Abstract
Many African countries currently use a sulfadoxine-pyrimethamine combination (SP) or amodiaquine (AQ) to treat uncomplicated Plasmodium falciparum malaria. Both drugs represent the last inexpensive alternatives to chloroquine. However, resistant P. falciparum populations are largely reported in Africa, and it is compulsory to know the present situation of resistance. The in vivo World Health Organization standard 28-day test was used to assess the efficacy of AQ and SP to treat uncomplicated falciparum malaria in Gabonese children under 10 years of age. To document treatment failures, molecular genotyping to distinguish therapeutic failures from reinfections and drug dosages were undertaken. A total of 118 and 114 children were given AQ or SP, respectively, and were monitored. SP was more effective than AQ, with 14.0 and 34.7% of therapeutic failures, respectively. Three days after initiation of treatment, the mean level of monodesethylamodiaquine (MdAQ) in plasma was 149 ng/ml in children treated with amodiaquine. In those treated with SP, mean levels of sulfadoxine and pyrimethamine in plasma were 100 μg/ml and 212 ng/ml, respectively. Levels of the three drugs were higher in patients successfully treated with AQ (MdAQ plasma levels) or SP (sulfadoxine and pyrimethamine plasma levels). Blood concentration higher than breakpoints of 135 ng/ml for MdAQ, 100 μg/ml for sulfadoxine, and 175 ng/ml for pyrimethamine were associated with treatment success (odds ratio: 4.5, 9.8, and 11.8, respectively; all P values were <0.009). Genotyping of merozoite surface proteins 1 and 2 demonstrated a mean of 4.0 genotypes per person before treatment. At reappearance of parasitemia, both recrudescent parasites (represented by common bands in both samples) and newly inoculated parasites (represented by bands that were absent before treatment) were present in the blood of most (51.1%) children. Only 3 (6.4%) therapeutic failures were the result not of treatment inefficacy but of new infection. In areas where levels of drug resistance and complexity of infections are high, drug dosage and parasite genotyping may be of limited interest in improving the precision of drug efficacy measurement. Their use should be weighted according to logistical constraints.
The spread of resistance of Plasmodium falciparum to chloroquine has pointed to the necessity for several African countries to identify alternative drugs as first-line antimalarial treatment, taking into account their activity against local parasite strains but also their tolerability, ease of administration, and cost (15, 17). Most African countries have now selected the combination of sulfadoxine and pyrimethamine (SP) as either first-line or second-line treatment for nonsevere malaria attacks (31). However, several studies also indicate that amodiaquine (AQ) is effective in treating chloroquine-resistant P. falciparum malaria parasites (10, 23), and recent studies report high cure rates in Africa (1, 4, 34), despite concerns about the hematological and hepatological side effects reported during prophylactic use of this drug (24).
In vivo and in vitro tests have been in use for more than 25 years for assessing the activity of drugs against P. falciparum (37). However, both tests possess their own drawbacks (3). In vitro tests reflect the mean susceptibility of the multiple parasite clonal lines present in an individual and may not be able to detect minor parasite populations that are resistant to the drug and that are at the origin of treatment failures. In addition, these tests circumvent the effects of individuals' immunity, which are added to that of the drug to achieve parasite clearance. Several point mutations (7, 16) or gene size polymorphisms (36) have been proposed as molecular markers of resistance to various antimalarial agents, but the correlations between molecular patterns and in vivo and in vitro drug sensitivity still need to be validated before these tests may be used for public health purposes. In vivo tests are a much more direct measurement of treatment efficacy in the target population but cannot distinguish late parasite recrudescences due to treatment failures from parasite reinfections in areas of malaria transmission. This is of utmost importance, since treatment failures usually occur more than 7 days after treatment, at a time when newly inoculated parasite strains may be present in the peripheral blood after having terminated their exoerythrocytic cycle. Moreover, pharmacokinetics of most antimalarial drugs are highly variable among patients, and it is important to differentiate a treatment failure due to parasite resistance or to poor metabolism, not allowing an efficient level of drug to be reached in the blood. Chromatography techniques have been designed that allow an accurate and sensitive determination of the level of most antimalarial drugs in blood (11, 12, 26). Molecular techniques, on their side, allow characterization of the parasite populations present in the blood (33) and therefore differentiation between recrudescences and reinfections. Although these techniques are quite sensitive, they also are money and time consuming and often require additional blood sampling that may not be easy to achieve during field studies. Thus, it is of interest to determine the genuine usefulness of such techniques in conducting drug sensitivity studies. In order to assess whether treatment failures observed during a standard in vivo test were related to treatment efficacy or whether they were the consequence of poor metabolism of the drug or of reinfection, we conducted such in vivo testing and measured the drug level achieved in plasma after administration of treatment. In parallel, we compared the polymorphism of merozoite protein 1 (MSP-1) and MSP-2 genes in the parasite population before treatment and at reappearance of parasitemia.
MATERIALS AND METHODS
Study area and population.
The study was conducted in Bakoumba, a village located in southeast Gabon in the Haut-Ogooué province, between January and June 2000. This village of 3,000 inhabitants is surrounded by the equatorial forest and belongs to an area to which P. falciparum malaria is mesoendemic to hyperendemic, where parasite transmission is perennial with seasonal variations according to the rains (13). Children of ages 6 months to less than 10 years, presenting at the outpatient clinic with nonsevere malaria attack, were enrolled for a 28-day follow-up study according to World Health Organization (WHO) protocol (37). Children were enrolled if the following criteria were met: age of <10 years, clinical illness compatible with malaria, presence of fever (axillary temperature, ≥37.5°C), P. falciparum parasite density of >2,000 asexual parasites/μl, hemoglobin rate of >5 g/dl, glycemia of >0.4 g/liter, and without any of the complications defined by WHO guidelines (38). Children presenting with coma or neurological signs, circulatory collapse, hyperparasitemia above 5%, or jaundice were not included, as they were considered to have severe malaria, and were referred to the hospital's physician. The study was approved by the Centre International de Recherches Médicales de Franceville ethical committee, and informed consent was obtained from all parents or guardians.
Treatment and follow-up of children.
At enrollment, a medical history was taken and a clinical examination was made. A finger-prick blood sample was obtained to measure parasite density, and children were orally given SP (Creat, Vernouillet, France) or AQ (Camoquin, Parke Davis, Dakar, Senegal) under supervision. Each treatment regimen was given to children enrolled every other week, and the dosage regimen was based on the child's weight: 25 mg of sulfadoxine/kg of body weight and 1.25 mg of pyrimethamine/kg as a single dose on day zero (D0), or 30 mg of AQ/kg given in three equal doses on D0 and days 1 and 2. Treatment was completed with three doses of paracetamol per day (10 mg/kg per day) at D0 and day 1. Each antimalarial dose was given at the hospital, whereas paracetamol doses were given by parents at home. When a child fulfilled criteria of early or late clinical failure (see data analysis), the child was given an additional treatment with AQ or SP, the drug he had not received as initial treatment, and was referred to hospital's physician. Children presenting with parasites in blood at the end of the follow-up were also given an additional treatment. Parents were asked to bring their child back on days 1, 2, 3, 7, 14, and 28 as well as any other day if the child was unwell. Temperature and parasite density were measured at each visit. Hemoglobin and glycemia rates were evaluated on D0 and the following days if values were, respectively, less than 8 g/dl and 0.6 g/liter at D0. Following finger-prick puncture, 3 drops of blood were collected on filter paper at D0, days 7, 14, and 28, and any other day if the child was unwell.
Parasite density, glycemia, and hemoglobin evaluation.
All thick blood smears were Giemsa stained and examined against 500 leukocytes. Parasite densities were recorded as the number of parasites/microliter of blood, assuming an average leukocyte count of 8,000/μl. All blood smears were examined twice. Glycemia was measured with a hemoglucotest (Glucotrend; Roche, Mannheim, Germany). To evaluate hemoglobin rates, blood was collected by finger-prick on filter paper, and color was compared to a color scale, as described elsewhere (35). Hemoglobin typing was determined by electrophoresis.
Concentrations of antimalarial drugs in plasma.
To determine monodesethyl-AQ (MdAQ), pyrimethamine, sulfadoxine, chloroquine, and quinine concentrations, venous blood samples were collected before treatment at D0 and at day 3 in EDTA tubes. Blood was conserved at 4°C before centrifugation and plasma separation within 4 h. Plasma was conserved at −20°C before analysis by high-performance liquid chromatography.
DNA preparation and PCR amplification.
After examination of blood smears, DNA extraction was performed on blood samples from subjects presenting with a clinical and/or parasitological failure. DNA was prepared from blood collected at enrollment and on the day when parasites reappeared in blood. Blood collected on Whatmann 3MM filter paper was dried and conserved at room temperature until extraction. DNA was prepared by Chelex extraction, as previously described (25). Briefly, blood-blotted filter papers were incubated with 1 ml of 0.5% saponin in phosphate-buffered saline (PBS) (pH 7.4) and stored overnight at 4°C. After microcentrifugation, the PBS-saponin solution was aspirated and replaced with 1 ml of PBS, and the tubes were incubated for 15 to 30 min at 4°C. After microcentrifugation, the PBS was aspirated and 100 μl of H2O was added with 50 μl of a stock solution of 20% Chelex-100 (Bio-Rad, Richmond, Calif.). The tubes were heated at 100°C for 10 min in a thermal cycler. After centrifugation at maximal speed for 5 min, supernatants were recovered, centrifuged again for 10 min, and collected into final tubes. Supernatants were stored at −20°C before being used for PCR.
The oligonucleotide primers were designed from published sequences, as listed in the nucleotide BLAST database, to amplify the polymorphic regions, block 2 of MSP-1 (20) and block 3 of MSP-2 (32) (Table 1). The two genes were amplified by nested PCR, with each amplification with a conserved or family-specific primer pair being done separately. All reactions were done in a 50-μl final volume, containing 100 mM Tris-HCl, 15 mM MgCl2, 500 mM KCl (pH 8.3), a 200 μM concentration (each) of the four dNTPs, a 450 μM concentration (each) of the two appropriate primers, and 2 U of Taq DNA polymerase (Roche, Mannheim, Germany). In the first reaction, 4 μl of Chelex-extracted DNA was added as a template, and 1 μl of the first PCR product was added in the second reaction. Denaturation at 94°C for 5 min preceded 30 amplification cycles: denaturation for 2 s at 94°C, annealing for 1 min 30 s at 55°C (first reactions for MSP-1 and MSP-2) or 58°C (all nested reactions for MSP-1, and the nested reaction for the MSP-2 3D7 family) or 61°C (the nested reaction for MSP-2 FC27 family), and extension for 2 min at 72°C. The last extension was carried out for 10 min. PCR products were electrophoresed on 1.5% agarose gels in TBE buffer (100 mM Tris, 100 mM boric acid, and 5 mM EDTA). DNA was visualized by UV transillumination after staining with ethidium bromide, and fragments obtained were compared by size.
TABLE 1.
Sequences of oligonucleotide primers used to amplify MSP-1 and MSP-2 polymorphic regions of P. falciparum isolates from Gabon, 2000
| Gene product | Primer | Sequence | Note |
|---|---|---|---|
| MSP-1 | MSP1-C1 | 5′-AAGCTTTAGAAGATGCAGTATTGAC | Conserved |
| MSP1-C2 | 5′-ATTCATTAATTTCTTCATATCCATC | Conserved | |
| K1a | 5′-GAAATTACTACAAAAGGTGCAAGTG | K1 family specific | |
| K1b | 5′-AGATGAAGTATTTGAACGAGGTAAAGTG | K1 family specific | |
| MAD20a | 5′-AAATGAAGGAACAAGTGGAACAGCTGTTAC | MAD20 family specific | |
| MAD20b | 5′-ATCTGAAGGATTTGTACGTCTTGAATTACC | MAD20 family specific | |
| RO33a | 5′-TAAAGGATGGAGCAAATACTCAAGTTGTTG | RO33 family specific | |
| RO33b | 5′-CATCTGAAGGATTTGCAGCACCTGGAGATC | RO33 family specific | |
| MSP-2 | MSP2-C1 | 5′-ATGAAGGTAATTAAAACATTGTCTATTATA | Conserved |
| MSP2-C2 | 5′-CTTTGTTACCATCGGTACATTCTT | Conserved | |
| FC27a | 5′-GCAAATGAAGGTTCTAATACTAATAG | FC27 family specific | |
| FC27b | 5′-GCTTTGGGTCCTTCTTCAGTTGATTC | FC27 family specific | |
| 3D7a | 5′-AGAAGTATGGCAGAAAGTAAGCCTCCTACT | 3D7 family specific | |
| 3D7b | 5′-GATTGTAATTCGGGGGATTCAGTTTGTTCG | 3D7 family specific |
Data analysis.
Both clinical and parasitological responses were considered in analyzing treatment efficacy, according to the revised WHO in vivo protocol for areas of intense transmission (39) (see Table 2), but the follow-up was extended to 28 days. This classification differs from the preceding one (37) by the recognition of an additional group (inside the late treatment failures group) of late parasitological failures defined by the presence of parasitemia on any day between days 14 and 28, without meeting any of the criteria of early treatment failure or late clinical failure.
TABLE 2.
Revised WHO classification of treatment failures in areas of intense malaria transmission
| Criterion |
|---|
| Early treatment failure |
| Development of danger signs or severe malaria on day 1, day 2 or day 3, in the presence of parasitemia |
| Parasitemia on day 3 with axillary temperature of ≥37.5°C |
| Parasitemia on day 2 higher than day 0 count |
| Parasitemia on day 3 ≥25% of count on day 0 |
| Late treatment failure |
| LCF |
| Development of danger signs or severe malaria after day 3 in the presence of parasitemia |
| Presence of parasitemia and axillary temperature >37.5°C on any day from day 4 to day 28, without previously meeting any of the criteria of early treatment failure |
| LPF |
| Presence of parasitemia on any day from day 14 to day 28, and axillary temperature <37.5°C, without previously meeting any of the criteria of early treatment failure or late clinical failure |
| ACPR |
| Absence of parasitemia on day 28 irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure or late clinical or parasitological failure |
To distinguish true therapeutic failures, with recrudescence of parasites that were present before treatment administration, from reinfections with blood proliferation of newly inoculated parasites, the DNA patterns of the parasitized blood collected on D0 and day of failure (DF) were compared according to bands' size and number. D0 and DF patterns were the combination of bands from the three families of MSP-1 and the two families of MSP-2 within each blood sample. Bands corresponding to alleles highly common (present in more than 85% of samples of a given family) were excluded from analysis. True therapeutic failure was considered when some or all bands observed in the DF pattern were also present in the D0 pattern, although additional bands may be present at D0. Reappearance of parasites in blood was considered to be due to reinfection when some or all bands present in the DF pattern were absent from the D0 pattern. Consequently, when the DF pattern exhibited both common and new bands as compared to that of D0, this was considered to involve both a recrudescence and a reinfection.
Distributions of qualitative data were assessed using a χ2 test. Distributions of qualitative data according to a quantitative variable were analyzed by Kruskal-Wallis test or Mann-Whitney U test. Dependence of quantitative values was controlled by correlation. Multivariate analyses were performed by logistic regression. Data were analyzed with the Statview software (SAS Institute Inc., Cary, N.C.) and BMDP (Los Angeles, Calif.) statistical software.
RESULTS
Treatment efficacy.
A total of 252 patients, less than 10 years old, were enrolled between January and June 2000. Twenty children (7.9%) (13 in the SP group and 7 in the AQ group) were not monitored after day 3 and were excluded from the analysis. Four children were followed until day 7 (three in the SP group and one in the AQ group) before being lost thereafter. Consequently, analysis was completed with the clinical follow-up of 118 children treated with AQ at enrolment and 114 treated with SP combination. The two groups did not differ in clinical and biological characteristics before treatment (Table 3; all P values, >0.10). For the whole group, the mean age (± standard error [SE]) was 3.98 (±0.15) years, and 53.8% of the patients were boys. Overall, 20.3% of the children presented with the A/S hemoglobin type. On admission, mean axillary temperature was 38.1°C (±0.06), mean glycemia was 0.92 g/liter (±0.01), and geometric mean parasite density (95% confidence interval) was 42,283 (31,313 to 53,254) asexual parasites/μl of blood. At enrollment, 13 children presented with a hemoglobin rate between 5 and 7 g/dl; these were controlled the following days to ensure that normal levels were restored. Three cases of posttherapeutic pruritus occurred in children treated with AQ.
TABLE 3.
Clinical and biological characteristics of children at enrollment for malaria attack, Bakoumba, Gabon, 2000
| Parametera | Value for group
|
|
|---|---|---|
| AQ (n = 118) | SP (n = 114) | |
| Age (mean ± SE), yr | 4.07 (±0.2) | 3.88 (±0.2) |
| Sex ratio (F/M) | 56/62 | 51/63 |
| Wt (mean ± SE), kg | 14.2 (±0.5) | 13.5 (±0.5) |
| Axillary temperature (mean ± SE), °C | 38.2 (±0.08) | 38.1 (±0.09) |
| A/S hemoglobin type, % | 19.5 | 21.1 |
| Glycemia (mean ± SE), g/liter | 0.92 (±0.02) | 0.92 (±0.02) |
| GMPD (95% CI), per μl of blood | 46,585 (29,983-63,188) | 38,248 (24,001-52,496) |
F, female; M, male; CI, confidence interval; GMPD, geometric mean parasite density.
Table 4 shows in vivo efficacies of AQ and SP treatments. AQ was clinically less efficient than SP, with, respectively, 65.3 and 86.0% of adequate clinical and parasitological responses (ACPR) and 34.7 and 14.0% of therapeutic failures (χ2 test, P = 0.0002). However, by day 3, parasite clearance occurred more often and mean temperature was lower, following AQ treatment (χ2 test, P = 0.003, and Mann Whitney U-test, P = 0.002, respectively). Late parasitological failures (LPF) occurred in 20.3 and 9.6% of children treated with AQ and SP, respectively, while late clinical failures (LCF) occurred in 13.6 and 2.6%, respectively. If the follow-up had been restricted to 14 days, as in the WHO standard in vivo test, 17 therapeutic failures would have been detected following AQ treatment, and 7 would have been detected following SP treatment. In the AQ group, age favored therapeutic success, since the proportion of adequate clinical response increased with age (Kruskal Wallis test, P < 0.05). In contrast, there was no significant association between age and treatment outcome following SP treatment. For the whole group, high parasite densities at enrollment were related to treatment failure (Kruskal Wallis test, P = 0.004).
TABLE 4.
In vivo efficacy of AQ and SP treatments of nonsevere P. falciparum malaria attacks in children from southeast Gabon in 2000, according to the 2001 WHO classification applied to a 28-day follow-up studya
| Parameter | Value for group
|
P value | |
|---|---|---|---|
| AQ (n = 118) | SP (n = 114) | ||
| In vivo treatment response [no. (%) of children]b | |||
| ACPR | 77 (65.3) | 98 (86.0) | 0.0002 |
| ETF | 1 (0.8) | 2 (1.8) | |
| LTFc | |||
| LCF | 16 (13.6) | 3 (2.6) | |
| LPF | 24 (20.3) | 11 (9.6) | |
| Parasitological clearance by day 3 [no. (%) of children] | 97 (82.2) | 73 (64.0) | 0.003 |
| Mean axillary temperature (± SE) on day 3 | 36.4 (±0.03) | 36.6 (±0.05) | 0.002 |
Treatment response is divided into adequate clinical and parasitological response (ACPR), and early (ETF) or late (LTF) treatment failures. According to the presence or not of clinical symptoms, LTF is divided into LCF or LPF. See Materials and Methods for details.
One child treated with AQ and 3 treated with SP were monitored only until day 7.
LTF, late treatment failure.
Antimalarial concentrations in blood.
To assess antimalarial drug consumption in the target population before treatment, antimalarial agents were measured at day zero in 44 plasma samples randomly selected. Four subjects (9.1%) presented with measurable levels of CQ, monodesethylchloroquine (MdCQ), AQ, and/or MdAQ (first subject [values in ng/ml]: CQ, 143, and MdCQ, 46; second subject: MdCQ, 20, AQ, 71, and MdAQ, 92; third subject: AQ, 422; fourth subject: MdAQ, 50), while traces of MdAQ and/or chloroquine were found in nine subjects, and traces of quinine were found in four.
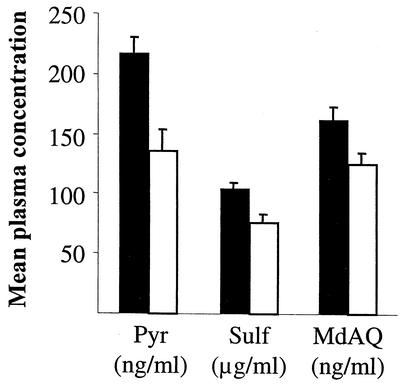
To assess posttreatment drug levels, MdAQ, sulfadoxine, and pyrimethamine were measured in 221 plasmas sampled at day 3. Mean (± SE) MdAQ, sulfadoxine, and pyrimethamine plasma concentrations were, respectively, 149.2 (±8.2) ng/ml, 100.0 (±4.2) μg/ml, and 212.0 (±14.4) ng/ml. These concentrations were positively related to treatment efficacy, as shown in Fig. 1. In both groups, the proportion of adequate clinical and parasitological responses was higher for subjects with a plasma drug concentration above the median (χ2 test, all P values < 0.04). The probability of having an ACPR according to plasma concentrations of MdAQ, sulfadoxine, and pyrimethamine (Table 5) and other covariates which appeared to be related to ACPR (such as age, parasite density, or glycemia) was assessed by multivariate analyses. Drug levels in plasma remained associated with treatment response. Different concentration thresholds were then tested in order to determine for each drug an optimal value of efficacy-to-failure ratio (highest estimated odds ratio). These breakpoints were, respectively, 135 ng/ml (MdAQ), 100 μg/ml (sulfadoxine), and 175 ng/ml (pyrimethamine).
FIG. 1.
Concentrations of pyrimethamine (Pyr) and sulfadoxine (Sulf) in plasma of 106 patients treated with sulfadoxine-pyrimethamine and of MdAQ in plasma of 112 patients treated with AQ in Gabon, 2000. All measurements were done 3 days after initiation of treatment. Concentrations of pyrimethamine and MdAQ are indicated in nanograms/milliliter, and those of sulfadoxine are in micrograms/milliliter. For each treatment, each group of children is divided according to treatment efficacy: adequate clinical and parasitological response (▪) and therapeutic failure (□).
TABLE 5.
Results of logistic regressions of ACPR on plasma concentrations of monodesethylamodiaquine (patients treated with AQ), sulfadoxine or pyrimethamine (patients treated with SP), and other covariatesa
| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Amodiaquine (> or ≤135 ng/ml) | 4.5 | 1.7-11.5 | 0.004 |
| Age | —b | — | 0.05 |
| Sulfadoxine (> or ≤100 μg/ml) | 9.8 | 1.9-50.3 | 0.009 |
| Glycemia | — | — | 0.06 |
| Pyrimethamine (> or ≤175 ng/ml) | 11.8 | 2.3-59.9 | 0.005 |
| Glycemia | — | — | 0.09 |
Drug thresholds were determined in order to maximize corresponding odds ratios (see the text for details). South-East Gabon, 2000.
—, odds ratios were not computed (quantitative variable).
MSP-1 and MSP-2 genotyping.
Of the 54 late treatment failures observed, DNA was extracted and polymerized from 52 paired samples of subjects, i.e., D0 and DF. Of these 52 pairs of samples, 5 had DF samples that gave no result at the MSP-1 or MSP-2 locus, and these pairs were subsequently not analyzed. Before treatment, allelic polymorphism of MSP-1 and MSP-2 loci showed high diversity of P. falciparum isolates, as 8 different alleles were obtained for MAD20 and 3D7 families, 11 for FC27, 13 for K1, and 3 for the RO33 family. Forty-seven (90.4%) D0 samples contained parasites belonging to the K1 family of MSP-1, 33 (63.5%)contained parasites belonging to the MAD20 family, and 19 (36.5%) contained parasites belonging to the RO33 family. For MSP-2, 34 (65.4%) isolates sampled contained parasites belonging to the FC27 family, and 43 (82.7%) contained parasites belonging to the 3D7 family. Before treatment, the mean number of genotypes per person (i.e., the mean value of the highest number of genotypes within any of the two markers) was 4.0.
Table 6 shows the classification of treatment failures with regard to recrudescence and reinfection. In the whole group of 47 recurrent parasitemia cases analyzed, 44 (93.6%) infections involved recrudescent parasites (represented by common bands in both samples), and 27 (57.4%) involved reinfections (represented by bands that were absent before treatment). The presence of both recrudescent and newly inoculated parasites was observed for 24 (53.2%) children, while 20 (42.6%) presented only recrudescent parasites, and 3 (6.4%) presented only new parasites. Among the 44 recrudescences, 7 occurred between days 7 and 13, 17 occurred between days 14 and 20, and 20 occurred between days 21 and 28. All “pure” reinfections had been treated with AQ and occurred at days 14 (LPF), 20 (LCF), and 21 (LPF).
TABLE 6.
Discrimination of therapeutic failures after AQ or SP administration, due to recrudescent parasites (presence of bands shared by the two patterns) from reinfections (appearance of new bands in the second pattern) using genotyping comparison of MSP-1 and MSP-2 molecular patterns of P. falciparum parasites in blood before treatment and at reappearance of parasites in blooda
| Drug | In vivo result | No. (%) of cases with:
|
||
|---|---|---|---|---|
| Recrudescence | Recrudescence + reinfection | Reinfection | ||
| AQ | LPF | 8 | 12 | 2 |
| LCF | 8 | 4 | 1 | |
| Total | 16 (46) | 16 (46) | 3 (8) | |
| SP | LPF | 4 | 7 | 0 |
| LCF | 0 | 1 | 0 | |
| Total | 4 (33) | 8 (67) | 0 | |
See Materials and Methods for details; southeast Gabon, 2000.
DISCUSSION
The efficacies of AQ and SP in treating nonsevere malaria attack in Gabonese children were analyzed according to the last classification proposed by the WHO (39), which integrates parasitological failures into late treatment failures. In the previous WHO classification (37), the reappearance of parasites in blood in the absence of clinical signs was classified not as a treatment failure but as an adequate clinical response. However, the reappearance of parasites in blood could be due to persistence of parasites that did not respond to treatment.
Our study demonstrates that the SP combination was more effective than AQ in treating uncomplicated malaria attacks in children less than 10 years old living in an area of Gabon to which the disease is highly endemic. Treatment failures with AQ occurred in 34.7% of children, while only 14.0% showed treatment failure after SP treatment. With both drugs, most failures were of the late type. During the present study, the efficacy of SP was somewhat better than in a previous study (70%) conducted in 1999 with a smaller group (66 children) from three different sites (one being Bakoumba) in two provinces of Gabon, all different in size and level of malaria endemicity (9). A similar level of resistance to SP was reported in Cameroon in 1997 and 1999 (30). Indeed, Central Africa seems to exhibit an intermediate prevalence of resistance to SP, between East Africa, where the resistance rate is around 30% in Uganda, Kenya, and Tanzania (18, 19, 22), and West Africa, where the rate of resistance remains lower, in the 0 to 10% range (5, 21). Our study revealed a worsening of the treatment efficacy of AQ, with more than 30% treatment failures. Twenty-eight percent resistance was already observed in Lambarene between 1994 and 1995 (27), and 13% resistance was observed in Libreville between 1996 and 1998 (6). This picture is very different from the one from Cameroon, where no case of resistance occurred in a recent study with adults and children (30). In East Africa, resistance rates are closer to our results, with 15 to 25% resistance in Kenya and Tanzania (18).
Field data on pharmacokinetics of AQ, sulfadoxine, or pyrimethamine are scarce. Despite an extensive search of the literature, we have been unable to find data related to the minimum AQ, sulfadoxine, or pyrimethamine concentration in human blood that is effective in curing a parasite strain susceptible in vitro to the corresponding drug. However, the day 3 plasma concentrations measured in the present study were in the same range as those obtained in Cameroon following administration of the same drug regimen (30). As in this study, interindividual variations were high. Interestingly, in our study, posttreatment MdAQ or SP plasma concentrations correlated to the treatment issue. In addition, we could determine by logistic regression optimal blood concentration breakpoints for MdAQ (135 ng/ml), sulfadoxine (100 μg/ml), and pyrimethamine (175 ng/ml), which best separated adequate responses from treatment failures. The quite high values of the odds ratios (4.5 to 11.8) demonstrate the importance of individual metabolism of drug in treatment efficacy.
Treatment failures were studied by molecular analysis of the MSP-1 and -2 genes to distinguish recrudescences from reinfections. Molecular genotyping is a suitable method for this purpose, since in vitro drug sensitivity data may not be sufficient to predict in vivo failures. Indeed, in vivo and in vitro data from high-transmission areas may not correlate, especially in the case of SP (3, 29, 30). The inhibitory concentration values derived from in vitro tests are the summation of the susceptibility values for the various clonal lines with which the infection originated, these lines being present in highly variable proportions. Since in areas of intense transmission of malaria, the majority of infections are due to multiple parasite lines, these mean values may hide minor clones with which recrudescence will originate. Furthermore, host immunity certainly plays an important role in adding its activity to that of the drug. The identification of molecular markers of resistance in parasite lines may be a useful alternative to in vitro tests. Currently the relevance of such markers still needs to be confirmed, in particular for 4-amino-quinoline compounds (36). In our study, the amplification of two markers, MSP-1 and MSP-2, was sufficient to distinguish reinfection from recrudescence. Both markers were useful since, if MSP-2 (the most polymorphic marker) were tested alone, results would have differed in 10 of the 47 instances: 9 cases of recrudescence plus reinfection would have been determined as recrudescences, and 1 case of recrudescence plus reinfection would have been established as pure reinfection. The RO33 family of MSP-1 exhibited a limited polymorphism and did not bring any information. This family was the least represented in our population of isolates, and the combination of the four remaining families was enough to distinguish reinfection from recrudescence. This may not be the case in areas of low transmission, where most infections are monoclonal.
In most instances, reappearance of parasites in blood following antimalarial treatment involved both the recrudescence of parasite clones present before treatment and the appearance of new clone(s) not present at D0. Such an observation was already reported from Gabon, where pre- and posttreatment samples from 108 children showed either identical parasites, a mixture of pretreatment and new parasites, or completely different parasites (28). This last group, corresponding to children presenting with a new infection, represented less than 13% of the whole group. In Papua New Guinea, AQ or chloroquine failures occurring in 12 children were all due to recrudescent parasites (2). Although reinfection with a clone presenting with a molecular profile similar to that of the D0 clone cannot be ruled out, the persistence of clones present at D0 most likely demonstrates that treatment failed to clear these parasites and thus demonstrates a true therapeutic failure. Conversely, although the multiplication of clones not present before treatment is most likely the consequence of reinfection, clones were shown to fluctuate in the peripheral blood with time, suggesting that some of them may have been hidden in the deep vasculature at D0. This phenomenon was demonstrated both in a low-transmission area during a several-month period of absence of transmission (40) and in holoendemic areas during a day-to-day study of genotypes (8, 14). Only three cases of pure reinfection, for which the molecular pattern of D0 and DF were completely different, were observed. Our method of analysis is likely to overestimate both recrudescences and reinfections, but this constitutes the limits of genotyping as an adjunct tool in treatment efficacy studies. The study of additional genetic polymorphic markers, such as GLURP, might have allowed the distinguishing of isolates that did not differ as regards MSP-1 and MSP-2 and would have even reduced the number of pure reinfections.
One of our objectives was to assess the usefulness of such a pharmacological and genotypic approach in better measuring drug efficacy. It is clear that interpretation of drug dosages requires the knowledge of the effective minimum concentrations of these drugs in blood, which seems to be still unknown. Before determination of such values, the usefulness of posttherapeutic drug dosages during field studies remains questionable at the individual level. From a pharmacokinetics point of view, the area under the curve better reflects the effective drug concentration, but such data cannot be collected in a field study, and low residual values indicate inadequate absorption and metabolism of the drug. Nevertheless, the importance of drug absorption and metabolism is demonstrated by the higher ACPR rate observed in patients with a high posttreatment concentration of drug in plasma. Among the 47 parasite cures followed by reappearance of parasites in blood (which were labeled “therapeutic failures” in the in vivo test), MSP genotyping was able to demonstrate that 3 (6.4%) of them were not the result of treatment inefficacy but only involved new infection. Therefore, the additional use of these techniques allows the assessment that therapeutic failures due to parasite resistance to treatment occurred in 32.2% (instead of 34.7%) of children treated with amodiaquine and in 14.0% (similar value) of children treated with SP. The use of parasite genotyping in addition to in vivo testing allows greater accuracy in determining the therapeutic efficacy of AQ and SP, but this gain was only moderate in the epidemiological conditions from Gabon. This combination of techniques allowed in vitro drug tests to be avoided in an area of high malaria transmission, where the complexity of infection is high (4.0 clones) and where immunity plays an important role, both phenomena reducing the relationship between in vitro test results and treatment efficacy. It is clear that in areas of such levels of drug resistance and such complexity of infections, the interest of assessing drug levels and parasite genotypes should be weighted with regard to the needed logistical constraints and the limited increase in the precision of the measurement of treatment efficacy. Nevertheless, the levels of resistance to AQ and the SP combination in this region of southeast Gabon begin to be alarming, and new antimalarial agents or new combinations urgently need to be tested and used in order to reduce the spread of resistance and to avoid the excessive use of quinine that is very frequent in this area.
Acknowledgments
This work was supported by the French Ministry of Research (VIHPAL grant). A. Aubouy was the recipient of a fellowship from the French Ministry of Research.
We are grateful to the children who participated in the study, as well as their mothers and guardians. We thank J. Mayombo for help in patients' management and J. Bourgeais, SODEPAL, for logistical support in Bakoumba. We also thank P. Ringwald, WHO EPH/DRS, P. Nguyen-Dinh, CDC, DPD, and W. K. Milhous, WRAIR, Washington, D.C., for helpful discussions.
REFERENCES
- 1.Adjuik, M., P. Agnamey, A. Babiker, S. Borrmann, P. Brasseur, M. Cisse, F. Cobelens, S. Diallo, J. F. Faucher, P. Garner, S. Gikunda, P. G. Kremsner, S. Krishna, B. Lell, M. Loolpapit, P. B. Matsiegui, M. A. Missinou, J. Mwanza, F. Ntoumi, P. Olliaro, P. Osimbo, P. Rezbach, E. Some, and W. R. Taylor. 2002. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomized, multicentre trial. Lancet 359:1365-1372. [DOI] [PubMed] [Google Scholar]
- 2.Al-Yaman, F., B. Genton, J. C. Reeder, R. F. Anders, and M. P. Alpers. 1997. Evidence that recurrent Plasmodium falciparum infection is caused by recrudescence of resistant parasites. Am. J. Trop. Med. Hyg. 56:436-439. [DOI] [PubMed] [Google Scholar]
- 3.Basco, L., and P. Ringwald. 2000. Chimiorésistance du paludisme: problèmes de la définition et de l'approche technique. Santé 10:47-50. [PubMed] [Google Scholar]
- 4.Basco, L. K., A. Same-Ekobo, V. F. Ngane, M. Ndounga, T. Metoh, P. Ringwald, and G. Soula. 2002. Therapeutic efficacy of sulfadoxine-pyrimethamine, amodiaquine and the sulfadoxine-pyrimethamine-amodiaquine combination against uncomplicated Plasmodium falciparum malaria in young children in Cameroon. Bull. W. H. O. 80:538-545. [PMC free article] [PubMed] [Google Scholar]
- 5.Bojang, K. A., G. Schneider, S. Forck, S. K. Obaro, S. Jaffar, M. Pinder, J. Rowley, and B. M. Greenwood. 1998. A trial of Fansidar plus chloroquine or Fansidar alone for the treatment of uncomplicated malaria in Gambian children. Trans. R. Soc. Trop. Med. Hyg. 92:73-76. [DOI] [PubMed] [Google Scholar]
- 6.Brasseur, P., R. Guiguemde, S. Diallo, V. Guiyedi, M. Kombila, P. Ringwald, and P. Olliaro. 1999. Amodiaquine remains effective for treating uncomplicated malaria in west and central Africa. Trans. R. Soc. Trop. Med. Hyg. 93:645-650. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, D. R., P. Wang, M. Read, W. M. Watkins, P. F. G. Sims, and J. E. Hyde. 1994. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthetase gene in lines of the human malaria parasite. Plasmodium falciparum, with differing resistance to sulfadoxine. Eur. J. Biochem. 2:397-405. [DOI] [PubMed] [Google Scholar]
- 8.Daubersies, P., S. Sallenave-Sales, S. Magne, J. F. Trape, H. Contamin, T. Fandeur, C. Rogier, O. Mercereau-Puijalon, and P. Druilhe. 1996. Rapid turnover of Plasmodium falciparum populations in asymptomatic individuals living in a high transmission area. Am. J. Trop. Med. Hyg. 54:18-26. [DOI] [PubMed] [Google Scholar]
- 9.Deloron, P., J. Mayombo, A. Le Cardinal, J. Mezui-Me-Ndong, C. Bruzi-Baert, F. Lekoulou, and N. Elissa. 2000. Sulfadoxine-pyrimethamine for the treatment of Plasmodium falciparum malaria in Gabonese children. Trans. R. Soc. Trop. Med. Hyg. 94:188-190. [DOI] [PubMed] [Google Scholar]
- 10.Deloron, P., J. D. Sexton, L. Bugilimfura, and C. Sezibera. 1988. Amodiaquine and sulfadoxine-pyrimethamine as treatment for chloroquine-resistant Plasmodium falciparum in Rwanda. Am. J. Trop. Med. Hyg. 38:244-248. [DOI] [PubMed] [Google Scholar]
- 11.Edstein, M. 1984. Quantification of antimalarial drugs. I. Simultaneous measurement of sulphadoxine, N4-acetylsulphadoxine and pyrimethamine in human plasma. J. Chromatogr. 305:502-507. [PubMed] [Google Scholar]
- 12.Edstein, M. 1984. Quantification of antimalarial drugs. II. Simultaneous measurement of dapsone, monoacetyldapsone and pyrimethamine in human plasma. J. Chromatogr. 307:426-431. [PubMed] [Google Scholar]
- 13.Elissa, N., S. Karch, P. Bureau, B. Ollomo, M. Lawoko, P. Yangari, B. Ebang, and A. J. Georges. 1999. Malaria transmission in a region of savanna-forest mosaic, Haut-Ogooue, Gabon. J. Am. Mosq. Control Assoc. 15:15-23. [PubMed] [Google Scholar]
- 14.Farnert, A., G. Snounou, I. Rooth, and A. Bjorkman. 1997. Daily dynamics of Plasmodium falciparum subpopulations in asymptomatic children in a holoendemic area. Am. J. Trop. Med. Hyg. 56:538-547. [DOI] [PubMed] [Google Scholar]
- 15.Fevre, E. M., and G. Barnish. 1999. Malaria-treatment policies: when and how should they be changed? Ann. Trop. Med. Parasitol. 93:549-560. [DOI] [PubMed] [Google Scholar]
- 16.Foote, S. J., D. Galatas, and A. F. Cowman. 1990. Amino acids in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum involved in cycloguanil resistance differ from those involved in pyrimethamine resistance. Proc. Natl. Acad. Sci. USA 87:3014-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster, S. 1994. Economic prospects for a new antimalarial drug. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):S55-S56. [DOI] [PubMed] [Google Scholar]
- 18.Gorissen, E., G. Ashruf, M. Lamboo, J. Bennebroek, S. Gikunda, G. Mbaruku, and P. A. Kager. 2000. In vivo efficacy study of amodiaquine and sulfadoxine/pyrimethamine in Kibwezi, Kenya and Kigoma, Tanzania. Trop. Med. Int. Health 5:459-463. [DOI] [PubMed] [Google Scholar]
- 19.Kamya, M. R., G. Dorsey, A. Gasasira, G. Ndeezi, J. N. Babirye, S. G. Staedke, and P. J. Rosenthal. 2001. The comparative efficacy of chloroquine and sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in Kampala, Uganda. Trans. R. Soc. Trop. Med. Hyg. 95:50-55. [DOI] [PubMed] [Google Scholar]
- 20.Miller, L. H., T. Roberts, M. Shahabuddin, and T. F. McCutchan. 1993. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol. Biochem. Parasitol. 59:1-14. [DOI] [PubMed] [Google Scholar]
- 21.Muller, O., M. B. van Hensbroek, S. Jaffar, C. Drakeley, C. Okorie, D. Joof, M. Pinder, and B. Greenwood. 1996. A randomized trial of chloroquine, amodiaquine and pyrimethamine-sulphadoxine in Gambian children with uncomplicated malaria. Trop. Med. Int. Health. 1:124-132. [DOI] [PubMed] [Google Scholar]
- 22.Ogutu, B. R., B. L. Smoak, R. W. Nduati, D. A. Mbori-Ngacha, F. Mwathe, and G. D. Shanks. 2000. The efficacy of pyrimethamine-sulfadoxine (Fansidar) in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children. Trans. R. Soc. Trop. Med. Hyg. 94:83-84. [DOI] [PubMed] [Google Scholar]
- 23.Olliaro, P., C. Nevill, J. LeBras, P. Ringwald, P. Mussano, P. Garner, and P. Brasseur. 1996. Systematic review of amodiaquine treatment in uncomplicated malaria. Lancet 348:1196-1201. [DOI] [PubMed] [Google Scholar]
- 24.Phillips-Howard, P. A., and L. J. West. 1990. Serious adverse drug reactions to pyrimethamine-sulphadoxine, pyrimethamine-dapsone and to amodiaquine in Britain. J. R. Soc. Med. 83:82-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plowe, C. V., A. Djimde, M. Bouare, O. Doumbo, and T. E. Wellems. 1995. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am. J. Trop. Med. Hyg. 52:565-568. [DOI] [PubMed] [Google Scholar]
- 26.Pussard, E., F. Verdier, and M. C. Blayo. 1986. Simultaneous determination of chloroquine, amodiaquine and their metabolites in human plasma, red blood cells, whole blood and urine by column liquid chromatography. J. Chromatogr. 374:111-118. [DOI] [PubMed] [Google Scholar]
- 27.Radloff, P. D., J. Philipps, M. Nkeyi, D. Hutchinson, and P. G. Kremsner. 1996. Atovaquone and proguanil for Plasmodium falciparum malaria. Lancet 347:1511-1514. [DOI] [PubMed] [Google Scholar]
- 28.Ranford-Cartwright, L. C., J. Taylor, T. Umasunthar, L. H. Taylor, H. A. Babiker, B. Lell, J. R. Schmidt-Ott, L. G. Lehman, D. Walliker, and P. G. Kremsner. 1997. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans. R. Soc. Trop. Med. Hyg. 91:719-724. [DOI] [PubMed] [Google Scholar]
- 29.Ringwald, P., and L. K. Basco. 1999. Comparison of in vivo and in vitro tests of resistance in patients treated with chloroquine in Yaounde, Cameroon. Bull. W. H. O. 77:34-43. [PMC free article] [PubMed] [Google Scholar]
- 30.Ringwald, P., A. Keundjian, A. Same Ekobo, and L. K. Basco. 2000. Chimiorésistance de Plasmodium falciparum en milieu urbain à Yaoundé, Cameroun. 2. Evaluation de l'efficacité de l'amodiaquine et de l'association sulfadoxine-pyriméthamine pour le traitement de l'accès palustre simple à Plasmodium falciparum à Yaoundé, Cameroun. Trop. Med. Int. Health. 5:620-627. [DOI] [PubMed] [Google Scholar]
- 31.Sibley, C. H., J. E. Hyde, P. F. Sims, C. V. Plowe, J. G. Kublin, E. K. Mberu, A. F. Cowman, P. A. Winstanley, W. M. Watkins, and A. M. Nzila. 2001. Pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: what next? Trends Parasitol. 17:582-588. [DOI] [PubMed] [Google Scholar]
- 32.Smythe, J. A., M. G. Peterson, R. L. Coppel, A. J. Saul, D. J. Kemp, and R. F. Anders. 1990. Structural diversity in the 45-kilodalton merozoite surface antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 39:227-234. [DOI] [PubMed] [Google Scholar]
- 33.Snewin, V. A., M. Herrera, G. Sanchez, A. Scherf, G. Langsley, and S. Herrera. 1991. Polymorphism of the alleles of the merozoite surface antigens MSA1 and MSA2 in Plasmodium falciparum wild isolates from Colombia. Mol. Biochem. Parasitol. 49:265-275. [DOI] [PubMed] [Google Scholar]
- 34.Staedke, S. G., M. R. Kamya, G. Dorsey, A. Gasasira, G. Ndeezi, E. D. Charlebois, and P. J. Rosenthal. 2001. Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: a randomised trial. Lancet 358:368-374. [DOI] [PubMed] [Google Scholar]
- 35.Stott, G. J., and S. M. Lewis. 1995. A simple and reliable method for estimating haemoglobin. Bull. W. H. O. 73:369-373. [PMC free article] [PubMed] [Google Scholar]
- 36.Su, X. Z., L. A. Kirkman, H. Fujioka, and T. E. Wellems. 1997. Complex polymorphisms in ∼330 kDa protein are linked to chloroquine-resistant P. falciparum in Southeast Asia and Africa. Cell 91:593-603. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. 1996. Assessment of therapeutic efficacy of antimalarial drugs for uncomplicated falciparum malaria in areas with intense transmission. Report no. MAL/96.1077. World Health Organization, Geneva, Switzerland.
- 38.World Health Organization. 2000. Communicable diseases cluster: severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 39.World Health Organization. 2002. Monitoring antimalarial drug resistance. Report no. CDS/CSR/EPH/2002.17. World Health Organization, Geneva, Switzerland.
- 40.Zwetyenga, J., C. Rogier, A. Spiegel, D. Fontenille, J. F. Trape, and O. Mercereau-Puijalon. 1999. A cohort study of Plasmodium falciparum diversity during the dry season in Ndiop, a Senegalese village with seasonal, mesoendemic malaria. Trans. R. Soc. Trop. Med. Hyg. 93:375-380. [DOI] [PubMed] [Google Scholar]