Abstract
Mycoplasma genitalium is associated with reproductive tract disease in women and may persist in the lower genital tract for months, potentially increasing the risk of upper tract infection and transmission to uninfected partners. Despite its exceptionally small genome (580 kb), approximately 4% is composed of repeated elements known as MgPar sequences (MgPa repeats) based on their homology to the mgpB gene that encodes the immunodominant MgPa adhesin protein. The presence of these MgPar sequences, as well as mgpB variability between M. genitalium strains, suggests that mgpB and MgPar sequences recombine to produce variant MgPa proteins. To examine the extent and generation of diversity within single strains of the organism, we examined mgpB variation within M. genitalium strain G-37 and observed sequence heterogeneity that could be explained by recombination between the mgpB expression site and putative donor MgPar sequences. Similarly, we analyzed mgpB sequences from cervical specimens from a persistently infected woman (21 months) and identified 17 different mgpB variants within a single infecting M. genitalium strain, confirming that mgpB heterogeneity occurs over the course of a natural infection. These observations support the hypothesis that recombination occurs between the mgpB gene and MgPar sequences and that the resulting antigenically distinct MgPa variants may contribute to immune evasion and persistence of infection.
Mycoplasma genitalium has been identified as a possible cause of several reproductive tract disease syndromes including urethritis in men and mucopurulent cervicitis, endometritis, pelvic inflammatory disease, and tubal factor infertility in women (3, 6, 7, 18, 23, 28, 30, 34, 35, 41, 48, 52, 55, 56). When untreated (or inappropriately treated), this organism persists at infected sites as demonstrated by its association with chronic nongonococcal urethritis in men (22, 53) and its persistence for months, if not years, in the lower genital tract of women (C. R. Cohen, M. Nosek, A. Meier, S. G. Astete, S. Iverson-Cabral, N. R. Mugo, and P. A. Totten, submitted for publication). The longevity of M. genitalium infection in the human reproductive tract suggests that this pathogen may have mechanism(s) to modulate or evade the host immune response.
At 580 kb, the M. genitalium G-37T genome is one of the smallest of any self-replicating cellular organism (9, 17, 49), yet 4% of the fully sequenced genome consists of repeated elements (43) designated as MgPa repeats or MgPar sequences (17). These repeat sequences are so named because they are composed of partial, incomplete copies of the mgpB gene, which encodes the MgPa protein that mediates attachment to various cell types, including the ciliated epithelium of human fallopian tubes (8). Although there is only a single mgpB expression site, there are nine distinct MgPar sequences found throughout the genome (17). The MgPa protein is antigenic and elicits an immune response in animal models (25, 37) and in women with tubal factor infertility (6). The presence of MgPar sequences and the immunogenicity of the MgPa protein suggests that that recombination between mgpB and MgPar sequences could be a mechanism that promotes MgPa antigenic diversity, potentially allowing for persistence of infection through mechanisms such as immune evasion (10, 17, 42). This hypothesis is supported by the heterogeneity of mgpB sequences observed between different M. genitalium clinical isolates in which variability could be explained by recombination between the mgpB expression site and MgPar sequences (42); however, to date, the extent of mgpB heterogeneity within a single M. genitalium strain has yet to be evaluated.
In the current study, we further define the architecture and sequence homology of the MgPar regions in relation to the mgpB gene, determine the heterogeneity of mgpB within the type strain of M. genitalium G-37T cultured in Seattle (G37-S), and demonstrate extensive mgpB sequence variability within a single M. genitalium strain in a persistently infected woman. The results of our analysis are consistent with extensive intrastrain mgpB heterogeneity both in vitro and in vivo and suggest that short sequences within the mgpB and MgPar sequences participate in recombination. These findings further suggest that this mechanism contributes to the persistence and survival of M. genitalium in the human reproductive tract.
(These data were presented, in part, at the 16th biennial meeting of the International Society for Sexually Transmitted Disease Research [ISSTDR], Amsterdam, The Netherlands, 10 to 13 July 2005.)
MATERIALS AND METHODS
Evaluation of mgpB repeat sequences in the M. genitalium G-37T genome.
The M. genitalium G-37T mgpB sequence was evaluated for homology and percent identity to each of the nine MgPar sequences using the BLAST (bl2seq) align two sequences program (http://www.ncbi.nlm.nih.gov/BLAST/bl2seq/wblast2.cgi) and the EMBOSS pairwise alignment algorithm (http://www.ebi.ac.uk/emboss/align/index.html?). To independently assess mgpB sequences that may be repeated elsewhere in the chromosome, overlapping 23-bp fragments (1-23, 2-24, 3-25, etc.) of mgpB were used to search the M. genitalium G-37T genome utilizing a modification of the Rocha and Blanchard computer algorithm (44). In this analysis, the M. genitalium G-37T genome was searched for sequence repetition using the overlapping sliding windows of the mgpB sequence, allowing for no mismatches. The program is written in C and is available from E. P. C. Rocha upon request. The “TAG” trinucleotide repeat sequence identified in this analysis (see Results below) was used to search for short, nearly exact matches to the M. genitalium G-37T genome using BLAST (http://www.nlm.nih.gov/BLAST/). The genome location and accession numbers of complete MgPar sequences used in this study are listed in Table 1; these sequences are more comprehensive than previously published MgPar sequences (also presented in Table 1). The G-37T mgpB gene used in these analyses is the second coding region (bp 1066 to 5400) in the MgPa operon available under accession number M31431 (27).
TABLE 1.
Genome location, previous nomenclature, and accession numbers for the complete MgPar sequences of M. genitalium G-37T
| MgPar sequence | Nomenclature of incomplete sequencea
|
Location of complete sequence in the G-37T genome (bp)d | Accession no. of complete MgPar sequence | |
|---|---|---|---|---|
| Clone identification | Accession no. | |||
| 1 | HB7 | U02105 | 85551-87720 | DQ248096 |
| 2 | SC2 | U34968 | 167179-169820 | DQ248097 |
| 3 | SD3a or HG4b | 174861-175680 | DQ248098 | |
| 4 | SA11c | U34967 | 213545-215898 | DQ248099 |
| 5 | ESH10 | U34970 | 229441-231630 | DQ248100 |
| 6 | SD3a or HG4b | 273331-273730 | DQ248101 | |
| 7 | SG8 | U34969 | 312807-315220 | DQ248102 |
| 8 | X5 | U01810 | 349231-351791 | DQ248103 |
| 9 | HSA7 | U01766 | 428281-430579 | DQ248104 |
Names and accession numbers assigned to clones containing partial MgPar sequences were published previously (42). New accession numbers (listed to the right) have been created for complete MgPar sequences.
Sequences published previously for SD3a and HG4 under accession numbers U02157 and U02110, respectively (42), were insufficient in length to determine whether they represented MgPar 3 or 6.
SA11, representing MgPar 4 (42), contains a complete MgPar sequence as well as irrelevant flanking sequences, so the new sequence has been modified to contain only mgpB and mgpC homologous sequences and internal AT-rich regions.
M. genitalium strains and culture methods.
The M. genitalium type strain G-37T was obtained from the American Type Culture Collection (ATCC 33530) and maintained in the laboratory of George Kenny (University of Washington). The stock culture of M. genitalium G-37T maintained and cultured in Seattle, Washington, will be referred to as G37-S throughout this study to distinguish it from the published type strain G-37T and from G37-DK, a G-37 stock separately maintained by Jørgen Jensen (Statens Serum Institute, Copenhagen, Denmark) (42). The G-37T strain was single-colony cloned using the standard threefold filtration procedure before it was deposited in the ATCC (58; J. G. Tully and D. Taylor-Robinson, personal communication); therefore, this M. genitalium strain G37-S is a clonal derivative of the original G-37 isolate (57, 58). Multiple 1-ml aliquots of G37-S have been frozen at −80°C and for the experiments described below, a 1-ml frozen aliquot was used to inoculate 100 ml of H-broth (0.02 g/ml soy peptone, 0.005 g/ml sodium chloride, 0.002% phenol red, 20% horse serum, 10% yeast dialysate, 200 units/ml penicillin G, and 5 mM glucose, pH 7.3) (32) in a 75-cm2 cell culture flask (Corning, Corning, NY) until the media changed color from red to orange, indicating growth of the organism through the production of acid. DNA was isolated from this culture using the Epicenter MasterPure DNA purification kit according to the manufacturer's directions (Epicenter, Madison, WI).
Amplification of mgpB from M. genitalium G37-S.
Designated regions of the mgpB gene were PCR amplified using the conditions and primers listed in Table 2. For consistency throughout the report, all base pair locations are listed relative to the presumed mgpB translational start, rather than the start of the MgPa operon (containing mgpA, mgpB, and mgpC), the numbering system that has been used previously (42).
TABLE 2.
Primers used to amplify various regions of the mgpB gene
| Region amplifieda | Primer name | Primer sequence | Location in mgpB gene (bp)b | Annealing temp (°C) | MgCl2 concn (mM) |
|---|---|---|---|---|---|
| Strain typing | 1Fc | GTGATGTTGTTAGTGATTGTGTG | −50 to −28c | 60 | 2.5 |
| 1R | GGGATTGAACTACTTCTACTGGA | 560-582 | |||
| Strain confirmation | 2F | GAAGTCTTGAGCCTTTCTAACC | 29-50 | 55 | 2.0 |
| 2R | CGAGTGGATGTAGCCCCd | 803-817 | |||
| Repeat region B | 3F | CAAAAATGGAAAACCCCTCAA | 436-456 | 60 | 2.5 |
| 3R | ATCATAGAAACTAACCACCGTCG | 1012-1034 | |||
| Repeat region EF | 4F | CAGGGCTAAGTGATAAGATTTTC | 2110-2132 | 55 | 2.0 |
| 4R | TCAGTAGAGTTGGTATTGGTGC | 2917-2938 | |||
| Repeat region G | 5F | AAATCCCTTCCAAATAGTTCA | 3253-3272 | 53 | 1.5 |
| 5R | GCCAGTTAGTTTACCATCCA | 3661-3680 |
The locations of each primer relative to repeat regions B, EF, and G are also shown schematically in Fig. 2.
The location of each primer in the mgpB gene of G-37T is numbered so that bp 1 represents the translational start of the gene.
This primer sequence is a modification of primer Mgpat-1010 (42), located 50 bp upstream of the mgpB translational start.
Primer 2R was designed based on the sequence obtained from mgpB of patient no. 10139 so that identity to the published G-37T mgpB gene exists for only 15 bases; the two underlined bases are not present in the mgpB sequence of G-37T.
All PCR assays were performed on a Perkin Elmer DNA thermal cycler, model 480, using the following conditions: 4 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at the annealing temperature designated in Table 2, 1 min at 72°C, and a final elongation step at 72°C for 10 min. PCR mixtures each contained 1× PCR buffer (Promega, Madison, WI), the indicated MgCl2 concentration (Table 2), 0.1 μM concentrations (each) of forward and reverse primers, 200 μmol/liter of each deoxynucleoside triphosphate, and 1 U Taq polymerase (Promega). PCR amplification was accomplished using 103 genome equivalents of purified M. genitalium G37-S DNA in a final volume of 100 μl. Fifty microliters of sterile, nuclease-free mineral oil was added to each reaction mixture to prevent loss of volume due to evaporation. All G37-S PCRs were performed using DNA from the same purified sample; in addition, all PCR protocols involved the use of PCR clean conditions to safeguard against PCR contamination.
Cloning of PCR products, sequencing, and in silico analyses.
Following PCR, resultant amplicons were concentrated using the MinElute PCR purification kit (QIAGEN, Valencia, CA) and ligated into the pCR2.1-Topo vector (Invitrogen, Carlsbad, CA) by following the manufacturer's standard protocols. Ligation reaction mixtures were transformed into TOP10-competent Escherichia coli cultures (Invitrogen), which were plated onto Luria agar media (36) containing 100 μg/ml ampicillin and 40 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). Well-isolated, white colonies from each transformation were inoculated into 2.5 ml of Luria broth (36) with 100 μg/ml ampicillin. Following overnight incubation at 37°C shaking, plasmids were isolated from the E. coli cultures using the QIAprep spin miniprep kit (QIAGEN), restriction enzyme digested with EcoRI, and analyzed by agarose gel electrophoresis to confirm insertion of the PCR product into the cloning vector. Plasmids containing inserts of the appropriate size were sequenced at the University of Washington Biochemistry DNA Sequencing Facility (http://depts.washington.edu/biowww/dna/) in the forward and reverse directions using the M13F or M13R primer provided in the Topo cloning kit (Invitrogen). The ClustalW program (http://www.ch.embnet.org/software/ClustalW.html) was used to align the resulting sequences, which were manually adjusted to correct for sequencing and/or alignment errors. To determine if mgpB sequences were heterogeneous at the amino acid level or contained in-frame stop codons, all nucleotide sequences were submitted to the European Bioinformatics Institute's (EMBL-EBI) EMBOSS Transeq program (http://www.ebi.ac.uk/emboss/transeq), where the DNA sequence was translated using both the mycoplasma genetic code (in which UGA encodes a tryptophan instead of a translational stop) (26) and the appropriate reading frame; the predicted amino acid sequences were then aligned using the ClustalW program referenced above. Because alternative sequences identified within mgpB are flanked by sequences of identity, regions of deviation for alternative sequences were defined by the first and last divergent nucleotide present. For our analysis of variation in the mgpB gene of M. genitalium G37-S, five PCRs were performed for each repeat region where five of the resulting transformed colonies were selected for sequencing, producing a total of 25 sequences analyzed for each repeat region.
Analysis of mgpB heterogeneity in cervical specimens from a persistently infected woman.
Cervical samples collected from women persistently infected with M. genitalium were used to analyze mgpB sequence variation in vivo. These samples were collected longitudinally approximately every 2 months from a cohort of 300 women monitored for up to 3 years to assess correlates of sexually transmitted infections among female commercial sex workers in Nairobi, Kenya (Cohen et al., submitted). Although these women resided in or near Kariobangi, a slum area inside Nairobi, Kenya, their clients included men from Nairobi, other parts of Kenya, and other countries (C. R. Cohen, unpublished data), suggesting strains detected in these women were not confined to a limited geographic region. All women gave written informed consent, and the study was approved by the Institutional Review Board of the University of Washington and the Ethical Review Committee at Kenyatta National Hospital. Cervical samples were tested for M. genitalium by our standard diagnostic PCR assay (16), and the samples from one woman with a long-term (21 months) M. genitalium infection, designated as participant no. 10139, were evaluated further. In addition, eight other women also identified as having a persistent M. genitalium infection (more than five M. genitalium-positive cervical samples over the course of 1 year) were chosen for strain typing of M. genitalium as described below. For PCR amplification, DNA was purified from 150 μl of cervical sample and inhibitors were removed using the MasterPure DNA purification kit (Epicenter) according to the manufacturer's standard protocol for the isolation of DNA from fluid samples. Samples were concentrated fivefold by rehydrating isolated DNA in 30 μl of 10 mM Tris, 1 mM EDTA, pH 8.0. Each PCR assay was performed as described above for G37-S, with the exception that 6 μl of purified DNA (representing 30 μl of the original cervical sample) was used in a final volume of 50 μl. Due to the limited volume of cervical specimens available for analysis, only one PCR assay from each time point was performed in the analysis of the mgpB repeat region B from participant no. 10139; from each PCR, 10 transformed colonies were isolated and sequenced so that among the four cervical samples amplified, a total of 40 sequences were evaluated.
M. genitalium strain typing.
The strain typing method developed by Jensen, et al. (29) was used to confirm that the culture of G37-S used for these analyses was not contaminated with other M. genitalium strains and to identify and differentiate M. genitalium strains among the cervical samples from nine persistently infected women. This strain typing system has been shown to have a high discriminatory index (0.95) for unrelated strains (21) and is based on single nucleotide polymorphisms that occur within the first 500 bases of mgpB, a region of the gene that is present in a single copy in the genome and is thus relatively conserved. All strain typing was performed using primer set 1F/1R (Table 2). Primer set 2F/2R was also used to further evaluate the M. genitalium strain present in cervical samples from participant no. 10139. PCR amplification, cloning, and sequencing were each performed as described above using conditions outlined in Table 2. Due to limited sample volume, as mentioned above, in the analysis of no. 10139, one PCR assay per time point was performed with five clones per PCR sequenced. Similarly, for the remaining persistently infected women, one PCR was performed with three clones per PCR sequenced.
Nucleotide sequence accession number.
All sequence data presented in this study have been submitted to the GenBank database and are available, as indicated in either Table 1 or any of the relevant figures, under accession numbers DQ248067 to DQ248104.
RESULTS
MgPar sequence architecture and homology to mgpB in M. genitalium G-37T.
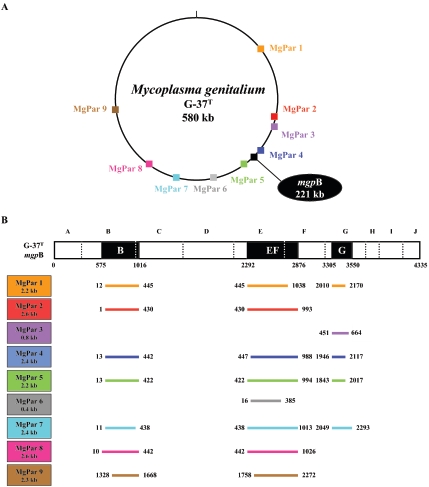
The mgpB expression site, which encodes the immunodominant MgPa adhesin protein, is present in only one copy on the G-37T chromosome (MG191 in The Institute for Genomic Research [TIGR] database [http://www.tigr.org]), yet incomplete copies of this gene (MgPar sequences) are present at nine distinct loci throughout the genome (Fig. 1A) (17). Before sequencing of the M. genitalium genome was complete, Dallo and Baseman (10) demonstrated that several portions of the mgpB gene (identified as restriction enzyme fragments B, EF, and G) (Fig. 1B) are repeated at multiple sites on the chromosome. To further define these mgpB-homologous regions, we performed pairwise alignments between the mgpB gene and each of the nine MgPars and determined that regions B, EF, and G localized to mgpB nucleotides 575 to 1016, 2292 to 2876, and 3305 to 3550, respectively (Fig. 1B; see also Fig. S1 to S3 in the supplemental material for alignment of mgpB and MgPar sequences). Our studies expanded on the results of Peterson et al. (42) in which mgpB repetition was evaluated using M. genitalium genomic clones, which except for MgPar 4 (SA11) (Table 1), contained incomplete MgPar sequences (42). Thus, our analysis presents the extent of mgpB homology in MgPars 1, 3, and 5 to 9 extends beyond what was previously reported (42).
FIG. 1.
Schematic drawing of M. genitalium G-37T MgPar sequences showing their genome location, architecture, and the location of homologous sequences in the mgpB gene. (A) Location of the full-length mgpB expression site and nine MgPar sequences in the M. genitalium G-37T genome. (B) Homology between the mgpB gene and the nine MgPar sequences in M. genitalium G-37T, as determined by pairwise alignment. Vertical dotted lines within mgpB define fragments A through J described by Dallo and Baseman (10). Shaded black boxes (labeled B, EF, and G) represent mgpB repeat regions identified in the analysis presented in Fig. 2. Below, each of the MgPar sequences (MgPar 1 to 9) is listed on the left with their corresponding size. Sequences within each MgPar site with homology to mgpB are shown diagrammatically by a colored line; the location of each line corresponds to the location in the mgpB gene in which homology was observed, numbered according to the GenBank entry (Table 1). For example, MgPar 1 has homology to repeat regions B, EF, and G between bp 12 to 445, 445 to 1038, and 2010 to 2170, respectively. The remainder of MgPar 1 is not homologous to sequences found within the mgpB gene. Sequence alignments for the mgpB and MgPar sequences diagrammatically pictured in panel B are presented in Fig. S1 to S3 in the supplemental material.
In addition to their homology to mgpB, most MgPar regions contain intervening sequences that are AT rich and/or have homology to the mgpC gene (MG192 in the TIGR database) located downstream of the mgpB gene in the MgPa operon (42). Again, because the MgPar sequences previously examined were incomplete (42), in MgPars 1 to 3, 5, and 7 to 9, we identified mgpC-homologous sequences that were absent from the previously examined genomic clones (data not shown). These AT-rich and mgpC homologous sequences were not analyzed further in our current study, which focuses on the sequence diversity of mgpB and its homology to the MgPar regions.
We next used our newly identified complete MgPar sequences to perform pairwise alignments with the mgpB gene and determined that sequences in mgpB repeat regions B, EF, and G are homologous to seven, eight, and five of the different MgPar sites, respectively (Fig. 1B). In addition, the MgPar sequences homologous to repeat regions B, EF, and G are colinear with the sequences in the mgpB expression site yet lack the intervening sequences that correspond to those regions that are unique to the mgpB gene. The MgPar sequences are not themselves predicted to serve as alternative, functional expression sites because they not only contain AT-rich sequences that contain stop codons in every frame but also because they lack over half of the mgpB expression site sequence that would be required for translation of a complete MgPa protein (42). MgPar sequences with homology to repeat regions B, EF, and G of mgpB are 79 to 90% identical to mgpB, including both strictly identical sequences that would be necessary for efficient homologous recombination (47, 59) as well as divergent sequences, highlighting their potential to generate mgpB heterogeneity via homologous recombination.
To validate the location of repeated sequences within mgpB, we analyzed the mgpB expression site for repetition elsewhere in the chromosome (not limiting the search to MgPar sequences) using a modification of the computer algorithm developed by Rocha and Blanchard (44). Consistent with previous studies (10, 42) and our pairwise alignment (Fig. 1B), sequence repetition was concentrated within the three distinct regions, B, EF, and G, while the remaining mgpB sequences were exclusive to the expression site (Fig. 2). These findings are different from a previously published in silico analysis in which an additional repeat region was identified between regions B and EF (44). This extra peak of repetition was not detected in this current study due to improvements in the analysis that made it more precise (see Materials and Methods).
FIG. 2.
Regions of mgpB sequence repetition in the M. genitalium G-37T genome. The computer-based algorithm of Rocha and Blanchard (44) was modified and used to probe the G-37T genome for sequences with 100% identity to overlapping 23-bp segments of the mgpB gene. Sequences of the mgpB gene found in multiple copies are concentrated within three regions that overlap with repeat regions B (bp 575 to 1016), EF (bp 2292 to 2876), and G (bp 3305 to 3550). Small arrows illustrate the locations of primers listed in Table 2.
In addition to confirming the concentration of genomic repeat sequences in mgpB regions B, EF, and G, the use of this algorithm allowed us to perform a more detailed examination of mgpB-MgPar homology not immediately apparent in the pairwise alignment. In this analysis, we identified sequences with exact identity between the mgpB repeat regions and external genome sequences as well as sequences that diverge. For example, in the region located between bp 2292 and 2876 (region EF) (Fig. 2), some sequences are unique to mgpB, while others are repeated outside the expression site as many as eight times. If homology within the MgPar sequences was constant (meaning an entire mgpB repeat region was duplicated in a MgPar sequence), the number of repeats would not vary over this region.
The in silico analysis described above also identified a sharp peak of repetition at bp 2964 (Fig. 2) that was found to result from seven consecutive “TAG” repeats that have identity to several other “TAG” trinucleotide repeats found elsewhere in the genome. A BLAST search of the M. genitalium G-37T genome revealed that this extended trinucleotide repeat (seven or more consecutive “TAG” repeats) was also present in the mgpC gene and MgPars 2, 8, and 9, each containing 11, 16, 10, and 9 “TAG” repeats, respectively. In addition, regardless of the number of “TAG” repeats at all five locations (mgpB, mgpC, and MgPars 2, 8, and 9) each trinucleotide repeat was flanked 5′ by “TGA” and 3′ by “TAC.” While the “TAG” codon encodes a translational stop in mycoplasmas, in silico translation of the trinucleotide repeat sequence in mgpB suggests that the corresponding MgPa protein would contain a run of seven serine residues (“AGT”). It is interesting, however, that a shift in the reading frame via slipped strand mispairing, for example, could change the expressed amino acid sequence to include seven valine residues (“GTA”) or seven stop codons (“TAG”).
In vitro heterogeneity of the mgpB gene in M. genitalium G37-S.
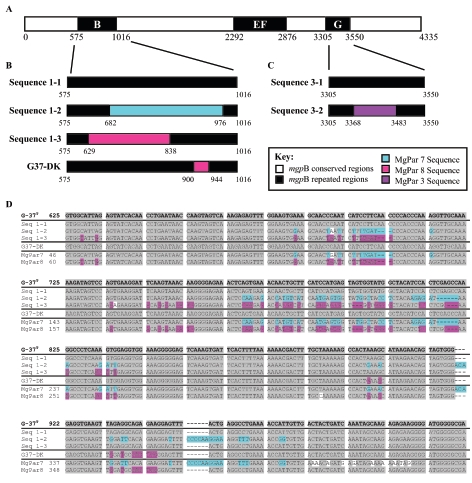
To determine the extent of mgpB sequence variation in M. genitalium G37-S, the stock of G-37 grown and maintained in Seattle, repeat regions B, EF, and G were PCR amplified from a single culture using primer sets 3F/3R, 4F/4R, and 5F/5R (Fig. 2), and the resulting amplicons were cloned into E. coli to separate individual PCR products, sequenced, and assessed for variability (Fig. 3). Three different sequences (sequences 1-1, 1-2, and 1-3) were identified in the clones analyzed from repeat region B (Fig. 3B). Sequence 1-1 was identical to the published G-37T mgpB sequence (identified in 23 of 25 clones), but sequences 1-2 and 1-3 (identified in one clone each) diverged over regions that instead had identity to MgPar 7 or 8, respectively (Fig. 3B and 3D). While variability in repeat region EF was not detected in this analysis, two different sequences were identified from repeat region G; sequence 3-1 (identified in 13 of 25 clones) was identical to the published G-37T mgpB sequence and sequence 3-2 (identified in 12 of 25 clones) contained divergent sequences identical to MgPar 3 (Fig. 3C). Variation of mgpB region B within the strain G-37 has been previously published for a separately maintained stock of G-37 (Fig. 3B, G37-DK) in which 45 bases had identity to MgPar 8 in place of the published G-37T sequence (42). The sequences of repeat region B are shown in the alignment in Fig. 3D in which the apparent insertion of MgPar sequences into the mgpB expression site can be better appreciated. Together, these results are consistent with multiple, independent recombination events between the mgpB and MgPar sequences.
FIG. 3.
Heterogeneity of the mgpB gene, determined by PCR amplification, cloning, and sequencing of M. genitalium G37-S. (A) Diagram of the mgpB gene showing the locations of repeat regions B, EF, and G. (B) Sequence heterogeneity identified in repeat region B. Sequence 1-1 was identical to the mgpB gene of the published M. genitalium G-37T sequence (17), while alternative sequences 1-2 and 1-3 contained 294 and 209 bases that were identical to MgPar 7 (103 to 391 bp) and MgPar 8 (65 to 263 bp), respectively, in place of the published mgpB sequence. The region B sequence for G37-DK (accession number X91075) (42) contains 45 bases that have identity to MgPar 8 (326 to 370 bp). (C) Sequence heterogeneity identified in the clones analyzed for repeat region G. Sequence 3-1 was identical to the M. genitalium G-37T published mgpB sequence, while sequence 3-2 contains 115 bp identical to MgPar 3 (474 to 592 bp) in place of the published mgpB sequence. (D) Nucleotide sequence alignment for repeat region B between the published sequences for G-37T mgpB, 1-1, 1-2, 1-3, G37-DK, and MgPars 7 and 8. The sequence heterogeneity is flanked by regions of sequence identity, and the conserved sequence at bp 838 to 900 is presumably where the recombination event between region B and MgPar 8 that generated sequence 1-3 and G37-DK took place. Bases identical to the G-37T sequence are highlighted in gray, while sequences identical to MgPars 7 and 8 are highlighted in aqua and pink, respectively. GenBank accession numbers for alternative G37-S sequences are as follows: 1-2, DQ248067; 1-3, DQ248068; 3-2, DQ248069. The accession numbers for MgPars 7 and 8 are listed in Table 1.
To predict whether the divergent nucleotide sequences identified in regions B and G would result in the expression of MgPa variants, the nucleotide sequences were assessed for variability at the amino acid level and the presence of UAG or UAA stop codons. When translated in silico, sequences 1-2, 1-3, and 3-2 all maintain the correct reading frame yet are predicted to encode alternative amino acid sequences (data not shown). Together, these experiments indicate that, even within a single stock strain of M. genitalium, the mgpB gene is heterogeneous, that sequence diversity observed is consistent with recombination between the mgpB expression site and alternative MgPar sequences, and that expression of these divergent sequences is predicted to result in the expression of variant MgPa proteins.
The development and evolution of mgpB repeat region B sequence heterogeneity in a woman persistently infected with M. genitalium.
The presence of nine MgPar sequences in the G-37T genome, as well as the heterogeneity of mgpB repeat regions B and G in a single culture of M. genitalium G37-S, prompted us to investigate the extent of mgpB heterogeneity in vivo. To accomplish this, we evaluated the scope and temporal relationships of mgpB sequence heterogeneity in mgpB repeat region B in the context of a persistent M. genitalium infection. A woman (participant no. 10139) from which 15 consecutive M. genitalium-positive cervical samples were obtained over a 21-month period was chosen for this analysis. Accordingly, repeat region B of the mgpB gene was amplified and sequenced from the DNA isolated from the first two (4 October 2001 and 14 February 2002) and last two (13 December 2002 and 9 July 2003) cervical samples collected to provide a longitudinal “snapshot” of the location and extent of in vivo mgpB region B diversity.
Among the 40 mgpB repeat region B sequences analyzed from participant no. 10139, 17 unique sequences (listed as A through Q in Fig. 4) were identified: three sequences (A to C) were identified at the first time point (4 October 2001), five (D to H) at the second time point (14 February 2002), three (I to K) at the third time point (13 December 2002), and six (L to Q) at the final time point (9 July 2003). Remarkably, in our analysis, none of the complete mgpB region B sequences were identified at more than one time point. For example, sequence G was identified in five different clones, all of which were derived from the 14 February 2002 cervical sample; this sequence was never identified in the analysis of the other three cervical samples (Fig. 4A). All sequences identified in this analysis contained regions with absolute identity to G-37T mgpB as well as regions that diverged from this published sequence (Fig. 4A). In some cases, these alternative sequences were identical to G-37T MgPar sequences (as noted in the legend to Fig. 4A), while others were not (and are designated “novel”). While all mgpB region B sequences share common elements (for example, sequences C, E to I, K, N, P, and Q all contained a 5′ sequence shown in Fig. 4A), each sequence is unique when mgpB region B is considered as a whole. Closer examination of these sequences revealed that sequences are chimeric and most likely originated from multiple recombination events with MgPar sequences (Fig. 4B). Several internal mgpB region B sequences are consistent with a temporal change in prevalence, as exemplified by the predominance and then absence of novel sequence 3 after the emergence, then dominance, of novel sequence 4 (Fig. 4A and C).
FIG. 4.
Repeat region B mgpB sequences identified in cervical specimens from participant no. 10139, a woman persistently infected with M. genitalium. A total of 40 sequences (10 per cervical sample) were analyzed, and 17 unique variants (A to Q) were detected in the four time points examined. (A) Schematic representation of the 17 sequences identified. White regions are homologous to the published G-37T sequence, while areas shaded as indicated by the key diverge from the mgpB sequence and instead have homology to G-37T MgPar sequences or have no homology to any sequences in the G-37T genome (designated “novel”). Block sizes shown in the key represent five nucleotides each. Sequence I contained an extra adenosine base (shown with an A) at bp 878, which is predicted to alter the reading frame and introduce three in-frame stop codons, indicated with red arrows. The schematically depicted sequences are presented in more detail in panels B and C. (B) Sequence alignment of mgpB bp 620 to 709 of G-37T; patient no. 10139 sequences A, B, D, E, and I; and the G-37T MgPar sequences showing the chimeric nature of mgpB sequences and possible explanations for their generation. Sequence A or B could have initially recombined with MgPar 4 to generate sequence D, which then could have recombined with novel sequence 1 to generate sequence E or I. (C) The shift in mgpB sequences over the course of a persistent infection exemplified by an alignment between sequences A through Q and the published G-37T mgpB sequence between bp 767 to 816. Bases with identity to the G-37T mgpB sequence are highlighted in gray, while bases identified as novel sequences 3 and 4 are shaded in red and yellow, respectively. The horizontal arrows at the top and bottom of panels A and C, respectively, indicate the conserved sequence targeted by primer 2R (Fig. 2; Table 2) used in the strain typing experiments. Sequences A through Q have been deposited in GenBank under accession numbers DQ248070 through DQ248086, respectively.
Similar to our observations with the alternative G37-S sequences discussed above, in silico translation of sequences A to Q (Fig. 4) predicts that each encodes divergent amino acid sequences (data not shown), with sequences A to H and J to Q remaining in the correct reading frame. Only sequence I was predicted to contain premature, in-frame translational stop codons (Fig. 4A), resulting from the insertion of an additional adenosine nucleotide (Fig. 4A) that alters the reading frame; other sequences contained six adenosines at this location. While we cannot rule out the possibility that the extra adenosine identified in sequence I is an artifact introduced during PCR amplification, it is not the result of a sequencing error, since both forward and reverse sequences contained seven adenosine residues. Despite this caveat, the identification of sequence I suggests the possibility that MgPa may undergo phase variation, since a full-length MgPa is not predicted to be expressed. Together, our results indicate that mgpB and, consequently, the expressed MgPa protein, are extremely diverse in vivo and may evolve over time in this persistently infected woman.
In vivo mgpB variability occurs within a single M. genitalium strain.
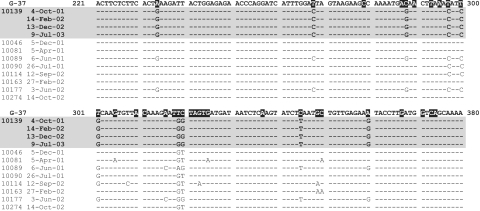
To investigate whether the mgpB heterogeneity identified in samples from participant no. 10139 was the result of infection by one or multiple M. genitalium strains, strain typing was performed on the same samples used to perform the repeat region B analysis (Fig. 4A). Primers 1F/1R (Fig. 2) were used to analyze the first 500 bp of the mgpB gene, which is present only once in the genome. In this experiment, all 20 sequences analyzed (five per time point × four time points) from participant no. 10139 were identical and clearly distinguishable from sequences obtained from eight other persistently infected women sampled at similar dates in the same cohort of women in Nairobi (Fig. 5). In addition, the longitudinal evaluation of seven women with infections lasting 10 months or longer has demonstrated that the persistence of one infecting strain over the course of a long-term M. genitalium infection, as seen with participant no. 10139, is not uncommon in this cohort of women (Cohen et al., submitted). These results are consistent with the conclusion that participant no. 10139 was infected with a single M. genitalium strain that is different from other strains circulating among this cohort during this study period.
FIG. 5.
Strain typing of M. genitalium sequences derived from cervical samples from women persistently infected with M. genitalium, suggesting that participant no. 10139 was infected with a single strain that can be distinguished from other strains present in this population during the same time period. Participant identification numbers and dates of sample collection are shown to the left. Dashed lines indicate sequence identity to the published G-37T sequence, between bp 221 and 380 of mgpB. Bases shaded in black are those previously shown to distinguish M. genitalium strains, which therefore serve as the basis of the strain typing system (29). For participant no. 10139, five clones were sequenced per cervical sample, while a minimum of three clones were sequenced from other persistently infected women; all sequences obtained from a given woman were identical. Sequences (with GenBank accession numbers) are as follows: 10139, DQ248092; 10046, DQ248087; 10081, DQ248088; 10089, DQ248089; 10090, DQ248090; 10114, DQ248091; 10163, DQ248093; 10177, DQ248094; 10274, DQ248095.
The close proximity of the mgpB strain typing region (29) and repeat region B allowed us to confirm that divergent region B sequences (Fig. 4) and conserved differential strain typing sequences (Fig. 5) occurred within the same genome. Therefore, primer set 2F/2R (Fig. 2 and 4) was used to amplify the entire strain typing region along with the first ∼260 bp of the heterogeneous region B from a cervical sample collected from participant no. 10139 at an intermediate time point (25 April 2002). In this analysis, five different sequences were obtained (data not shown). All contained the heterogeneity characteristic of bp 575 to 817 of sequences D, G, H, I, J, and K (Fig. 4) linked to the same characteristic strain typing sequence of the infecting M. genitalium strain (Fig. 5 and data not shown). These results, including the conservation of the same strain typing sequence over the period of observation, confirms that participant no. 10139 was persistently infected for almost 2 years with a single strain of M. genitalium and that the apparent evolution of mgpB repeat region B heterogeneity observed was not the result of coinfection with multiple strains.
DISCUSSION
In the current study, we analyzed the architecture and sequence homology between mgpB and the complete MgPar sequences in the M. genitalium type strain G-37T and showed that this strain contains multiple, partial copies of mgpB that have regions of identity and divergence with the mgpB expression site. These studies establish that mgpB sequence variation occurs in vitro within our laboratory strain M. genitalium G37-S and additionally demonstrate that sequence heterogeneity in vivo is extensive and evolves over time in a woman persistently infected with a single strain of M. genitalium. Altogether, these observations support the hypothesis that MgPar sequences serve as a reservoir of alternative mgpB sequences that, through recombination, allow antigenic variation of the MgPa protein, immune evasion, and persistence of infection (10, 17, 42).
The availability of a fully sequenced M. genitalium genome allowed us to expand on the number and architecture of the MgPar sequences, as well as their potential to generate variants of mgpB within a single strain. The M. genitalium G-37T strain available from ATCC was derived from a clonal isolate (57, 58); however, in the analysis of our stock culture of M. genitalium G37-S, we identified variant mgpB repeat region B and G sequences that were identical to sequences within identified MgPar sequences, consistent with recombination between the mgpB expression sites and these putative MgPar donor sites. Strain typing confirmed that the G37-S stock was not contaminated with other M. genitalium strains (data not shown), indicating that mgpB variability developed within this strain during in vitro passage of the organism. The diversity of mgpB sequences from M. genitalium cultured under laboratory conditions, however, may not be representative of its diversity in vivo due to the absence of the selective pressures pathogens may experience within a host, such as immune pressure, and/or to the possible selection of individual M. genitalium clones either at the time of isolation or during the unknown number of subcultures since its initial isolation in 1981 (57). Given this limitation, we evaluated the heterogeneity and variability of mgpB sequences from a woman persistently infected with a single strain of M. genitalium, and while limited sample volume prevented the performance of multiple PCRs, we remarkably identified a minimum of 17 different mgpB region B variants. Many of the regions containing divergent sequences in these variants were identical to the MgPar sequences published for M. genitalium G-37T, which is consistent with the conservation of at least some MgPar sequences between strains. However, other mgpB sequences differed from the reported mgpB and MgPar sequences of G-37T, suggesting that in other strains, MgPar sequences may diverge from those of the published M. genitalium genome. While extensive MgPar heterogeneity, including the identification of sequences not present in the G-37T genome, has been detected in other M. genitalium strains (S. L. Iverson-Cabral and P. A. Totten, unpublished data), further studies are needed to determine the variability within sequences and, possibly, numbers of MgPar sequences among and within M. genitalium strains. Any such findings would emphasize the potential for, and generation of, population diversity within this species.
The mgpB region B sequences identified in our analysis of the persistently infected woman suggests that sequential multiple recombination events occurred over the course of infection, although at present, we cannot determine if these mgpB variants were present in the initial inoculum or whether they were generated by recombination during infection. The chimeric nature of mgpB sequences and possible evolution over time is exemplified in the alignment shown in Fig. 4. The mgpB hybrid sequences detected in vivo are consistent with the pattern of mgpB heterogeneity within G37-S and the previously published G37-DK (42) in which different portions of MgPar 8 have recombined into a single mgpB repeat region at least two different ways (Fig. 3D). These in vitro and in vivo data demonstrate the occurrence of multiple recombination events between mgpB and the MgPar sequences, which would enhance the potential for MgPa diversity.
Heterogeneity and antigenic variation of immunodominant proteins has been characterized in other sexually transmitted pathogens, including Treponema pallidum and Neisseria gonorrhoeae. In T. pallidum, heterogeneity has been shown to accumulate within distinct variable regions of the tprK gene, with alternative sequences linked to putative tprK donor sequences found elsewhere in the genome (5). For N. gonorrhoeae, variant pilin proteins are expressed when silent, alternative pilS sequences recombine into the pilE expression site (38, 39). The variability of pilS sequences and the ongoing recombination between pilE and pilS sites allow N. gonorrhoeae an almost unlimited repertoire of pilin protein antigenic epitopes, which is dramatically increased by the variability and number of pilS sequences between N. gonorrhoeae strains (19). Pilin proteins help facilitate host cell attachment, and different pilE sequence variants have been associated with differences in host cell tropism (31), while antigenic and phase variation of another surface-expressed antigen, the opacity proteins (using mechanisms that differ from the pilE/pilS system described above), is responsible for the induction of different signaling pathways in target host cells (12, 20). It is currently unknown if MgPa variability similarly alters M. genitalium host cell tropism, facilitates different cell signaling pathways, and/or allows the organism to escape the host immune response.
The architecture and sequence similarity between MgPar and mgpB sequences strongly suggests that alternative sequences in mgpB are generated by homologous recombination. This process has been better characterized in other bacterial species, including E. coli and N. gonorrhoeae, in which recombination is dependent on the length and degree of sequence identity. In E. coli, homologous recombination requires flanking sequences containing a minimum of 20 bp of identity, with 25 to 100 bp as an average range (47, 59). In N. gonorrhoeae, antigenic variation occurs by homologous recombination between pilS and pilE sequences, as described above (38, 39), with variant sequences bordered by sequences of identity that range from 3 to 68 bp in length (24). Similar to these variant pilE sequences, we identified stretches of homologous sequences that range from less than 10 bp to more than 60 bp (Fig. 3D and 4B; see also Fig. S1 to S3 in the supplemental material) that flank variant mgpB sequences. These regions of identical sequences are not only found at the termini of mgpB repeat regions but also located within the repeat regions that flank internal regions of divergence, each of which could independently recombine to create an almost unlimited potential for mgpB heterogeneity and, therefore, MgPa diversity.
While very little is known about recombination in mycoplasma species, annotation of the M. genitalium genome suggests that the components for basic recombination are present, including homologues of the RecA, RuvA, RuvB, RecU, and PolA proteins (17, 45). A comparative analysis of the homologous recombination components present in various bacteria genomes suggests that the recombination machinery in M. genitalium is reduced and lacks important initiation enzymes such as RecBCD, RecQ, or RecFOR (4, 45). The lack of such important presynaptic proteins has also been observed for other bacterial pathogens, including the highly recombinant Helicobacter pylori (45, 54), suggesting that for many organisms, presynaptic functions are performed by other enzymes not identified in current genome annotations. Interestingly, in M. genitalium, the mgpA gene (MG190 in TIGR database) found upstream of mgpB and mgpC in the MgPa operon has a phosphoesterase motif and homology to the RecJ protein in E. coli (2). While both mgpB and mgpC are repeated throughout the genome (10, 42), the sequences within mgpA are not. It is tempting to speculate that mgpA provides the exonuclease activity required to produce a free 3′ single-stranded DNA to which RecA can bind for the initiation of homologous recombination (1). The mechanisms and requirements for recombination in the reduced M. genitalium genome, including the accessory proteins involved, needs to be evaluated not only to elucidate the recombination machinery of M. genitalium and other Firmicutes but also to clarify the requirements for recombination in other bacterial pathogens, such as H. pylori, that appear to lack large portions of a complete recombination system.
Regardless of how recombination occurs, the variant mgpB sequences identified in our analysis of a persistently infected woman imply that sequences change over time, presumably in response to immune pressure. As can be seen in the example given in Fig. 4C, novel sequence 4 was not identified at earlier time points but emerged in the third cervical sample and came to dominate sequences identified in the final sample. This coincides with the apparent elimination of novel sequence 3, which was predominant in earlier specimens. These observations, as well as our analysis of mgpB heterogeneity in vitro, is in agreement with the hypothesis that MgPa antibodies produced during persistent infection select new mgpB variants generated via mgpB-MgPar recombination until they in turn are eliminated by the immune system. Clearly, antibodies against the MgPa protein are induced during M. genitalium infection (6, 25, 37; Iverson-Cabral and Totten, unpublished), yet the antigenic epitopes of MgPa, the biologic activity of anti-MgPa antibodies, and the contribution of antibodies to the generation of protein diversity have not been established. The hypothesis of immune evasion relies on the assumption that variable MgPa regions (i.e., those encoded by mgpB regions B, EF, or G) correspond with immunogenic epitopes, and consistent with this model, Opitz and Jacobs (40) identified three regions of the MgPa protein that overlap with mgpB repeat regions B, EF, and G, which mediate attachment and are therefore assumed to be surface exposed. In addition to facilitating survival under immune pressure, variation of this dominant attachment protein may also contribute to colonization in different anatomical niches, promote evasion of uptake and killing by professional phagocytes, and/or enhance transmission of infection.
The architecture and repetition of mgpB sequences in M. genitalium are very similar to the P1 gene in the respiratory pathogen Mycoplasma pneumoniae, in which portions of the P1 gene are repeated throughout the M. pneumoniae genome (46, 60); the P1 protein of M. pneumoniae is the homologue of MgPa of M. genitalium (11, 37). Based on these similarities, one would presume that P1 antigenic variation occurs in M. pneumoniae (46); however, only minimal P1 heterogeneity has been documented among M. pneumoniae isolates with strains classified into one of two groups (50, 51). Subsequent restriction fragment length polymorphism analysis of over 200 clinical M. pneumoniae strains identified additional subtypes within these two groups (13, 14, 33). The sequencing of variant P1 genes indicated that sequence variation could be explained by recombination, with the repeated elements found outside the P1 expression site (13, 14, 33). Furthermore, M. pneumoniae strains expressing different P1 variants were shown to differ in their ability to colonize the respiratory tract of guinea pigs, suggesting that pathogenicity may be influenced by P1 variation (15). It is reasonable that MgPa expression similarly influences M. genitalium colonization, survival, and persistence in the genital tract, although future experiments will be required to establish this possibility. As the mgpB diversity reported here is much more extensive than what has been previously observed in M. pneumoniae, the study of M. genitalium mgpB sequence heterogeneity and its effect on persistence in vivo may serve as a model for future studies of M. pneumoniae P1 diversity and its relationship with chronic respiratory infection.
As indicated throughout this discussion, much remains to be discovered about the pathogenicity of M. genitalium, including the mechanisms and consequences of antigenic variation. This study provides compelling evidence that M. genitalium persistence is due to antigenic variation of the immunodominant MgPa protein. Our results are consistent with the recombination of alternative MgPar sequences into the mgpB expression site both in a strain of M. genitalium cultured in vitro as well as among sequences identified from a strain in a persistently infected woman. We hypothesize that the almost unlimited generation of mgpB diversity contributes to the chronic nature of M. genitalium infection. Future studies establishing the surface exposure of MgPa, the nature of mgpB-MgPar recombination, the contribution of immune response to the generation and selection of alternative mgpB sequences in vivo, the requirements for homologous recombination, and possible differences in binding and/or host cell signaling will promote a better understanding of the sophisticated mechanisms of persistence, survival, and pathogenesis of this genomically challenged organism.
Supplementary Material
Acknowledgments
This work was supported by NIH grant AI/HD48634 and the University of Washington STD Cooperative Research Center grant AI31448. S. Iverson-Cabral is supported by STD/AIDS Research Training Grant NIH/NIAID T32 AI007140.
We thank Gwen Wood, Arturo Centurion-Lara, and Steven Moseley for critical reading of the manuscript, George Kenny for assistance with M. genitalium growth, and Sheila Lukehart for scientific discussion and suggestions.
Editor: D. L. Burns
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Amundsen, S. K., and G. R. Smith. 2003. Interchangeable parts of the Escherichia coli recombination machinery. Cell 112:741-744. [DOI] [PubMed] [Google Scholar]
- 2.Aravind, L., and E. V. Koonin. 1998. A novel family of predicted phosphoesterases includes Drosophila prune protein and bacterial RecJ exonuclease. Trends Biochem. Sci. 23:17-19. [DOI] [PubMed] [Google Scholar]
- 3.Bjornelius, E., P. Lidbrink, and J. S. Jensen. 2000. Mycoplasma genitalium in non-gonococcal urethritis—a study in Swedish male STD patients. Int. J. STD AIDS 11:292-296. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho, F. M., M. M. Fonseca, S. Batistuzzo De Medeiros, K. C. Scortecci, C. A. Blaha, and L. F. Agnez-Lima. 2005. DNA repair in reduced genome: the mycoplasma model. Gene 360:111-119. [DOI] [PubMed] [Google Scholar]
- 5.Centurion-Lara, A., R. E. LaFond, K. Hevner, C. Godornes, B. J. Molini, W. C. Van Voorhis, and S. A. Lukehart. 2004. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol. Microbiol. 52:1579-1596. [DOI] [PubMed] [Google Scholar]
- 6.Clausen, H. F., J. Fedder, M. Drasbek, P. K. Nielsen, B. Toft, H. J. Ingerslev, S. Birkelund, and G. Christiansen. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16:1866-1874. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, C. R., L. E. Manhart, E. A. Bukusi, S. Astete, R. C. Brunham, K. K. Holmes, S. K. Sinei, J. J. Bwayo, and P. A. Totten. 2002. Association between Mycoplasma genitalium and acute endometritis. Lancet 359:765-766. [DOI] [PubMed] [Google Scholar]
- 8.Collier, A. M., J. L. Carson, P. C. Hu, S. S. Hu, C. H. Huang, and M. F. Barile. 1990. Attachment of Mycoplasma genitalium to the ciliated epithelium of human fallopian tubes, p. 730-732. In G. H. C. G. Stanek, J. G. Tully, and R. F. Whitcomb (ed.), Recent advances in mycoplasmology. Gustav Fischer Verlag, Stuttgart, Germany.
- 9.Colman, S. D., P. C. Hu, W. Litaker, and K. F. Bott. 1990. A physical map of the Mycoplasma genitalium genome. Mol. Microbiol. 4:683-687. [DOI] [PubMed] [Google Scholar]
- 10.Dallo, S. F., and J. B. Baseman. 1991. Adhesin gene of Mycoplasma genitalium exists as multiple copies. Microb. Pathog. 10:475-480. [DOI] [PubMed] [Google Scholar]
- 11.Dallo, S. F., J. R. Horton, C. J. Su, and J. B. Baseman. 1989. Homologous regions shared by adhesin genes of Mycoplasma pneumoniae and Mycoplasma genitalium. Microb. Pathog. 6:69-73. [DOI] [PubMed] [Google Scholar]
- 12.Dehio, C., S. D. Gray-Owen, and T. F. Meyer. 1998. The role of neisserial Opa proteins in interactions with host cells. Trends Microbiol. 6:489-495. [DOI] [PubMed] [Google Scholar]
- 13.Dorigo-Zetsma, J. W., J. Dankert, and S. A. Zaat. 2000. Genotyping of Mycoplasma pneumoniae clinical isolates reveals eight P1 subtypes within two genomic groups. J. Clin. Microbiol. 38:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorigo-Zetsma, J. W., B. Wilbrink, J. Dankert, and S. A. Zaat. 2001. Mycoplasma pneumoniae P1 type 1- and type 2-specific sequences within the P1 cytadhesin gene of individual strains. Infect. Immun. 69:5612-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumke, R., I. Catrein, E. Pirkil, R. Herrmann, and E. Jacobs. 2003. Subtyping of Mycoplasma pneumoniae isolates based on extended genome sequencing and on expression profiles. Int. J. Med. Microbiol. 292:513-525. [DOI] [PubMed] [Google Scholar]
- 16.Dutro, S. M., J. K. Hebb, C. A. Garin, J. P. Hughes, G. E. Kenny, and P. A. Totten. 2003. Development and performance of a microwell-plate-based polymerase chain reaction assay for Mycoplasma genitalium. Sex. Transm. Dis. 30:756-763. [DOI] [PubMed] [Google Scholar]
- 17.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, R. D. Fritchman, J. F. Weidman, K. V. Small, M. Sandusky, J. Fuhrmann, D. Nguyen, T. R. Utterback, D. M. Saudek, C. A. Phillips, J. M. Merrick, J. F. Tomb, B. A. Dougherty, K. F. Bott, P. C. Hu, T. S. Lucier, S. N. Peterson, H. O. Smith, C. A. Hutchison III, and J. C. Venter. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 18.Gambini, D., I. Decleva, L. Lupica, M. Ghislanzoni, M. Cusini, and E. Alessi. 2000. Mycoplasma genitalium in males with nongonococcal urethritis: prevalence and clinical efficacy of eradication. Sex. Transm. Dis. 27:226-229. [DOI] [PubMed] [Google Scholar]
- 19.Hamrick, T. S., J. A. Dempsey, M. S. Cohen, and J. G. Cannon. 2001. Antigenic variation of gonococcal pilin expression in vivo: analysis of the strain FA1090 pilin repertoire and identification of the pilS gene copies recombining with pilE during experimental human infection. Microbiology 147:839-849. [DOI] [PubMed] [Google Scholar]
- 20.Hauck, C. R., and T. F. Meyer. 2003. ‘Small’ talk: Opa proteins as mediators of Neisseria-host-cell communication. Curr. Opin. Microbiol. 6:43-49. [DOI] [PubMed] [Google Scholar]
- 21.Hjorth, S. V., E. Björnelius, P. Lidbrink, L. Falk, B. Dohn, L. Berthelsen, L. Ma, D. H. Martin, and J. S. Jensen. Sequence based typing of Mycoplasma genitalium reveals sexual transmission. J. Clin. Micriobiol., in press. [DOI] [PMC free article] [PubMed]
- 22.Horner, P., B. Thomas, C. Gilroy, M. Egger, M. McClure, and D. Taylor-Robinson. 2003. Antibodies to Chlamydia trachomatis heat-shock protein 60 kDa and detection of Mycoplasma genitalium and Ureaplasma urealyticum are associated independently with chronic nongonococcal urethritis. Sex. Transm. Dis. 30:129-133. [DOI] [PubMed] [Google Scholar]
- 23.Horner, P. J., C. B. Gilroy, B. J. Thomas, R. O. Naidoo, and D. Taylor-Robinson. 1993. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 342:582-585. [DOI] [PubMed] [Google Scholar]
- 24.Howell-Adams, B., and H. S. Seifert. 2000. Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Mol. Microbiol. 37:1146-1158. [DOI] [PubMed] [Google Scholar]
- 25.Hu, P. C., U. Schaper, A. M. Collier, W. A. Clyde, Jr., M. Horikawa, Y. S. Huang, and M. F. Barile. 1987. A Mycoplasma genitalium protein resembling the Mycoplasma pneumoniae attachment protein. Infect. Immun. 55:1126-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inamine, J. M., K. C. Ho, S. Loechel, and P. C. Hu. 1990. Evidence that UGA is read as a tryptophan codon rather than as a stop codon by Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum. J. Bacteriol. 172:504-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inamine, J. M., S. Loechel, A. M. Collier, M. F. Barile, and P. C. Hu. 1989. Nucleotide sequence of the MgPa (mgp) operon of Mycoplasma genitalium and comparison to the P1 (mpp) operon of Mycoplasma pneumoniae. Gene 82:259-267. [DOI] [PubMed] [Google Scholar]
- 28.Jensen, J. S. 2004. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 18:1-11. [DOI] [PubMed] [Google Scholar]
- 29.Jensen, J. S., E. Bjornelius, B. Dohn, and P. Lidbrink. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J. Clin. Microbiol. 42:683-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen, J. S., R. Orsum, B. Dohn, S. Uldum, A. M. Worm, and K. Lind. 1993. Mycoplasma genitalium: a cause of male urethritis? Genitourin. Med. 69:265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsson, A. B., D. Ilver, P. Falk, J. Pepose, and S. Normark. 1994. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol. Microbiol. 13:403-416. [DOI] [PubMed] [Google Scholar]
- 32.Kenny, G. E. 1985. Mycoplasmas, p. 407-411. In H. W. Balows and H. J. Shadomy (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, D.C.
- 33.Kenri, T., R. Taniguchi, Y. Sasaki, N. Okazaki, M. Narita, K. Izumikawa, M. Umetsu, and T. Sasaki. 1999. Identification of a new variable sequence in the P1 cytadhesin gene of Mycoplasma pneumoniae: evidence for the generation of antigenic variation by DNA recombination between repetitive sequences. Infect. Immun. 67:4557-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maeda, S., M. Tamaki, M. Nakano, M. Uno, T. Deguchi, and Y. Kawada. 1998. Detection of Mycoplasma genitalium in patients with urethritis. J. Urol. 159:405-407. [DOI] [PubMed] [Google Scholar]
- 35.Manhart, L. E., C. W. Critchlow, K. K. Holmes, S. M. Dutro, D. A. Eschenbach, C. E. Stevens, and P. A. Totten. 2003. Mucopurulent cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187:650-657. [DOI] [PubMed] [Google Scholar]
- 36.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Morrison-Plummer, J., A. Lazzell, and J. B. Baseman. 1987. Shared epitopes between Mycoplasma pneumoniae major adhesin protein P1 and a 140-kilodalton protein of Mycoplasma genitalium. Infect. Immun. 55:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nassif, X., C. Pujol, P. Morand, and E. Eugene. 1999. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol. Microbiol. 32:1124-1132. [DOI] [PubMed] [Google Scholar]
- 39.Nassif, X., and M. So. 1995. Interaction of pathogenic neisseriae with nonphagocytic cells. Clin. Microbiol. Rev. 8:376-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opitz, O., and E. Jacobs. 1992. Adherence epitopes of Mycoplasma genitalium adhesin. J. Gen. Microbiol. 138:1785-1790. [DOI] [PubMed] [Google Scholar]
- 41.Pepin, J., A. C. Labbe, N. Khonde, S. Deslandes, M. Alary, A. Dzokoto, C. Asamoah-Adu, H. Meda, and E. Frost. 2005. Mycoplasma genitalium: an organism commonly associated with cervicitis among west African sex workers. Sex. Transm. Infect. 81:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson, S. N., C. C. Bailey, J. S. Jensen, M. B. Borre, E. S. King, K. F. Bott, and C. A. Hutchison III. 1995. Characterization of repetitive DNA in the Mycoplasma genitalium genome: possible role in the generation of antigenic variation. Proc. Natl. Acad. Sci. USA 92:11829-11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson, S. N., P. C. Hu, K. F. Bott, and C. A. Hutchison III. 1993. A survey of the Mycoplasma genitalium genome by using random sequencing. J. Bacteriol. 175:7918-7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocha, E. P., and A. Blanchard. 2002. Genomic repeats, genome plasticity and the dynamics of Mycoplasma evolution. Nucleic Acids Res. 30:2031-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rocha, E. P., E. Cornet, and B. Michel. 2005. Comparative and evolutionary analysis of the bacterial homologous recombination systems. PLoS Genet. 1:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruland, K., R. Wenzel, and R. Herrmann. 1990. Analysis of three different repeated DNA elements present in the P1 operon of Mycoplasma pneumoniae: size, number and distribution on the genome. Nucleic Acids Res. 18:6311-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen, P., and H. V. Huang. 1986. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics 112:441-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simms, I., K. Eastick, H. Mallinson, K. Thomas, R. Gokhale, P. Hay, A. Herring, and P. A. Rogers. 2003. Associations between Mycoplasma genitalium, Chlamydia trachomatis and pelvic inflammatory disease. J. Clin. Pathol. 56:616-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su, C. J., and J. B. Baseman.. 1990. Genome size of Mycoplasma genitalium. J. Bacteriol. 172:4705-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su, C. J., A. Chavoya, S. F. Dallo, and J. B. Baseman. 1990. Sequence divergency of the cytadhesin gene of Mycoplasma pneumoniae. Infect. Immun. 58:2669-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su, C. J., S. F. Dallo, A. Chavoya, and J. B. Baseman. 1993. Possible origin of sequence divergence in the P1 cytadhesin gene of Mycoplasma pneumoniae. Infect. Immun. 61:816-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor-Robinson, D. 2002. Mycoplasma genitalium--an up-date. Int. J. STD AIDS 13:145-151. [DOI] [PubMed] [Google Scholar]
- 53.Taylor-Robinson, D., C. B. Gilroy, B. J. Thomas, and P. E. Hay. 2004. Mycoplasma genitalium in chronic non-gonococcal urethritis. Int. J. STD AIDS 15:21-25. [DOI] [PubMed] [Google Scholar]
- 54.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 55.Totten, P. A., L. E. Manhart, and A. Centurion-Lara. 2004. PCR detection of Haemophilus ducreyi, Treponema pallidum, and Mycoplasma genitalium, p. 349-360. In D. H. Persing, F. C. Tenover, J. Versalovic, Y.-W. Tang, E. R. Unger, D. A. Relman, and T. J. White (ed.), Molecular microbiology: diagnostic principles and practice. ASM Press, Washington, D.C.
- 56.Totten, P. A., M. A. Schwartz, K. E. Sjostrom, G. E. Kenny, H. H. Handsfield, J. B. Weiss, and W. L. Whittington. 2001. Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J. Infect. Dis. 183:269-276. [DOI] [PubMed] [Google Scholar]
- 57.Tully, J. G., D. Taylor-Robinson, R. M. Cole, and D. L. Rose. 1981. A newly discovered mycoplasma in the human urogenital tract. Lancet i:1288-1291. [DOI] [PubMed] [Google Scholar]
- 58.Tully, J. G., D. Taylor-Robinson, D. L. Rose, R. M. Cole, and J. M. Bove. 1983. Mycoplasma genitalium, a new species from the human urogenital tract. Int. J. Syst. Bacteriol. 33:387-396. [Google Scholar]
- 59.Watt, V. M., C. J. Ingles, M. S. Urdea, and W. J. Rutter. 1985. Homology requirements for recombination in Escherichia coli. Proc. Natl. Acad. Sci. USA 82:4768-4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wenzel, R., E. Pirkl, K. Ruland, and R. Herrmann. 1990. Multiple copies of Mycoplasma pneumoniae gene P1 sequences, p. 193-198. In G. H. C. G. Stanek, J. G. Tully, and R. F. Whitcomb (ed.), Recent advances in mycoplasmology. Gustav Fischer Verlag, Stuttgart, Germany.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.