Abstract
Orientia tsutsugamushi, a causative agent of scrub typhus, is an obligate intracellular bacterium that requires the exploitation of the endocytic pathway in the host cell. We observed the localization of O. tsutsugamushi with clathrin or adaptor protein 2 within 30 min after the infection of nonprofessional phagocytes. We have further confirmed that the infectivity of O. tsutsugamushi is significantly reduced by drugs that block clathrin-mediated endocytosis but not by filipin III, an inhibitor that blocks caveola-mediated endocytosis. In the present study, with a confocal microscope, O. tsutsugamushi was sequentially colocalized with the early and late endosomal markers EEA1 and LAMP2, respectively, within 1 h after infection. The colocalization of O. tsutsugamushi organisms with EEA1 and LAMP2 gradually disappeared until 2 h postinfection, and then free O. tsutsugamushi organisms were found in the cytoplasm. When the acidification of endocytic vesicles was blocked by treating the cells with NH4Cl or bafilomycin A, the escape of O. tsutsugamushi organisms from the endocytic pathway was severely impaired, and the infectivity of O. tsutsugamushi was drastically reduced. To our knowledge, this is the first report that the invasion of O. tsutsugamushi is dependent on the clathrin-dependent endocytic pathway and the acidification process of the endocytic vesicles in nonprofessional phagocytes.
Orientia tsutsugamushi, an obligate intracellular bacterium, is the causative agent of scrub typhus (12). The disease is characterized by fever, rash, eschar, pneumonitis, meningitis, and disseminated intravascular coagulation, which leads to severe multiorgan failure in untreated cases (4, 6, 51). This bacterium infects a variety of mouse and human cells in vitro, including macrophages, polymorphonuclear leukocytes, and endothelial cells (21, 29, 38), and replicates in the cytoplasm of the infected cells (22). Previously, we have shown that O. tsutsugamushi utilizes microtubules and dynein to move from the cell periphery to the microtubule organizing center (22). Even though prior studies have reported that O. tsutsugamushi induces the phagocytosis of host cells and starts to escape from the phagosome within 30 min (37, 49), the precise mechanisms of entry into host cells and escape from the endocytic pathway have not yet been clearly defined.
Most of the intracellular bacteria exploit at least two distinct pathways, clathrin- and caveola-mediated endocytic pathways, to enter the nonprofessional phagocytes after making contact with the cell surface (40). It remains unclear whether O. tsutsugamushi enters the host cells by a clathrin-mediated or caveola-mediated pathway. Clathrin coats are known to be involved in receptor-mediated and fluid-phase endocytosis, from plasma membranes to early endosomes (42). Several intracellular bacteria, such as Chlamydia spp. (26), Neisseria gonorrhoeae (15), Citrobacter freundii (30), and enterohemorrhagic Escherichia coli (25), are known to use the clathrin-mediated endocytosis pathway. Caveolae are small, flask-shaped invaginations of the plasma membrane, characterized by high contents of cholesterol and glycosphingolipids and by the presence of caveolin (34). FimH-expressing E. coli (44), Mycobacterium bovis (10), Campylobacter jejuni (53), and Mycobacterium kansasii (35) have been shown to exploit the caveola-mediated endocytosis pathway to enter the host cells.
Internalized bacteria are usually carried by a membrane-bound endosome. The invading microorganisms may remain in the developing endosomes and exploit them to survive in the low-pH environment of lysosomes (27). During this process, the dynamic changes of endosomal vesicles, including the delivery of hydrolytic enzymes and proton pumps from other intracellular compartments, occur, and the intraphagosomal pH of the endocytic vesicles is lowered (3). To cope with this harsh environment, the intracellular bacteria have developed different strategies. For example, Coxiella burnetii (27) thrives in an acidic compartment. Mycobacterium avium (8) and Legionella pneumophila (17) attenuate the acidic pH of the compartment in which they reside. Salmonella enterica serovar Typhimurium has developed mechanisms to modulate the redistribution of endosomal and lysosomal markers (16). Other intracellular pathogens, such as Listeria monocytogenes (36) or Rickettsia prowazekii (52), have developed mechanisms to lyse the phagosomal membrane and escape into the cytoplasm. In the case of O. tsutsugamushi, the organism lyses the phagosomal membranes with an unknown mechanism and is released into the cytoplasm at an early stage of infection. In this study, we used a variety of approaches to elucidate the endocytosis and infection pathways of O. tsutsugamushi in nonprofessional phagocytes. Our results show that O. tsutsugamushi exploits clathrin-dependent endocytosis to enter the nonprofessional phagocytes. The infectivity of this intracellular bacterium was significantly reduced by pharmacological inhibitors that block the clathrin-dependent endocytic pathway but not by filipin III, which inhibits the caveola-dependent pathway. In addition, we also demonstrated that O. tsutsugamushi organisms were colocalized with early and late endosomes sequentially in an early stage of infection and that the movement of this intracellular bacterium from the endocytic pathway is dependent on the acidification process of the endocytic vesicle.
MATERIALS AND METHODS
Cell culture.
ECV304 (an immortalized human umbilical vein endothelial cell line) and L929 (a mouse fibroblast line), used as the nonprofessional phagocytes, and J774A.1 (a mouse macrophage cell line), used as the phagocytes, were obtained from the American Type Culture Collection (Rockville, Md.). ECV304 cells were cultured in medium 199. L929 and J774A.1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL). All media were supplemented with 10% fetal bovine serum (GIBCO BRL), 5 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified atmosphere containing 5% CO2. O. tsutsugamushi strain Boryong was propagated in monolayers of L929 cells as described previously (20, 43). Briefly, when more than 90% of the cells were infected, as determined by an indirect immunofluorescence antibody technique (5), the cells were collected, homogenized using a glass Dounce homogenizer (Wheaton, Inc.), and centrifuged at 500 × g for 5 min. The supernatant was stored in liquid nitrogen until use. The titer of infectivity of the inoculum was determined as described previously with slight modification (18). Briefly, the bacterial stock was serially diluted and inoculated onto L929 cell layers in a 24-well tissue culture plate containing 12-mm-diameter glass coverslips. After the cells were infected with O. tsutsugamushi for 6 h in a humidified 5% CO2 atmosphere at 34°C, the culture medium was removed. The cells were washed with phosphate-buffered saline (PBS), fixed in 100% methanol for 15 min at −20°C, and stained by indirect immunofluorescence antibody assay as described below (18). The ratio of infected cells to the counted number of cells was determined microscopically, and the number of infected-cell-counting units (ICU) of the O. tsutsugamushi was calculated (47). A total of 2.4 × 106 or 2.4 × 107 ICU of O. tsutsugamushi was used to infect cultured cells in a 24-well tissue culture plate.
Inhibition assays.
Cells were grown on 12-mm-diameter glass coverslips and pretreated for 1 h with chemical reagents before infection by O. tsutsugamushi Boryong (2.4 × 106 or 2.4 × 107 ICU). The concentration of each chemical was as follows: chlorpromazine hydrochloride, 10 μM in distilled water (Calbiochem); monodansylcadaverine, 0.2 mM in acetic acid (Sigma); sucrose, 0.3 M in DMEM (Sigma); filipin III, 1.5 mM in dimethyl sulfoxide (Sigma); NH4Cl, 20 mM in DMEM (Sigma); and bafilomycin A, 20 nM in DMEM (Calbiochem). Control cells were incubated in medium without inhibitors. After infection by O. tsutsugamushi for designated times, each cell was stained with antibody against O. tsutsugamushi, and the infectivity of O. tsutsugamushi was examined. One hundred cells were counted for each experimental set, and the results were expressed as the mean number of bacteria/counted cells ± the standard deviation of the means. To check the effects of these chemicals on the viability and function of cells, cells were assayed, and the viability and infectivity of those that had been pretreated were compared to those from which the chemicals had been washed.
Immunofluorescence assay.
O. tsutsugamushi was stained with monoclonal antibody (KI-37) against the Boryong strain's 56-kDa outer membrane protein (33) or human patient polyserum. Anti-clathrin heavy chain, anti-caveolin 1, anti-early endosome antigen 1 (EEA1), anti-lysosome-associated protein 2 (LAMP2) (BD Biosciences), or anti-α-adaptin (Santa Cruse Biotechnology) antibody was used for the staining of cellular organelles. Fluorescein isothiocyanate (FITC) or tetramethyl-rhodaminyl isothiocyanate (TRITC)-conjugated goat anti-mouse immunoglobulin G (IgG), TRITC-conjugated goat anti-rabbit IgG (Santa Cruse Biotechnology), or Alexa Fluor 568 or Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes) was used as the secondary antibody. Immunofluorescence assays were performed as described previously (22). In brief, the infected monolayer of cells was washed three times with PBS, fixed in PBS containing 4% paraformaldehyde for 10 min at room temperature, and permeabilized in a 0.2% Triton X-100 solution. Subsequently, cells were incubated for 30 min at 37°C with primary antibodies. After incubation, cells were washed three times and incubated at 37°C with secondary antibodies diluted in PBS. Cells were examined under a confocal microscope (Carl Zeiss AG, Germany). Images of cellular sections were produced every 1 μm, and all images were analyzed and processed using Adobe Photoshop v. 6.0 software (Adobe Systems).
RESULTS
Orientia tsutsugamushi colocalized with clathrin-coated vesicles at an early stage of infection.
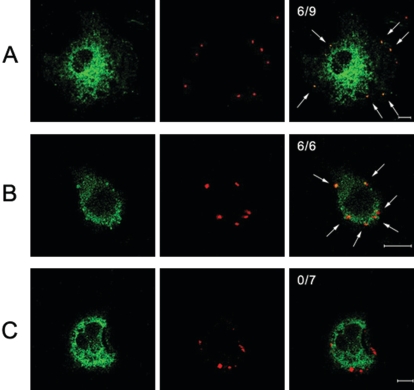
Orientia tsutsugamushi can infect a wide range of nonprofessional phagocytes, including fibroblasts, epithelial cells, and endothelial cells. In the present study, we tried to identify the endocytic pathway that O. tsutsugamushi utilized to become internalized in the host cells. To determine whether the entry of O. tsutsugamushi is mediated by clathrin-dependent endocytosis, we analyzed the localization of O. tsutsugamushi at various time points after infection using a confocal microscope. Clathrin-coated pits contain two major structural proteins, clathrin and adaptor protein 2 (AP-2). These proteins play a critical role in the attachment of clathrin to membranes and the recruitment of membrane proteins that localize to the clathrin-rich membrane region (50). FITC-labeled clathrin or AP-2- and TRITC-labeled O. tsutsugamushi could be visualized as punctuate clusters within cells as early as 10 min postinfection (data not shown). By 30 min after infection, most of the TRITC-labeled O. tsutsugamushi cells and FITC-labeled clathrin (Fig. 1A) or AP-2 (Fig. 1B) could be seen as dots in the periphery of the cytoplasm of the infected cells. Overlaying the red and green channels showed that O. tsutsugamushi cells were colocalized with clathrin-coated vesicles. Six O. tsutsugamushi bacteria were colocalized with clathrin among nine dots (Fig. 1A) or α-adaptin among six dots (Fig. 1B). In contrast, none of the O. tsutsugamushi colocalized with caveolin 1 (Fig. 1C). This suggests that O. tsutsugamushi and clathrin-coated vesicles coexist in the same endocytic compartments. By contrast, there was no evident colocalization of O. tsutsugamushi with caveolin 1, a marker for the caveola-dependent pathway (Fig. 1C). Colocalization of clathrin or α-adaptin with O. tsutsugamushi within 30 min after infection showed that O. tsutsugamushi was internalized by the host cells by clathrin-mediated endocytosis. With L929 cells, other nonprofessional phagocytes infected with O. tsutsugamushi, similar results could be observed (data not shown).
FIG. 1.
Colocalization of O. tsutsugamushi with clathrin, α-adaptin, or caveolin 1. ECV304 cells were infected with O. tsutsugamushi for 30 min. The cells were subjected to anti-clathrin (A), anti-α-adaptin (B), or anti-caveolin 1 (C) antibodies with anti-O. tsutsugamushi antibody. Colocalization of each protein (green) with O. tsutsugamushi (red) was examined with a confocal microscope. Colocalization of each protein and O. tsutsugamushi produced yellow fluorescence. The ratios are the number of O. tsutsugamushi organisms that colocalized with clathrin (A), α-adaptin (B), or caveolin 1 (C) to the total number of infecting O. tsutsugamushi organisms. The colocalizations of O. tsutsugamushi with clathrin, α-adaptin, or caveolin 1 are indicated by arrows. Bars, 10 μm.
Infection by O. tsutsugamushi was dependent on clathrin-mediated endocytosis, but not on caveola-mediated endocytosis in nonprofessional phagocytes.
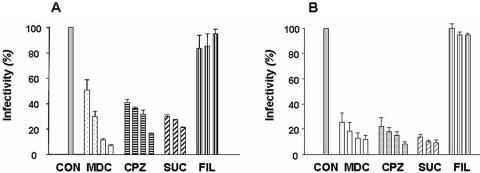
In order to examine which pathway O. tsutsugamushi takes during the endosomal stage, we used several drugs that selectively inhibit the two known endocytic pathways, clathrin- or caveola-mediated endocytosis. Chlorpromazine hydrochloride (CPZ), monodansylcadaverine (MDC), and sucrose (SUC) were used to prevent clathrin-mediated endocytosis (9, 31, 45), whereas filipin III (2) was used to block the caveola-dependent pathway. As shown in Fig. 2A, the infectivity of O. tsutsugamushi was inhibited in the group of cells treated with MDC, CPZ, or SUC, suggesting that O. tsutsugamushi uses clathrin-mediated endocytosis in nonprofessional phagocytes. Once ECV304 cells were exposed to 0.4 mM of MDC, O. tsutsugamushi invasion was inhibited by over 90%. When the clathrin-mediated endocytosis pathway was blocked using CPZ, O. tsutsugamushi infection was dramatically inhibited (up to 70%) in a dose-dependent manner. However, the treatment of cells with filipin III, which blocks the caveola-dependent pathway, did not significantly alter the O. tsutsugamushi infection at all doses tested (Fig. 2A). When L929 mouse fibroblasts were treated with the same chemicals, the effects on the infectivity of O. tsutsugamushi were similar to those observed in ECV304 cells (Fig. 2B). It is worthwhile to note that the inhibitory effect on the infectivity of O. tsutsugamushi in the mouse macrophage cell line J774A.1 was not observed (data not shown) when these chemicals, which block the clathrin- or caveola-dependent pathway, were individually tested. These results show that infection by O. tsutsugamushi in nonprofessional phagocytes is mediated by clathrin-dependent endocytosis.
FIG. 2.
Inhibitory effects of endocytosis-disrupting agents on O. tsutsugamushi invasion of nonprofessional phagocytes. ECV304 (A) and L929 (B) cells were infected at 2 × 105 ICU (about 15 O. tsutsugamushi bacteria were found per cell) for 2 h in the presence or absence of the inhibitors. Cells were labeled with KI-37 (monoclonal antibody against the Boryong strain's 56-kDa outer membrane protein) and goat anti-mouse IgG-FITC. The number of infecting bacteria in every 100 cells was counted by observation with a fluorescence microscope. The concentrations of inhibitors were maintained throughout the study. Each experiment was performed a minimum of three times. Inhibition assays were performed by preincubation of inhibitors for 30 min followed by O. tsutsugamushi infection for 2 h. The concentrations of inhibitors were as follows: MDC, 0.1, 0.2, 0.3, and 0.4 mM; CPZ, 1, 5, 10, and 25 μM; SUC, 0.1, 0.2, and 0.3 M; and filipin III (FIL), 0.375, 0.75, and 1.5 mM. CON, control.
O. tsutsugamushi organisms escaped from late endosomal organelles.
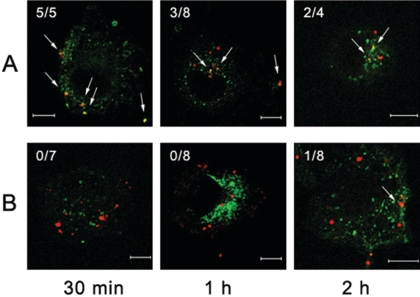
To further define the internalization pathway after the entry of O. tsutsugamushi into the cells, we examined the colocalization of these organisms with established markers for endocytic pathways. We used anti-EEA1 (28) antibody to detect early endosomes and anti-LAMP2 (39) antibody to visualize late endosomes and lysosomes. Five out of 6 O. tsutsugamushi organisms were colocalized at 30 min postinfection, and 6 out of 11 cells were colocalized at 1 h postinfection, whereas there was no colocalization at 2 h postinfection (Fig. 3A). It is intriguing that O. tsutsugamushi colocalized with LAMP2 in 2 out of 9, 6 out of 7, and 1 out of 9 cells at 30 min, 1 h, and 2 h postinfection, respectively (Fig. 3B). This result demonstrated that O. tsutsugamushi moved through the early endosome and late endosome/lysosome within 1 h after infection. By 2 h postinfection, the colocalization of O. tsutsugamushi and LAMP2 was not observed. Thus, our interpretation of this result is that most bacteria escaped from the late endosome/lysosome, and then they existed in the cytoplasm.
FIG. 3.
Colocalization of O. tsutsugamushi with EEA1 (A) and LAMP2 (B). Representative immunofluorescence with the confocal microscopic images of ECV304 cells infected with O. tsutsugamushi for the time periods indicated below. Cells were fixed, permeabilized, and double labeled with human serum against O. tsutsugamushi (goat anti-human rhodamine conjugate) and anti-EEA1 (Alexa Fluor 488) or LAMP2 (Alexa Fluor 488). (A) Merged images of EEA1 (green) and O. tsutsugamushi (red) at 30 min (left panels), 1 h (middle panels), and 2 h (right panels). (B) Merged images of LAMP2 (green) and O. tsutsugamushi (red) at 30 min, 1 h, and 2 h. The ratios are the number of O. tsutsugamushi organisms that colocalized with EEA1 (A) or LAMP2 (B) to the total number of infecting O. tsutsugamushi organisms. The colocalization of O. tsutsugamushi with EEA1 or LAMP2 is indicated by arrows. Bars, 10 μm.
The escape of O. tsutsugamushi from endocytic vesicles was dependent on the acidification process.
Since the above results suggested that the internalized O. tsutsugamushi moved through early and late endosomal vesicles, we tried to determine whether the infecting bacteria require a change in pH for their escape from endocytic vesicles to the cytoplasm by pretreating cells with NH4Cl or bafilomycin A. NH4Cl is a weak lysosomotropic base that diffuses into acidic endosomes, where it becomes protonated. Once protonated, it is unable to diffuse out, thereby increasing the pH (24). Bafilomycin A is a potent inhibitor of vacuolar H+ ATPase, which is the proton pump responsible for acidification of the intracellular compartments of eukaryotic cells (13, 32).
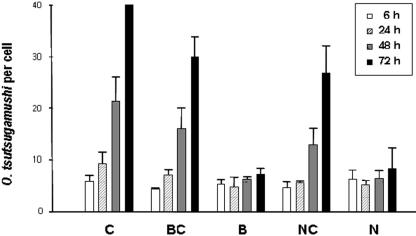
The results showed the effect that the treatment of cells with NH4Cl or bafilomycin A had on the colocalization of O. tsutsugamushi with EEA1 or LAMP2. There was colocalization of O. tsutsugamushi with EEA1 (Fig. 4A) when cells were treated with NH4Cl or bafilomycin A. In contrast, none of the O. tsutsugamushi organisms colocalized with LAMP2 at the times indicated in Fig. 4 in experiments with the same design (Fig. 4B). Furthermore, to clarify this result, O. tsutsugamushi infection was performed with or without these inhibitors for 6 h, 24 h, 48 h, and 72 h. It was interesting that there was replication of O. tsutsugamushi in the ECV304 cells without treatment with inhibitors (Fig. 5 bar C), whereas there was no replication in the cells treated with inhibitors (Fig. 5, bars B and N). In order to further analyze the status of O. tsutsugamushi 2 h postinfection in the cells treated with NH4Cl or bafilomycin A, we replaced inhibitor-supplemented medium with regular medium at 2 h postinfection. The results showed that the number of O. tsutsugamushi organisms increased in a time-dependent manner, as in the control (Fig. 5, bars BC and NC). In conclusion, we observed that growth and replication absolutely required the intracellular acidic compartments and vacuolar H+ ATPase, supporting our previous finding that intracellular O. tsutsugamushi moves toward the microtubule organizing center.
FIG. 4.
Colocalization of O. tsutsugamushi and early endosome antigen 1 or LAMP2 in the presence of NH4Cl (20 mM). Representative immunofluorescence as seen in confocal microscopic images of ECV304 cells infected with O. tsutsugamushi for the indicated time periods. Infected cells were washed, fixed, permeabilized, and double labeled with human serum against O. tsutsugamushi (goat anti-human rhodamine conjugate) together with anti-clathrin, anti-EEA1, or anti-LAMP2 (Alexa Fluor 488). (A) Colocalization of EEA1 (green) with O. tsutsugamushi (red) at 30 min, 1 h, and 2 h. (B) Colocalization of LAMP2 (green) with O. tsutsugamushi (red) at 30 min, 1 h, and 2 h. Ratios are the number of O. tsutsugamushi organisms that colocalized with EEA1 (A) or LAMP2 (B) to the total number of infecting O. tsutsugamushi organisms. The colocalization of O. tsutsugamushi with EEA1 or LAMP2 is indicated by arrows. Bars, 10 μm.
FIG. 5.
Effects of bafilomycin A and NH4Cl on O. tsutsugamushi infection. Cells were infected at 2 × 105 ICU for the indicated time periods in the presence or absence of bafilomycin A (20 nM) or NH4Cl (20 nM). ECV304 cells were fixed and labeled with KI-37 and goat anti-mouse IgG-FITC conjugate. Infective bacteria in every 100 cells were counted with a fluorescence microscope. Values represent the means from three independent experiments. C, control; BC, bafilomycin A-supplemented medium replaced with regular medium (without bafilomycin A); B, bafilomycin A-treated cells; NC, NH4Cl-supplemented medium replaced with regular medium (without NH4Cl); N, NH4Cl-treated cells.
DISCUSSION
To infect nonprofessional phagocytes, O. tsutsugamushi must bind to a specific surface receptor in the host cells and become internalized by passing through the plasma membrane. We have previously identified and characterized the transmembrane heparin sulfate proteoglycan, which is responsible for the attachment of O. tsutsugamushi (19). We have further proved that O. tsutsugamushi replicates in the microtubule organizing center at a later stage (22). Therefore, in the present study, we have attempted to identify the endocytosis pathway and endosomal escape stage.
Two major pathways, namely, the clathrin- and caveola-mediated pathways, are the most widely accepted mechanisms used by bacteria to become internalized by host cells (40). A number of studies have identified the invasive mechanism using a model system which introduces genes or proteins that are involved in a certain pathway or that uses biochemical inhibitors to block the expected steps. For instance, MDC, CPZ, and SUC are the biochemical inhibitors best known to interfere with the formation or recycling of the coated pit (9, 31, 45). In the clathrin-mediated endocytosis pathway, clathrin and α-adaptin serve as structural proteins to form the coated pit and are found during the early stage of bacterial invasion (50). Our result showed that O. tsutsugamushi was colocalized with clathrin or α-adaptin but not with caveolin 1 30 min postinfection and that MDC, CPZ, and SUC, but not filipin III, inhibited entry of the bacteria, indicating that the invasive mechanism of O. tsutsugamushi is the clathrin-dependent pathway. Furthermore, the colocalization of O. tsutsugamushi and transferrin was blocked by chlorpromazine HCl (data not shown), as expected. The bacterial invasive mechanism appeared to be largely dependent on the characteristics of the invasive microorganism and types of host cells. For instance, the exposure of primary human urethral epithelial cells to the transglutaminase inhibitor MDC resulted in an inhibition of Neisseria gonorrhoeae invasion of over 90% (15). In contrast, the pretreatment of Caco-2 cell monolayers with filipin III, which disrupts caveolae by chelating cholesterol, significantly reduced the ability of Campylobacter jejuni to enter these cells (30). Notably, it has been shown that Rickettsia, a very close family member of O. tsutsugamushi, uses a phagocytic pathway, not an endocytic pathway (48). In addition, when the chemicals that inhibit both endocytosis pathways (i.e., the clathrin- and caveola-dependent endocytosis pathways) were added to macrophages and infected with O. tsutsugamushi, no inhibition was found (data not shown), suggesting that the endocytic pathway is minimally involved in this experimental setting.
Once O. tsutsugamushi attaches to the receptor, it enters the endosomal compartments. Our data showed complete colocalization of O. tsutsugamushi with EEA1 between 10 and 30 min postinfection, indicating that the bacterial organisms target the early endosome at the beginning stage of invasion. Therefore, O. tsutsugamushi rapidly enters the host cell, and the majority of bacteria remain within the endosomal compartment for up to 1 h after invasion and are gradually trafficked to another compartment, such as the late endosome/lysosome. We then visualized the colocalization of O. tsutsugamushi with the lysosomal marker LAMP2 at 1 h postinfection. To our surprise, O. tsutsugamushi still remained in the late endosomes/lysosomes. Among intracellular bacteria, Listeria has been shown to escape the early endosome and multiply in the cytoplasm of infected cells (1). In most cases, specific mechanisms which lead to the disruption of the phagosomal membrane have been identified, thus allowing bacterial entry into the cytoplasm from the early endosome stage. In L. monocytogenes, the essential proteins that escape at the early endosome stage have been characterized, and they are the pore-forming lysteriolysin (LLO) and two phospholipase C proteins, PlcA and PlcB (46). Recently, it has been shown that Francisella tularensis utilizes mechanisms to escape from the late endosome/lysosome (14) which are distinct from those used by other intracellular bacterial pathogens, such as L. monocytogenes and Rickettsia conorii (48), to escape from the phagosome. The strategy of O. tsutsugamushi to escape from a compartment containing markers of a late endosome/lysosome is unusual for intracellular pathogens. A recent report has indicated that Bacillus anthracis, traditionally considered to have nonhemolytic genes, is capable, however, of expressing both hemolysins and phospholipases under anaerobic conditions (23). Gene sequencing of O. tsutsugamushi by our group has revealed the presence of hemolysin and hemolysin C genes (data not shown), and studies are under way to determine the functions and effects of these genes on the endosome lysis.
Exposure to the mildly acidic environment of endosomes causes conformational changes and triggers a fusion of bacterial membranes as well as endosomal membranes (7, 11, 41). Thus, we hypothesize that O. tsutsugamushi also undergoes the conformational changes associated with membrane fusion activity in the endosomes or lysosomes, which may be an essential process during the infectious life cycle of O. tsutsugamushi. In fact, we found that these acidic compartments are necessary for O. tsutsugamushi infection, since an inhibitor of vacuole-type ATPase, such as NH4Cl and bafilomycin A (BFLA1), completely blocks the bacterial protein synthesis. We have treated cells with NH4Cl or BFLA1 and observed the localization of O. tsutsugamushi in order to study when the escape occurs. There was no colocalization of LAMP2 with O. tsutsugamushi in the cells treated with NH4Cl or BFLA1. In addition, the replication rate of BFLA1- or NH4Cl-treated cells is very low compared to that of nontreated cells. This result suggests that the proton pump and low pH are essential requirements for O. tsutsugamushi to escape from the endosome to the cytosol.
In summary, the current study has demonstrated that O. tsutsugamushi enters endothelial cells and fibroblasts through the clathrin-mediated endocytic pathway and moves from the endosome to the cytosol at the phagosome/lysosome stage.
Acknowledgments
This study was supported by a grant from the Ministry of Health & Welfare, Republic of Korea (HMP-99-M-04-0002).
Editor: F. C. Fang
REFERENCES
- 1.Alvarez-Dominguez, C., R. Roberts, and P. D. Stahl. 1997. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 110:731-743. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beron, W., C. Alvarez-Dominguez, L. Mayorga, and P. D. Stahl. 1995. Membrane trafficking along the phagocytic pathway. Trends Cell Biol. 5:100-104. [DOI] [PubMed] [Google Scholar]
- 4.Caruana, S. 1974. Rickettsial diseases. Trop. Dis. Bull. 71:781-786. [PubMed] [Google Scholar]
- 5.Chang, W.-H., J.-S. Kang, W.-K. Lee, M.-S. Choi, and J.-H. Lee. 1990. Serological classification by monoclonal antibodies of Rickettsia tsutsugamushi isolated in Korea. J. Clin. Microbiol. 28:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chi, W. C., J. J. Huang, J. M. Sung, R. R. Lan, W. C. Ko, and F. F. Chen. 1997. Scrub typhus associated with multiorgan failure: a case report. Scand. J. Infect. Dis. 29:634-635. [DOI] [PubMed] [Google Scholar]
- 7.Clague, M. J., S. Urbe, F. Aniento, and J. Gruenberg. 1994. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 269:21-24. [PubMed] [Google Scholar]
- 8.Crowle, A. J., R. Dahl, E. Ross, and M. H. May. 1991. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect. Immun. 59:1823-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, P. J., D. R. Davies, A. Levitzki, F. R. Maxfield, P. Milhaud, M. C. Willingham, and I. H. Pastan. 1980. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature 283:162-167. [DOI] [PubMed] [Google Scholar]
- 10.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 11.Geisow, M. J., and W. H. Evans. 1984. pH in the endosome. Measurements during pinocytosis and receptor-mediated endocytosis. Exp. Cell Res. 150:36-46. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert, D. N., W. L. Moore, Jr., C. L. Hedberg, and J. P. Sanford. 1968. Potential medical problems in personnel returning from Vietnam. Ann. Intern. Med. 68:662-678. [DOI] [PubMed] [Google Scholar]
- 13.Girolomoni, G., D. K. Stone, P. R. Bergstresser, and P. D. Cruz, Jr. 1991. Vacuolar acidification and bafilomycin-sensitive proton translocating ATPase in human epidermal Langerhans cells. J. Investig. Dermatol. 96:735-741. [DOI] [PubMed] [Google Scholar]
- 14.Golovliov, I., V. Baranov, Z. Krocova, H. Kovarova, and A. Sjöstedt. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey, H. A., M. P. Jennings, C. A. Campbell, R. Williams, and M. A. Apicella. 2001. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol. Microbiol. 42:659-672. [DOI] [PubMed] [Google Scholar]
- 16.Hashim, S., K. Mukherjee, M. Raje, S. K. Basu, and A. Mukhopadhyay. 2000. Live Salmonella modulate expression of Rab proteins to persist in a specialized compartment and escape transport to lysosomes. J. Biol. Chem. 275:16281-16288. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz, M. A., and F. R. Maxfield. 1984. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 99:1936-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kee, S.-H., I.-H. Choi, M.-S. Choi, I.-S. Kim, and W.-H. Chang. 1994. Detection of Rickettsia tsutsugamushi in experimentally infected mice by PCR. J. Clin. Microbiol. 32:1435-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, H. R., M. S. Choi, and I. S. Kim. 2004. Role of Syndecan-4 in the cellular invasion of Orientia tsutsugamushi. Microb. Pathog. 36:219-225. [DOI] [PubMed] [Google Scholar]
- 20.Kim, I.-S., S.-Y. Seong, S.-G. Woo, M.-S. Choi, and W.-H. Chang. 1993. High-level expression of a 56-kilodalton protein gene (bor56) of Rickettsia tsutsugamushi Boryong and its application to enzyme-linked immunosorbent assays. J. Clin. Microbiol. 31:598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, M.-K., S.-Y. Seong, J.-Y. Seoh, T.-H. Han, H.-J. Song, J.-E. Lee, J.-H. Shin, B.-U. Lim, and J.-S. Kang. 2002. Orientia tsutsugamushi inhibits apoptosis of macrophages by retarding intracellular calcium release. Infect. Immun. 70:4692-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, S.-W., K.-S. Ihn, S.-H. Han, S.-Y. Seong, I.-S. Kim, and M.-S. Choi. 2001. Microtubule- and dynein-mediated movement of Orientia tsutsugamushi to the microtubule organizing center. Infect. Immun. 69:494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klichko, V. I., J. Miller, A. Wu, S. G. Popov, and K. Alibek. 2003. Anaerobic induction of Bacillus anthracis hemolytic activity. Biochem. Biophys. Res. Commun. 303:855-862. [DOI] [PubMed] [Google Scholar]
- 24.Lencer, W. I., G. Strohmeier, S. Moe, S. L. Carlson, C. T. Constable, and J. L. Madara. 1995. Signal transduction by cholera toxin: processing in vesicular compartments does not require acidification. Am. J. Physiol. 269:G548-G557. [DOI] [PubMed] [Google Scholar]
- 25.Liu, S. H., M. L. Wong, C. S. Craik, and F. M. Brodsky. 1995. Regulation of clathrin assembly and trimerization defined using recombinant triskelion hubs. Cell 83:257-267. [DOI] [PubMed] [Google Scholar]
- 26.Majeed, M., and E. Kihlström. 1991. Mobilization of F-actin and clathrin during redistribution of Chlamydia trachomatis to an intracellular site in eucaryotic cells. Infect. Immun. 59:4465-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurin, M., A. M. Benoliel, P. Bongrand, and D. Raoult. 1992. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun. 60:5013-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu, F. T., J. M. Callaghan, O. Steele-Mortimer, H. Stenmark, R. G. Parton, P. L. Campbell, J. McCluskey, J. P. Yeo, E. P. Tock, and B. H. Toh. 1995. EEA1, an early endosome-associated protein. EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J. Biol. Chem. 270:13503-13511. [DOI] [PubMed] [Google Scholar]
- 29.Ng, F. K., S. C. Oaks, Jr., M. Lee, M. G. Groves, and G. E. Lewis, Jr. 1985. A scanning and transmission electron microscopic examination of Rickettsia tsutsugamushi-infected human endothelial, MRC-5, and L-929 cells. Jpn. J. Med. Sci. Biol. 38:125-139. [DOI] [PubMed] [Google Scholar]
- 30.Oelschlaeger, T. A., P. Guerry, and D. J. Kopecko. 1993. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc. Natl. Acad. Sci. USA 90:6884-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamoto, Y., H. Ninomiya, S. Miwa, and T. Masaki. 2000. Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J. Biol. Chem. 275:6439-6446. [DOI] [PubMed] [Google Scholar]
- 32.Papini, E., M. Bugnoli, M. De Bernard, N. Figura, R. Rappuoli, and C. Montecucco. 1993. Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol. Microbiol. 7:323-327. [DOI] [PubMed] [Google Scholar]
- 33.Park, C. S., I. C. Kim, J. B. Lee, M. S. Choi, S. B. Choi, W. H. Chang, and I. Kim. 1993. Analysis of antigenic characteristics of Rickettsia tsutsugamushi Boryong strain and antigenic heterogeneity of Rickettsia tsutsugamushi using monoclonal antibodies. J. Korean Med. Sci. 8:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelkmans, L., T. Burli, M. Zerial, and A. Helenius. 2004. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118:767-780. [DOI] [PubMed] [Google Scholar]
- 35.Peyron, P., C. Bordier, E. N. N′Diaye, and I. Maridonneau-Parini. 2000. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J. Immunol. 165:5186-5191. [DOI] [PubMed] [Google Scholar]
- 36.Portnoy, D. A., T. Chakraborty, W. Goebel, and P. Cossart. 1992. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 60:1263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rikihisa, Y., and S. Ito. 1982. Entry of Rickettsia tsutsugamushi into polymorphonuclear leukocytes. Infect. Immun. 38:343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rikihisa, Y., and S. Ito. 1979. Intracellular localization of Rickettsia tsutsugamushi in polymorphonuclear leukocytes. J. Exp. Med. 150:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roszek, K., and J. Gniot-Szulzycka. 2005. Lysosomal membrane glycoprotein lamp2a—receptor for chaperone-mediated degradation of cytosolic proteins. Postepy Biochem. 51:88-94. (In Polish.) [PubMed] [Google Scholar]
- 40.Roy, C. R., and F. G. van der Goot. 2003. Eukaryotic cells and microbial pathogens: a familiar couple take centre stage. Nat. Cell Biol. 5:16-19. [DOI] [PubMed] [Google Scholar]
- 41.Schmid, J. A. 1994. The acidic environment in endocytic compartments. Biochem. J. 303:679-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmid, S. L. 1997. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu. Rev. Biochem. 66:511-548. [DOI] [PubMed] [Google Scholar]
- 43.Seong, S.-Y., M.-S. Huh, W.-J. Jang, S.-G. Park, J.-G. Kim, S.-G. Woo, M.-S. Choi, I.-S. Kim, and W.-H. Chang. 1997. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect. Immun. 65:1541-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin, J. S., and S. N. Abraham. 2001. Caveolae as portals of entry for microbes. Microbes Infect. 3:755-761. [DOI] [PubMed] [Google Scholar]
- 45.Sofer, A., and A. H. Futerman. 1995. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the Golgi apparatus and the subsequent elevation of cyclic AMP. J. Biol. Chem. 270:12117-12122. [DOI] [PubMed] [Google Scholar]
- 46.Stachowiak, R., and J. Bielecki. 2001. Contribution of hemolysin and phospholipase activity to cytolytic properties and viability of Listeria monocytogenes. Acta Microbiol. Pol. 50:243-250. [PubMed] [Google Scholar]
- 47.Tamura, A., and H. Urakami. 1981. Easy method for infectivity titration of Rickettsia tsutsugamushi by infected cell counting. Nippon Saikingaku Zasshi 36:783-785. (In Japanese.) [PubMed] [Google Scholar]
- 48.Teysseire, N., J. A. Boudier, and D. Raoult. 1995. Rickettsia conorii entry into Vero cells. Infect. Immun. 63:366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urakami, H., T. Tsuruhara, and A. Tamura. 1983. Penetration of Rickettsia tsutsugamushi into cultured mouse fibroblasts (L cells): an electron microscopic observation. Microbiol. Immunol. 27:251-263. [DOI] [PubMed] [Google Scholar]
- 50.Vigers, G. P., R. A. Crowther, and B. M. Pearse. 1986. Location of the 100 kd-50 kd accessory proteins in clathrin coats. EMBO J. 5:2079-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watt, G., and D. Strickman. 1994. Life-threatening scrub typhus in a traveler returning from Thailand. Clin. Infect. Dis. 18:624-626. [DOI] [PubMed] [Google Scholar]
- 52.Whitworth, T., V. L. Popov, X.-J. Yu, D. H. Walker, and D. H. Bouyer. 2005. Expression of the Rickettsia prowazekii pld or tlyC gene in Salmonella enterica serovar Typhimurium mediates phagosomal escape. Infect. Immun. 73:6668-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wooldridge, K. G., P. H. Williams, and J. M. Ketley. 1996. Host signal transduction and endocytosis of Campylobacter jejuni. Microb. Pathog. 21:299-305. [DOI] [PubMed] [Google Scholar]