Abstract
Obligate human-pathogenic Neisseria gonorrhoeae expresses numerous variant surface proteins mediating adherence to and invasion of target cells. The invariant major outer membrane porin PorB of serotype A (P.IA) gonococci triggers invasion into Chang cells only if the medium is devoid of phosphate. Since gonococci expressing PorBIA are frequently isolated from patients with severe disseminating infections, the interaction initiated by the porin may be of major relevance for the development of this serious disease. Here, we investigated the low-phosphate-dependent invasion and compared it to the well-known pathways of entry initiated by Opa proteins. P.IA-triggered invasion requires clathrin-coated pit formation and the action of actin and Rho GTPases. However, in contrast to Opa-initiated invasion via heparan sulfate proteoglycans, microtubules, acidic sphingomyelinase, phosphatidylinositol 3-kinase, and myosin light chain kinase are not involved in this entry pathway. Nor are Src kinases required, as they are in invasion, e.g., via the CEACAM3 receptor. Invasion by PorBIA occurs in a wide spectrum of cell types, such as primary human epithelial and endothelial cells and in cancer cells of human and animal origin. Low-phosphate-dependent invasion is thus a pathway of gonococcal entry distinct from Opa-mediated invasion.
Due to emerging antibiotic resistances, Neisseria gonorrhoeae, the etiological agent of the sexually transmitted disease gonorrhea, is still a serious threat to world health, with an estimated 62 million infections occurring each year. During the infection process, the human-specific pathogen colonizes mucosal tissues and gains access to deeper tissues. In most cases the infection remains localized. However, in approximately 1 to 3% of cases, the gonococcus spreads within the host organism and causes systemic infections leading to serious conditions, such as endocarditis, meningitis, and pneumonia. The gonococcus expresses a remarkable variety of adhesins, like the type IV pili (28, 36), different phase-variable colony opacity-associated (Opa) outer membrane proteins (35) and the principal outer membrane protein, PorB (P.IA) (12, 37), the ribosomal protein L12 (34), and the lipooligosaccharide (19, 33). The adhesins recognize different cellular receptors (29), enabling the pathogen to interact with diverse tissues during the course of infection. Pili are thought to mediate the initial adhesion, followed by a close, primarily Opa-dependent interaction leading to the pathogen's engulfment. The family of the Opa proteins comprises 11 different proteins (2), which are subdivided into two different classes according to their differential binding specificities. The first class, the Opa50 adhesin, binds to surface heparan sulfate proteoglycan (HSPG) receptors (7, 39). The second class of Opa proteins, the Opa51-60 adhesins, targets CD66 epitope-containing members of the carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs).
Interaction with the different receptor classes induces the activation of distinct signaling cascades. Binding of gonococci to HSPG receptors activates the lipid-hydrolyzing enzymes phosphatidylcholine-specific phospholipase C and the acid sphingomyelinase (ASM), generating ceramide (16). As a consequence, bacteria are phagocytosed in a microfilament-dependent and, perhaps, a microtubule-dependent process (17). In some cell lines, however, efficient bacterial entry requires the additional interaction with the serum-derived extracellular matrix proteins fibronectin and vitronectin (10, 11, 14, 38). In turn, vitronectin mediates binding to integrins and activation of protein kinase C (10, 13). Gonococcal engulfment via CEACAM1, -3, -5, and -6 depends on distinct signaling pathways, involving serine/threonine and/or tyrosine kinases for the CEACAM1 pathway (20, 21, 26) or Src-family kinases, phospholipase C as well as Rac and the phosphatidylinositol 3-kinase (PI3-kinase), in the case of the neutrophil-specific CEACAM3 receptor (3, 4, 8, 26, 27).
PorB porin has been recognized as a pathogenicity factor involved in serum resistance, immune stimulation, host cell survival, and invasion (25). N. gonorrhoeae strains express PorB porins of either serotype A (P.IA) or B (P.IB). Evidence exists for different roles of the PorB serotypes during infection. Expression of P.IA correlates with serious systemic gonococcal infection (5, 6, 30). Moreover, P.IA, but not P.IB, mediates the efficient uptake of gonococci in a phosphate-sensitive manner (37). It is not known yet whether the function of P.IA as an adhesin and invasin is the basis for the natural courses of infections with P.IA- and P.IB-bearing strains. Moreover, the host cell specificity and the signaling pathways involved in the P.IA-mediated adherence and invasion are poorly understood.
Here, we demonstrate that P.IA-dependent adherence and invasion appear to constitute an invasion pathway distinct from the HSPG and CEACAM route of entry.
MATERIALS AND METHODS
Neisseria strains.
N. gonorrhoeae strains used in this study were derived from MS11 and have been described previously: strain N303 [MS11: P− Opa50 (opaC::cat; pTH6a::opaC), PorBIB] (23) and the isogenic strains (MS11: P− Opa−) expressing different porB genes, N924 (PorBIB), N927 (PorBIA), N928 (Neisseria cinerea PorB), N929 (Neisseria lactamica PorB), and N930 (Neisseria mucosa PorB) (1). N1105 (N. meningitidis PorB class II) expressed PorB of meningococcal clinical isolates 9649 (kindly provided by U. Vogel; University Würzburg) and N1106 (N. meningitidis PorB class III) of meningococcal strain MC58. Gonococci were routinely grown on GC agar base plates (Becton Dickinson, Difco, and Remel) supplemented with Proteose Peptone no. 3 (Difco) and 1% vitamin mix for 14 to 20 h at 37°C in 5% CO2 in a humidified atmosphere.
Construction of PorBP.IA-expressing E. coli.
PorBP.IA-producing Escherichia coli was obtained by subcloning the porB region with flanking antibiotic resistance cassettes from N. gonorrhoeae strain N920 (1) into pGEMT (Promega). In this construct, PorB expression is under the control of its own promoter, similar to what has been described previously (15). Briefly, genomic gonococcal DNA was prepared from N920, and the fragment was amplified using primer FBJ4 and ISO3 (1) and cloned into pGEMT (Promega).
Quantification of total cell-associated and intracellular CFU.
To quantify total cell-associated bacteria, washed monolayers were lysed with 1% saponin in RPMI 1640 for 7 min at 37°C, releasing adherent and intracellular gonococci. After vigorous pipetting, serial dilutions of the lysates were plated on GC agar plates and CFU were determined after a 24-h incubation period. For quantification of intracellular viable bacteria, extracellular bacteria were selectively killed by incubating monolayers with 50 μg/ml gentamicin in RPMI 1640 for 2 h at 37°C, 5% CO2, prior to saponin lysis and plating. Values are given as mean percentages of gonococci per 24 wells and represent the means ± standard deviations for at least two experiments done in duplicate.
Immunocytochemistry.
For differentiating extra- from intracellular bacteria, the following staining method was applied at room temperature, while preventing exposure of specimens to light. After several washes with phosphate-buffered saline (PBS), unspecific binding sites were blocked with 0.2% bovine serum albumin in PBS for 20 min. For staining of the extracellular antigen, the coverslips were incubated for 1.5 h with a suitable dilution of the first antibody, polyclonal rabbit anti-Neisseria gonorrhoeae (1:150; AK213) or polyclonal rabbit anti-E. coli (1:200; K43) in 0.2% bovine serum albumin in PBS, followed by several washes with PBS and an incubation for 1 h with a dilution of the second antibody (1:150), Cy-conjugated goat anti-rabbit immunoglobulin G (Jackson ImmunoResearch). After extensive washing with PBS, cells were permeabilized with 0.2% Triton X-100 for 15 min. Blocking and staining of the extra- and intracellular antigen was subsequently done as described above using a second antibody coupled to a different fluorochrome. To label F-actin, tetramethylrhodamine isothiocyanate-phalloidin (1:300; Sigma) was included in the last antibody solution. After extensive washing, the specimens were mounted in Mowiol (Hoechst) and analyzed by confocal laser scanning microscopy using a Leica TCS NT microscope equipped with an argon-krypton mixed gas laser. Images were taken using appropriate excitation-and-emission filters for the fluorescence dyes used. Overlay images of the single channels were obtained using Adobe Photoshop 6.0.
RESULTS
P.IA is sufficient for the low-phosphate-dependent invasion.
Infection via the low-phosphate-dependent invasion (LPDI) pathway has been previously described as depending on the expression of the A serotype of PorB (37). However, Neisseria expresses numerous different adhesins, which could cooperate with P.IA in host cell binding as has previously been described for P.IB and the Opa50 protein (1). We therefore expressed P.IA in E. coli (Fig. 1A) and tested whether recombinant bacteria have adherence and invasion properties similar to those of N927. Recombinant E. coli expressing PorBIA (H3378) but not the vector harboring E. coli (H3379) efficiently bound to Chang cells in a phosphate-dependent manner (Fig. 1B), suggesting that indeed no additional neisserial factor besides PorBIA is required for low-phosphate infection. Invasive H3378 was only rarely detected, which could be due to the relatively low expression of PorB compared to recombinant N920 (Fig. 1A). We then investigated whether PorB of other Neisseria species, Neisseria meningitidis or commensal strains, mediate LPDI. First, the infection system was set up using isogenic N927 (PorBIA) and N924 (PorBIB) under low-phosphate conditions, essentially as previously described (37) with the exception that prior to infection bacteria were grown in chemically defined HEPES-buffered medium containing 15 mM phosphate. HEPES-buffered medium without supplements was then used for infection (see experimental procedures in the supplemental material). In order to generate isogenic strains expressing PorB of N. meningitidis, we made use of an allelic exchange strategy to integrate a foreign porB gene and at the same time delete the porBIB allele of strain MS11, as previously described (1). Using this procedure, recombinant derivatives of strain MS11 were made, expressing PorB of strain 9649 (N1105; class II, P.IB) and MC58 (N1106; class III, P.IA). None of the strains interacted with Chang cells under low-phosphate conditions, suggesting that the meningococcal PorBs tested do not confer binding. Similar results were obtained with isogenic strains (see Fig. S1 in the supplemental material) expressing PorB of Neisseria cinerea (PorBNci) (N928), Neisseria lactamica (PorBNla) (N929) and Neisseria mucosa (PorBNmu) (N930) (1). Thus, LPDI depends on the expression of the structurally distinct P.IA of N. gonorrhoeae.
FIG. 1.
PorBIA of N. gonorrhoeae but not PorB of other neisserial species mediates interaction with host cells. (A) Immunoblot of N920 and E. coli expressing PorBIA. (B) Chang cells were infected with E. coli expressing PorBIA or vector control under low-phosphate (HepM) or high-phosphate (HepM+PO4; RPMI) conditions and stained for extracellular (yellow) and intracellular (red) bacteria.
LPDI is rapid, efficient, and serum sensitive.
To further characterize LPDI, we compared the kinetics and efficiency of binding and uptake of N927 and N303. N927 expresses the P.IA allele of PorB but no Opa proteins (not shown), whereas N303 expresses the P.IB allele and harbors a plasmid, which drives the expression of the Opa50 protein, promoting the invasion via heparan sulfate proteoglycans (23). Only a few bacteria of strain N927 bound during the initial 90 min, but then binding increased constantly up to 5 h postinfection (Fig. 2A). In contrast, N303 adhered more efficiently after being added to the cells, plateaued finally after 90 min, and started to bind again after 180 min (Fig. 2B). Interestingly, the kinetics of LPDI were in contrast to the respective adhesion process. N927 rapidly invaded during the initial 60 min and continued to invade after 120 min, again in a constant but less-efficient manner (Fig. 2A). The kinetics of Opa50-mediated invasion, however, did not show the same effect; N303 invasion paralleled the kinetics of adherence at a lower level (Fig. 2B).
FIG. 2.
LPDI- and Opa50-mediated invasion differ in kinetics and serum sensitivity. Infection with N927 (A) or N303 (B) was performed under low-phosphate conditions, and adherence (black lines) and invasion (gray lines) were determined. Adherence and invasion of N927 at 120 min were set as 100%. (C) Adherence (black bars) and invasion (gray bars) of N927 and N303 were analyzed in the absence and presence of 7.5% serum (fetal calf serum) under low-phosphate conditions (HepM). N927 does not bind to Chang cells in RPMI medium (not shown here; see Fig. S2 in the supplemental material), but binding and invasion of N303 are similar in HepM and RPMI. Intracellular bacteria were quantified by gentamicin survival assays.
Since LPDI may play a role during disseminating gonococcal infection, the influence of serum levels on adherence and infection was investigated and compared to Opa50 invasion. Attachment of strains N927 and N303 to Chang cells was only slightly different in the absence or presence of 7.5% heat-inactivated fetal calf serum (Fig. 2C). Interestingly, in the presence of serum, invasion of N927 increased by a factor of approximately 2.5, whereas the invasion of N303 was dramatically reduced. Denaturation of serum proteins by boiling completely abrogated the observed invasion stimulation by serum (data not shown). Thus, kinetics and serum sensitivity of invasion clearly differ between the PorB- and Opa50-initiated invasion pathways.
Similar host cell repertoire of Opa50- and PorBIA-dependent attachment and invasion.
To test whether the different kinetics of uptake are also reflected by the engagement of different receptors, we tested the host cell spectrum of isogenic strains expressing Opa50 and P.IB (N303) or no Opa proteins and P.IA (N927). The binding patterns and efficiencies of N927 and N303 with a whole range of different transformed and nontransformed cell lines of human or nonhuman origin were similar. Strong binding of both strains could be observed, for example to HeLa, HBMEC, COS-7, and CHO-KI cells (see Fig. S2 in the supplemental material), suggesting that host cell receptors are restricted neither to epithelial nor to human cells. Next, we tested whether the two strains interacted via the same general mechanism involving active energy metabolism of the host cell. The two strains bind to paraformaldehyde fixed cells with about the same efficiencies (data not shown), suggesting no major involvement of host cell metabolism in the adherence.
Uptake of bacteria is often accompanied by structural rearrangements of the host cell's cytoplasmic membrane (9). To investigate whether the different downstream signaling pathways under investigation ultimately resulted in the generation of different membrane structures, infected samples were analyzed by scanning (SEM) and transmission (TEM) electron microscopy. Cells infected with N927 occasionally formed long filamentous structures, but typical membrane ruffles were never observed (Fig. 3A). The bacteria seemed to sink into the cells rather than being actively taken up (Fig. 3B, panels 1 to 7). In contrast, N303 was engulfed by the host cell in protruding membrane structures surrounding the bacteria (Fig. 3A and B, panels 8 to 10). Interestingly, TEM analysis of N927-infected cells also unveiled small membrane invaginations lined by electron-dense material reminiscent of clathrin-coated pits where bacterial and host cell membranes interacted (Fig. 3B, panels 1 to 4). The same structures were only rarely found in N303-infected cells (Fig. 3B, panel 10). In summary, these analyses showed clear differences in the mode of uptake initiated by P.IA and Opa50 adhesins, respectively, with clathrin-coated pits possibly being involved in LPDI.
FIG. 3.
N927 and N303 trigger different ultrastructural modifications of the cell surface under low-phosphate conditions. (A) SEMs of cells showing pseudopods and protrusions that are infected with N303 but not with N927. The left pictures show enlargements of the boxed areas. (B1 to -6) TEM of cells infected with N927 at different stages of the infection and (B7) overview at lower magnification. The arrows point to structures reminiscent of coated pits. Details (B8-9) and overview (B10) TEM pictures of N303-infected cells. Arrows point to pseudopodia surrounding the bacteria.
P.IA- and Opa50-mediated invasions require common and distinct signaling pathways.
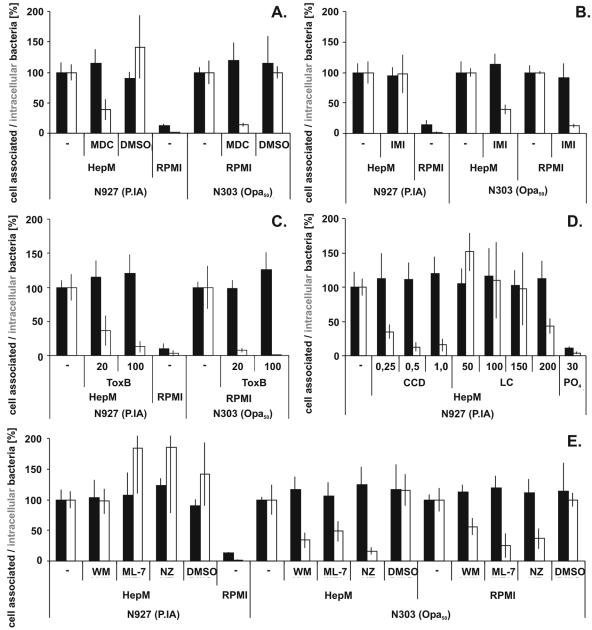
Receptor-mediated endocytosis via clathrin-coated pits is one entry route used by some pathogenic bacteria (9). The transamidase inhibitor monodansylcadaverine (MDC) interferes with the formation of clathrin-coated pits and was therefore used to test whether this pathway is engaged during LPDI. This and all other inhibitors were used under conditions where no cytotoxic effect on the Chang cells was visible by microscopical examination (not shown). Pretreatment of Chang cells with 150 μM MDC reduced LPDI by more than 50% compared to results with mock-treated Chang cells, as well as reducing the uptake of N303 dramatically (Fig. 4A).
FIG. 4.
P.IA invasion resembles a new gonococcal invasion pathway. Chang cells were pretreated with the individual inhibitors or the respective solvent as described in the experimental procedures and infected under low-phosphate (HepM) or high-phosphate (RPMI) conditions with either N927 or N303 as indicated. Adherence (black bars) and invasion (white bars) were quantified by gentamicin survival assays. (A) MDC; (B) imipramine (IMI); (C) Clostridium difficile toxin B (ToxB); (D), cytochalasin D (CCD) or latrunculin (LC); (E), wortmannin (WM), ML-7, or nocodazol (NZ). The graph shows the mean for at least three independent experiments ± standard deviation.
Uptake of Opa50-expressing gonococci by Chang cells has been shown to depend on active signaling via ASM (17). ASM was, however, not an essential component of LPDI, since pretreatment with 100 μM imipramine had, in contrast to uptake of N303, no effect on invasion of N927 into Chang cells (Fig. 4B). An influence of the phosphate-free medium on the inhibitor could be ruled out, since N303 invasion was blocked independently of the infection medium. Interestingly, the uptake of both N927 and N303 into gentamicin-resistant compartments required active Rho GTPases (Fig. 4C) and intact actin filaments (Fig. 4D) (17), since toxin B as well as cytochalasin D and latrunculin A, respectively, blocked invasion. Only Opa50-initiated, but not PorBIA-initiated, uptake was also sensitive to nocodazol (Fig. 4E) acting on tubulin filaments. In addition, only Opa50 entry could be prevented by the PI3-kinase inhibitor wortmannin or ML-7 blocking the myosin light chain kinase acting on myosin motor activity (Fig. 4E). The data obtained by using different biochemical inhibitors thus provided further evidence for different downstream entry pathways of Opa50 and PorBIA.
DISCUSSION
A large number of different surface proteins of Neisseria gonorrhoeae serve as adhesins or/and invasins (29, 32). The genes for most of these proteins are present in multiple copies and exhibit significant intra- and interstrain variability. For example, in the case of the family of Opa proteins, structurally different Opa proteins interact with different receptors and thereby may influence the tissue tropism of the bacteria. In contrast to this group of adhesins/invasins, PorB is predominantly conserved, not subjected to phase variation, and present in one copy. Moreover, the fact that only the P.IA type of PorB exhibits the function of an adhesin and invasin (37) suggests that interaction via PorB serves a different function than tissue adaptation.
To investigate the still-enigmatic role of the P.IA-host cell interaction, we first expressed P.IA in E. coli and proved that P.IA is sufficient for LPDI. Only a few recombinant E. coli bacteria, however, invaded Chang cells (not shown). This could either mean that additional neisserial factors are required for the PorB-mediated invasion under low-phosphate conditions or, on the other hand, the relatively low expression of PorB in E. coli could affect the proper engagement of putative PorB receptors, leading to reduced uptake of the recombinant E. coli. We then tested a repertoire of PorB molecules from different Neisseria species for their capability of binding to host cells under low-phosphate conditions. P.IA of N. gonorrhoeae, but none of the N. meningitidis or commensal PorBs, mediated binding in an isogenic strain background. This was unexpected, since the N. meningitidis class III (GI 45213) proteins exhibit amino acid sequences 79% identical to those of P.IA of strain VPI, more than PorB of N. lactamica (GI 45009; 74%) and P.IB of strain MS11 (GI 150343; 69%). Therefore, the interaction of N. gonorrhoeae with host cells under low-phosphate conditions probably depends on a distinct structural difference in one of the P.IA loops.
Several studies have shown that P.IA-expressing N. gonorrhoeae is associated with disseminating infections characterized by entry of the bacteria into the bloodstream (5, 6, 30). Moreover, serum of human adults contains phosphate in the range of 0.84 to 1.45 mM, making low-phosphate-dependent interaction via P.IA in this environment possible. We therefore investigated the influence of serum on the interaction of P.IA with host cells and found that a heat-labile serum component significantly stimulated the uptake of P.IA-expressing N. gonorrhoeae into Chang cells. In contrast, invasion of an isogenic P.IB strain interacting via the HSPG-specific Opa50 adhesin was blocked by serum. These data suggest that serum is a favorable environment for P.IA-triggered host cell invasion. Currently, we do not know the molecular basis for the enhanced PorB-dependent invasion in the presence of serum. A similar effect has been described for the invasion of Opa50-expressing gonococci into HeLa cells, where extracellular matrix proteins present in the serum interact with integrins to mediate efficient uptake (10, 11, 14, 38).
While Opa proteins interact only with CEACAM receptors of human origin (18), the interaction of Opa50 with HSPG occurs also with cells of nonhuman origin (7, 11, 14). We found that the cell type and species pattern of P.IA binding is similar to that of Opa50. In fact, both adhesins recognize receptors on human primary epithelia (unpublished data), on endothelial cells, and on hamster and monkey cells. Van Putten et al. (38) could not block binding of P.IA-expressing gonococci by heparin, which efficiently prevents Opa50-HSPG interaction, suggesting that these adhesins use highly conserved but different receptors. Interesting candidates for P.IA receptors were Toll-like receptor 2 (TLR2) and complement receptor 3, which bind to class 3 porin of N. meningitidis and P.IA and P.IB of N. gonorrhoeae, respectively (12, 24). However, since N927 binds efficiently to TLR2-negative HOSE cells (31) and TLR2 knockout mouse embryonic fibroblasts (C. Rechner and T. Rudel, unpublished), as well as to complement receptor 3-negative CHO-KI cells, these receptors can be excluded. The efficient and phosphate-sensitive binding of P.IA to fixed cells suggests that the receptor is already constantly expressed by the cell before infection. We can thus also virtually rule out that PorB translocated to the host cell membrane binds to gonococci and mediates binding and invasion, as has been shown for the Tir protein of enteropathogenic E. coli (22).
In contrast to N303, N927 interacting with host cell surface receptors under low-phosphate conditions failed to induce massive membrane ruffling. Rather, N927 seemed to sink into the cells, while pseudopodia reaching from the host cell surrounded N303, leading to engulfment. These obvious differences already suggested that the uptake mechanisms of N927 and N303 are unrelated. Blocking uptake via clathrin-coated pits (CCPs) by monodansylcadaverine treatment massively blocked uptake of N927 and N303. However, ultrastructural evidence for the presence of CCPs was frequently found in the case of N927 infection and only rarely in N303 infection, suggesting that MDC may cause inhibition of invasion via a different mechanism than dissolution of CCPs (16). Acidic sphingomyelinase, PI3-kinase, myosin light chain kinase, and microtubuli, all essential for Opa50-triggered invasion, were not involved in P.IA-mediated invasion. Also, blocking Src-kinases involved in the CEACAM entry route had no effect on invasion of N927 (unpublished data). The latter finding was confirmed in experiments with Src knockout mouse embryonic fibroblasts which were heavily infected by N927 (unpublished data), suggesting that signaling pathways engaged by Opa-CEACAM interaction also differ from that initiated by P.IA.
In summary, we have identified the phosphate-sensitive P.IA-triggered invasion as a new way for N. gonorrhoeae to enter cells. The correlation of P.IA-expressing N. gonorrhoeae and disseminating gonococcal infection points to an important and heretofore underestimated role of this pathway in the pathogenesis of a rare, but sometimes fatal, progression of the infection.
Supplementary Material
Acknowledgments
We thank Gerald Wenig for technical assistance with the cloning of P.IA and Volker Brinkmann and Ulrike Reichard for the SEM and TEM analysis and provision of the images. Jenny Gebhardt is thanked for critically reading the manuscript.
This work was supported by the DFG SPP1131 to T.F.M. and T.R.
Editor: J. N. Weiser
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bauer, F. J., T. Rudel, M. Stein, and T. F. Meyer. 1999. Mutagenesis of the Neisseria gonorrhoeae porin reduces invasion in epithelial cells and enhances phagocyte responsiveness. Mol. Microbiol. 31:903-913. [DOI] [PubMed] [Google Scholar]
- 2.Bhat, K. S., C. P. Gibbs, O. Barrera, S. G. Morrison, F. Jahnig, A. Stern, E. M. Kupsch, T. F. Meyer, and J. Swanson. 1991. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol. Microbiol. 5:1889-1901. [DOI] [PubMed] [Google Scholar]
- 3.Billker, O., A. Popp, V. Brinkmann, G. Wenig, J. Schneider, E. Caron, and T. F. Meyer. 2002. Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1- and Cdc42-dependent and -independent pathways. EMBO J. 21:560-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth, J. W., D. Telio, E. H. Liao, S. E. McCaw, T. Matsuo, S. Grinstein, and S. D. Gray-Owen. 2003. Phosphatidylinositol 3-kinases in carcinoembryonic antigen-related cellular adhesion molecule-mediated internalization of Neisseria gonorrhoeae. J. Biol. Chem. 278:14037-14045. [DOI] [PubMed] [Google Scholar]
- 5.Britigan, B. E., M. S. Cohen, and P. F. Sparling. 1985. Gonococcal infection: a model of molecular pathogenesis. N. Engl. J. Med. 312:1683-1694. [DOI] [PubMed] [Google Scholar]
- 6.Cannon, J. G., T. M. Buchanan, and P. F. Sparling. 1983. Confirmation of association of protein I serotype of Neisseria gonorrhoeae with ability to cause disseminated infection. Infect. Immun. 40:816-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, T., R. J. Belland, J. Wilson, and J. Swanson. 1995. Adherence of pilus− Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J. Exp. Med. 182:511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, T., S. Bolland, I. Chen, J. Parker, M. Pantelic, F. Grunert, and W. Zimmermann. 2001. The CGM1a (CEACAM3/CD66d)-mediated phagocytic pathway of Neisseria gonorrhoeae expressing opacity proteins is also the pathway to cell death. J. Biol. Chem. 276:17413-17419. [DOI] [PubMed] [Google Scholar]
- 9.Cossart, P. 1997. Host/pathogen interactions. Subversion of the mammalian cell cytoskeleton by invasive bacteria. J. Clin. Investig. 99:2307-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehio, M., O. G. Gomez-Duarte, C. Dehio, and T. F. Meyer. 1998. Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves alpha(v) integrin receptors. FEBS Lett. 424:84-88. [DOI] [PubMed] [Google Scholar]
- 11.Duensing, T. D., and J. P. van Putten. 1997. Vitronectin mediates internalization of Neisseria gonorrhoeae by Chinese hamster ovary cells. Infect. Immun. 65:964-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards, J. L., E. J. Brown, S. Uk-Nham, J. G. Cannon, M. S. Blake, and M. A. Apicella. 2002. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol. 4:571-584. [DOI] [PubMed] [Google Scholar]
- 13.Freissler, E., H. A. Meyer auf der, G. David, T. F. Meyer, and C. Dehio. 2000. Syndecan-1 and syndecan-4 can mediate the invasion of OpaHSPG-expressing Neisseria gonorrhoeae into epithelial cells. Cell Microbiol. 2:69-82. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Duarte, O. G., M. Dehio, C. A. Guzman, G. S. Chhatwal, C. Dehio, and T. F. Meyer. 1997. Binding of vitronectin to Opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells. Infect. Immun. 65:3857-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorby, G. L., A. F. Ehrhardt, M. A. Apicella, and C. Elkins. 2001. Invasion of human fallopian tube epithelium by Escherichia coli expressing combinations of a gonococcal porin, opacity-associated protein, and chimeric lipo-oligosaccharide. J. Infect. Dis. 184:460-472. [DOI] [PubMed] [Google Scholar]
- 16.Grassme, H., E. Gulbins, B. Brenner, K. Ferlinz, K. Sandhoff, K. Harzer, F. Lang, and T. F. Meyer. 1997. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 91:605-615. [DOI] [PubMed] [Google Scholar]
- 17.Grassme, H. U., R. M. Ireland, and J. P. van Putten. 1996. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect. Immun. 64:1621-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray-Owen, S. D., D. R. Lorenzen, A. Haude, T. F. Meyer, and C. Dehio. 1997. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol. Microbiol. 26:971-980. [DOI] [PubMed] [Google Scholar]
- 19.Harvey, H. A., N. Porat, C. A. Campbell, M. Jennings, B. W. Gibson, N. J. Phillips, M. A. Apicella, and M. S. Blake. 2000. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol. Microbiol. 36:1059-1070. [DOI] [PubMed] [Google Scholar]
- 20.Hauck, C. R., E. Gulbins, F. Lang, and T. F. Meyer. 1999. Tyrosine phosphatase SHP-1 is involved in CD66-mediated phagocytosis of Opa52-expressing Neisseria gonorrhoeae. Infect. Immun. 67:5490-5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauck, C. R., T. F. Meyer, F. Lang, and E. Gulbins. 1998. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 17:443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 23.Kupsch, E. M., B. Knepper, T. Kuroki, I. Heuer, and T. F. Meyer. 1993. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 12:641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 25.Massari, P., S. Ram, H. Macleod, and L. M. Wetzler. 2003. The role of porins in neisserial pathogenesis and immunity. Trends Microbiol. 11:87-93. [DOI] [PubMed] [Google Scholar]
- 26.McCaw, S. E., E. H. Liao, and S. D. Gray-Owen. 2004. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect. Immun. 72:2742-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCaw, S. E., J. Schneider, E. H. Liao, W. Zimmermann, and S. D. Gray-Owen. 2003. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol. Microbiol. 49:623-637. [DOI] [PubMed] [Google Scholar]
- 28.McGee, Z. A., A. P. Johnson, and D. Taylor-Robinson. 1981. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J. Infect. Dis. 143:413-422. [DOI] [PubMed] [Google Scholar]
- 29.Merz, A. J., and M. So. 2000. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu. Rev. Cell Dev. Biol. 16:423-457. [DOI] [PubMed] [Google Scholar]
- 30.Morello, J. A., and M. Bohnhoff. 1989. Serovars and serum resistance of Neisseria gonorrhoeae from disseminated and uncomplicated infections. J. Infect. Dis. 160:1012-1017. [DOI] [PubMed] [Google Scholar]
- 31.Muenzner, P., O. Billker, T. F. Meyer, and M. Naumann. 2002. Nuclear factor-kappa B directs carcinoembryonic antigen-related cellular adhesion molecule 1 receptor expression in Neisseria gonorrhoeae-infected epithelial cells. J. Biol. Chem. 277:7438-7446. [DOI] [PubMed] [Google Scholar]
- 32.Nassif, X., C. Pujol, P. Morand, and E. Eugene. 1999. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol. Microbiol. 32:1124-1132. [DOI] [PubMed] [Google Scholar]
- 33.Porat, N., M. A. Apicella, and M. S. Blake. 1995. Neisseria gonorrhoeae utilizes and enhances the biosynthesis of the asialoglycoprotein receptor expressed on the surface of the hepatic HepG2 cell line. Infect. Immun. 63:1498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spence, J. M., and V. L. Clark. 2000. Role of ribosomal protein L12 in gonococcal invasion of Hec1B cells. Infect. Immun. 68:5002-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern, A., M. Brown, P. Nickel, and T. F. Meyer. 1986. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47:61-71. [DOI] [PubMed] [Google Scholar]
- 36.Swanson, J. 1973. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J. Exp. Med. 137:571-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Putten, J. P., T. D. Duensing, and J. Carlson. 1998. Gonococcal invasion of epithelial cells driven by P.IA, a bacterial ion channel with GTP binding properties. J. Exp. Med. 188:941-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Putten, J. P., T. D. Duensing, and R. L. Cole. 1998. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol. Microbiol. 29:369-379. [DOI] [PubMed] [Google Scholar]
- 39.van Putten, J. P., and S. M. Paul. 1995. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 14:2144-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.