter the LPS composition of its surfaces very rapidly. This takes place as a response to high temperature (45°C) (27) but also in response to other environmental stress factors, such as the presence of the membrane-active antibiotic gentamicin (16) and to oxygen stress (36).
Indeed, for mutants of Escherichia coli showing higher tolerance towards solvents, the hydrophobicities of cell surfaces have been reported to decrease (1). However, an explanation for the physiological advantage of a more hydrophobic cell surface as an adaptive response to the presence of a very hydrophobic solvent seems very difficult. This had already been discussed in 1998 by de Bont (6), who had assumed a decrease in cell hydrophobicity in order to repel the solvent. The outer membrane is known to be a very good barrier for hydrophobic compounds. This very low permeability for hydrophobic compounds is usually more affected by the outer membrane porins than by variations in the LPS content. However, taking into consideration that the major mechanism creating the phenotype of highly solvent-tolerant bacteria is the presence of at least three efflux pumps (39) that permanently remove the toxic solvents from the cytoplasmic membrane and transport them to the outer layer of the outer membrane, the observed modification of the surface properties makes sense because this hydrophobic layer is able to take up more of the solvents.
Additionally, the release of solvent-containing membrane vesicles was discussed as a mechanism of adaptation to toxic solvents (19). A detailed study of the LPS content of P. putida DOT-T1E growing in the presence and absence of 1-decanol, including antibodies for the different LPS bands, will be carried out in the near future.
Bacteria belonging to the genus Pseudomonas are famous not only because of their high solvent tolerance but mainly because of their capability of degrading a wide range of pollutants, even at very low concentrations. Also, in the presence of nontoxic crude oil components, such as hexadecane, an increase in cell hydrophobicity has been observed (30). The major reason for this change in surface properties seems to be an increased adhesion to the surfaces of very poorly water-soluble compounds that leads to an increase in the bioavailability of the compounds (49, 50). As in adaptation to poorly water-soluble substrates, uptake systems seem to also be involved, because a hydrophobic surface also works as a kind of source for the compounds that accumulates them at the cell surface and allows better uptake (2). Thus, a more hydrophobic surface can work as a kind of sink for toxic concentrations of solvents that are excluded by efflux pumps but also as a kind of source for less bioavailable substrates that are transported into the cells by several uptake systems.
P. putida DOT-T1E and other highly solvent-tolerant bacteria were shown to be capable of adapting to the presence of very toxic solvents, such as toluene or 1-decanol, without being highly affected in their growth properties and cellular energetics. Thus, these bacteria can be handled at technical scales for the production of fine chemicals of interest in high, economically sound concentrations (13, 47). Since these strains are also very accessible to genetic modifications and the introduction of foreign genes, a wide range of products can be synthesized by using a two-phase biotransformation system.
Acknowledgments
This work was partially supported by contract no. QLRT-2001-00435 of the European Commission within its Fifth Framework Programme.
We thank Karin Lange, Jana Reichenbach, Rita Remer, Birgit Würz, and Andreas Zehnsdorf (all UFZ) for their help with the experiments.
REFERENCES
- 1.Aono, R., and H. Kobayashi. 1997. Cell surface properties of organic solvent-tolerant mutants of Escherichia coli K-12. Appl. Environ. Microbiol. 63:3637-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias-Barrau, E., A. Sandoval, E. R. Olivera, J. M. Luengo, and G. Naharro. 2005. A two-component hydroxylase involved in the assimilation of 3-hydroxyphenyl acetate in Pseudomonas putida. J. Biol. Chem. 280:26435-26447. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson, D. E., and G. M. Walton. 1967. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J. Biol. Chem. 342:3239-3241. [PubMed] [Google Scholar]
- 4.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Chapman, A. G., L. Fall, and D. E. Atkinson. 1971. Adenylate energy charge in Escherichia coli during growth and starvation. J. Bacteriol. 108:1072-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bont, J. A. M. 1998. Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol. 16:493-499. [Google Scholar]
- 7.Hartig, C., N. Loffhagen, and H. Harms. 2005. Formation of trans fatty acids is not involved in growth-linked membrane adaptation of Pseudomonas putida. Appl. Environ. Microbiol. 71:1915-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmans, S., J. P. Smits, M. J. van der Werf, F. Volkering, and J. A. M. de Bont. 1989. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl. Environ. Microbiol. 55:2850-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heipieper, H. J., R. Diefenbach, and H. Keweloh. 1992. Conversion of cis-unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl. Environ. Microbiol. 58:1847-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heipieper, H. J., H. Keweloh, and H. J. Rehm. 1991. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl. Environ. Microbiol. 57:1213-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heipieper, H. J., F. J. Weber, J. Sikkema, H. Keweloh, and J. A. M. de Bont. 1994. Mechanisms behind resistance of whole cells to toxic organic solvents. Trends Biotechnol. 12:409-415. [Google Scholar]
- 12.Hiementz, P. C. 1986. Principles of colloid and surface chemistry. Marcel Dekker, Inc., New York, N.Y.
- 13.Husken, L. E., M. C. F. Dalm, J. Tramper, J. Wery, J. A. M. de Bont, and R. Beeftink. 2001. Integrated bioproduction and extraction of 3-methylcatechol. J. Biotechnol. 88:11-19. [DOI] [PubMed] [Google Scholar]
- 14.Isken, S., A. Derks, P. F. G. Wolffs, and J. A. M. de Bont. 1999. Effect of organic solvents on the yield of solvent-tolerant Pseudomonas putida S12. Appl. Environ. Microbiol. 65:2631-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadurugamuwa, J. L., and T. J. Beveridge. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol. 177:3998-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadurugamuwa, J. L., J. S. Lam, and T. J. Beveridge. 1993. Interaction of gentamicin with the A band and B band lipopolysaccharides of Pseudomonas aeruginosa and its possible lethal effect. Antimicrob. Agents Chemother. 37:715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayser, A., J. Weber, V. Hecht, and U. Rinas. 2005. Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. I. Growth-rate-dependent metabolic efficiency at steady state. Microbiology 151:693. [DOI] [PubMed] [Google Scholar]
- 18.Kelly, N. M., M. H. MacDonald, N. Martin, T. Nicas, and R. E. W. Hancock. 1990. Comparison of the outer membrane protein and lipopolysaccharide profiles of mucoid and nonmucoid Pseudomonas aeruginosa. J. Clin. Microbiol. 28:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi, H., K. Uematsu, H. Hirayama, and K. Horikoshi. 2000. Novel toluene elimination system in a toluene-tolerant microorganism. J. Bacteriol. 182:6451-6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laane, C., S. Boeren, K. Vos, and C. Veeger. 1987. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 30:81-87. [DOI] [PubMed] [Google Scholar]
- 21.Lambert, P. A., and S. M. Hammond. 1973. Potassium fluxes, first indication of membrane damage in micro-organisms. Biochem. Biophys. Res. Commun. 54:796-799. [DOI] [PubMed] [Google Scholar]
- 22.Leon, R., P. Fernandes, H. M. Pinheiro, and J. M. S. Cabral. 1998. Whole-cell biocatalysis in organic media. Enzyme Microb. Technol. 23:483-500. [Google Scholar]
- 23.Loffhagen, N., and W. Babel. 1985. pH-linked control of energy charge in Acetobacter methanolicus sp. MB 70. J. Basic Microbiol. 25:575-580. [Google Scholar]
- 24.Lundin, A., M. Hasenson, J. Persson, and A. Pousette. 1986. Estimation of biomass in growing cell lines by adenosine triphosphate assay. Methods Enzymol. 133:27-44. [DOI] [PubMed] [Google Scholar]
- 25.Lundin, A., and A. Thore. 1975. Analytical information obtainable by evaluation of time course of firefly bioluminescence in assay of ATP. Anal. Biochem. 66:47-63. [DOI] [PubMed] [Google Scholar]
- 26.Makin, S. A., and T. J. Beveridge. 1996. The influence of A-band and B-band lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology 142:299-307. [DOI] [PubMed] [Google Scholar]
- 27.Makin, S. A., and T. J. Beveridge. 1996. Pseudomonas aeruginosa PAO1 ceases to express serotype-specific lipopolysaccharide at 45°C. J. Bacteriol. 178:3350-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller, S., N. Loffhagen, T. Bley, and W. Babel. 1996. Membrane-potential-related fluorescence intensity indicates bacterial injury. Microbiol. Res. 151:127-131. [Google Scholar]
- 29.Neumann, G., N. Kabelitz, A. Zehnsdorf, A. Miltner, H. Lippold, D. Meyer, A. Schmid, and H. J. Heipieper. 2005. Prediction of the adaptability of Pseudomonas putida DOT-T1E to a second phase of a solvent for economically sound two-phase biotransformations. Appl. Environ. Microbiol. 71:6606-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norman, R. S., R. Frontera-Suau, and P. J. Morris. 2002. Variability in Pseudomonas aeruginosa lipopolysaccharide expression during crude oil degradation. Appl. Environ. Microbiol. 68:5096-5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinkart, H. C., J. W. Wolfram, R. Rogers, and D. C. White. 1996. Cell envelope changes in solvent-tolerant and solvent-sensitive Pseudomonas putida strains following exposure to o-xylene. Appl. Environ. Microbiol. 62:1129-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos, J., E. Duque, M. Huertas, and A. Haidour. 1995. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J. Bacteriol. 177:3911-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos, J. L., E. Duque, M. T. Gallegos, P. Godoy, M. I. Ramos-Gonzalez, A. Rojas, W. Teran, and A. Segura. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 34.Rijnaarts, H. H. M., W. Norde, J. Lyklema, and A. J. B. Zehnder. 1995. The isoelectric point of bacteria as indicator for the presence of cell surface polymers that inhibit adhesion. Colloids Surf. B 4:191-197. [Google Scholar]
- 35.Rojas, A., E. Duque, A. Schmid, A. Hurtado, J.-L. Ramos, and A. Segura. 2004. Biotransformation in double-phase systems: physiological responses of Pseudomonas putida DOT-T1E to a double phase made of aliphatic alcohols and biosynthesis of substituted catechols. Appl. Environ. Microbiol. 70:3637-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabra, W., H. Lunsdorf, and A. P. Zeng. 2003. Alterations in the formation of lipopolysaccharide and membrane vesicles on the surface of Pseudomonas aeruginosa PAO1 under oxygen stress conditions. Microbiology 149:2789-2795. [DOI] [PubMed] [Google Scholar]
- 37.Segura, A., E. Duque, G. Mosqueda, J. L. Ramos, and F. Junker. 1999. Multiple responses of gram-negative bacteria to organic solvents. Environ. Microbiol. 1:191-198. [DOI] [PubMed] [Google Scholar]
- 38.Segura, A., P. Godoy, P. van Dillewijn, A. Hurtado, N. Arroyo, S. Santacruz, and J.-L. Ramos. 2005. Proteomic analysis reveals the participation of energy- and stress-related proteins in the response of Pseudomonas putida DOT-T1E to toluene. J. Bacteriol. 187:5937-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segura, A., H. J. Heipieper, W. Terán, M. E. Guazzaroni, A. Rojas, E. Duque, M. T. Gallegos, and J. L. Ramos. 2004. Enzymatic activation of the cis-trans isomerase and transcriptional regulation of efflux pumps in solvent tolerance in Pseudomonas putida, p. 479-508. In J. L. Ramos (ed.), Pseudomonas, vol. 2. Kluwer Press, Dordrecht, The Netherlands. [Google Scholar]
- 40.Sikkema, J., J. A. de Bont, and B. Poolman. 1994. Interactions of cyclic hydrocarbons with biological membranes. J. Biol. Chem. 269:8022-8028. [PubMed] [Google Scholar]
- 41.Sikkema, J., J. A. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran, Q. H., and G. Unden. 1998. Changes in the proton potential and the cellular energetics of Escherichia coli during growth by aerobic and anaerobic respiration or by fermentation. Eur. J. Biochem. 251:538. [DOI] [PubMed] [Google Scholar]
- 43.Van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. B. Zehnder. 1987. Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 53:1898-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Loosdrecht, M. C. M., J. Lyklema, W. Norde, G. Schraa, and A. J. B. Zehnder. 1987. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 53:1893-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vrionis, H. A., A. M. Kropinski, and A. J. Daugulis. 2002. Enhancement of a two-phase partitioning bioreactor system by modification of the microbial catalyst: demonstration of concept. Biotechnol. Bioeng. 79:587-594. [DOI] [PubMed] [Google Scholar]
- 46.Weber-Frick, C., and W. Schmidt-Lorenz. 1988. The growth and lipopolysaccharide production of Escherichia coli and Pseudomonas aeruginosa. Zentbl. Bakteriol. Mikrobiol. Hyg. B 186:478-493. [PubMed] [Google Scholar]
- 47.Wery, J., D. I. M. da Silva, and J. A. M. de Bont. 2000. A genetically modified solvent-tolerant bacterium for optimized production of a toxic fine chemical. Appl. Microbiol. Biotechnol. 54:180-185. [DOI] [PubMed] [Google Scholar]
- 48.Wery, J., and J. A. M. de Bont. 2004. Solvent-tolerance of pseudomonads: a new degree of freedom in biocatalysis, p. 609-634. In J.-L. Ramos (ed.), Pseudomonas, vol. 3. Biosynthesis of macromolecules and molecular metabolism. Kluwer Academic/Plenum, Boston, Mass. [Google Scholar]
- 49.Wick, L. Y., A. R. de Munain, D. Springael, and H. Harms. 2002. Responses of Mycobacterium sp. LB501T to the low bioavailability of solid anthracene. Appl. Microbiol. Biotechnol. 58:378-385. [DOI] [PubMed] [Google Scholar]
- 50.Wick, L. Y., N. Pasche, S. M. Bernasconi, O. Pelz, and H. Harms. 2003. Characterization of multiple-substrate utilization by anthracene-degrading Mycobacterium frederiksbergense LB501T. Appl. Environ. Microbiol. 69:6133-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]





