Abstract
Performing patch-clamp experiments on human macrophages, we show that the K+ channel MaxiK is activated by lipopolysaccharide, peptidoglycan, and interleukin-1. Cytokine production initiated by several Toll-like receptor (TLR) ligands and by interleukin-1 is inhibited by MaxiK blockade. This provides evidence for functional association of the MaxiK channel and TLR signaling complexes.
Lipopolysaccharide (LPS) from gram-negative bacteria is the most potent bacterial stimulus and a critical virulence factor in gram-negative sepsis (4). Essential for LPS-initiated transmembrane signaling and macrophage activation are the transport of LPS to and its interaction with the Toll-like receptor 4 (TLR4)-MD2 complex (1, 5, 8, 10, 12). For other bacterial virulence factors such as peptidoglycan (PG), lipopeptide (LP), and lipoteichoic acid (LTA) from gram-positive bacteria, TLR2 has been shown to be required in transmembrane signaling (13). Macrophage activation can also be initiated by the cytokine interleukin-1 (IL-1). In this case, transmembrane signaling is mediated by the IL-1 receptor, which exhibits a cytoplasmic domain highly homologous to that of TLRs. TLRs and the IL-1 receptor trigger basically the same intracellular core signaling cascade, finally leading to NF-κB translocation and the production and release of inflammatory cytokines (2). Investigations into the cellular distribution of components of the LPS receptor complex have shown that TLR4/MD2, CD14, and a variety of other proteins associate with each other and form complex receptor clusters. Also, the IL-1 receptor associates several subunits into an active receptor complex. However, until now no indication of a common use of membrane-associated accessory proteins by these receptors has been found. We have shown that the large-conductance calcium- and voltage-activated potassium channel MaxiK (BK channel) is involved in the activation of human macrophages by LPS (3, 11). However, the specificity and mechanism of the activation of this ion channel and its precise role in the generation of proinflammatory signals are not yet fully understood.
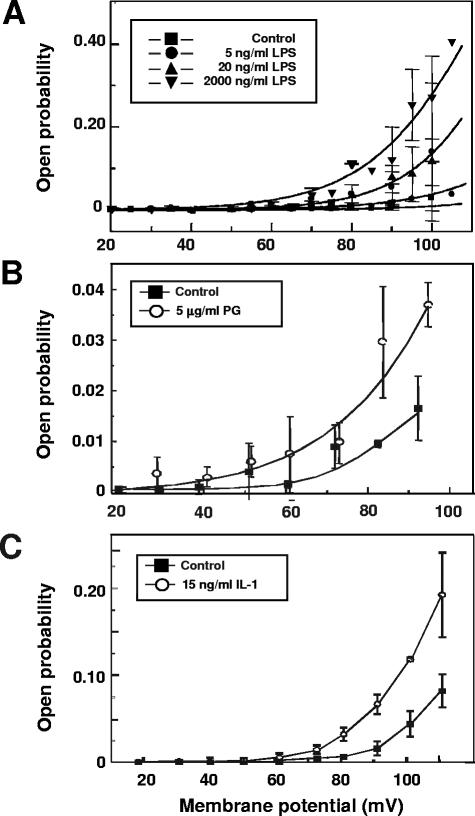
In order to investigate possible interactions of MaxiK and TLR/IL-1-receptor in macrophage activation by different virulence factors and IL-1, we performed electrophysiological measurements on human macrophages. In excised outside-out patches from the macrophage cytoplasmic membrane prepared as described in reference 3, the application of either LPS from Salmonella enterica serovar Minnesota strain R595 (extracted according to the phenol-chloroform/petrol ether procedure [6]), soluble PG (isolated from penicillin-treated Staphylococcus aureus as described elsewhere [9]), or recombinant human IL-1 (R&D Systems) to the extracellular face of the patch led to a strong increase in channel activity as demonstrated by the increase in the open probability (determined according to the method of Glasbey and Martin [6a]) of MaxiK compared to untreated controls (Fig. 1A to C). Single-channel conductance, however, was not affected by LPS, PG, or IL-1. Channel activation by IL-1 could be suppressed by adding an IL-1 receptor antagonist (ra IL-1; 400 ng/ml; R&D Systems) to the bathing solution (data not shown), proving that channel activation is induced by the interaction of the cytokine with its receptor.
FIG. 1.
Lipopolysaccharide, peptidoglycan, and interleukin-1 activate MaxiK in the cytoplasmic membranes of human macrophages. Shown are open probabilities (means ± standard deviations) of the MaxiK channel in excised outside-out membrane patches in a bathing solution of Hanks balanced salt solution after the application of either LPS at the indicated concentrations (A), 5 μg/ml PG (B), or 15 ng/ml IL-1 (C) as a function of the applied membrane potential. Current/voltage curves were fitted to sigmoid functions. The data shown are representative of three independent experiments.
Channel activation by ligands of the TLR/IL-1 receptor family can be observed in excised membrane patches, implying that channel activation is mediated by membrane-associated structures of the signaling machinery. Independently of the mechanism leading to the activation of MaxiK, we can conclude that its activation is an initial event involved in cell activation.
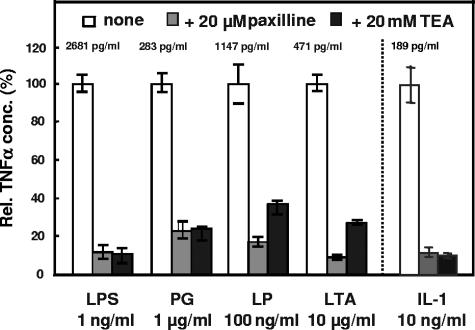
To show the requirement for MaxiK in the process of cytokine release upon macrophage activation, we employed pharmacological channel blockade. Macrophages differentiated from human peripheral blood mononuclear cells were stimulated for 4 h with LPS, PG, the synthetic LP Pam3CSK4, or LTA isolated from penicillin-treated Staphylococcus aureus as described elsewhere (7) in the absence or presence of the MaxiK-specific blocker paxilline (Alomone Labs) or the nonspecific K+ channel blocker tetraethylammonium (TEA) (Sigma). For all stimuli, the release of tumor necrosis factor alpha (TNF-α) was inhibited in the presence of paxilline or TEA (Fig. 2). The inhibitory effects of the blockers did not affect the integrity of the cells, as checked by trypan blue exclusion. As was the case for the bacterial virulence factors, the application of paxilline or TEA—and of the IL-1 receptor antagonist (data not shown)—also inhibited IL-1-induced cytokine release from macrophages (Fig. 2). In contrast, when cells were stimulated with TNF-α, paxilline did not inhibit the release of cytokines (data not shown), indicating that the inhibitory effect of paxilline does not interfere with TNF receptor-linked signaling pathways. Our results allow us to conclude that activation of MaxiK in human macrophages represents a general principle in transmembrane signaling initiated by members of the TLR and IL-1 receptor family. The dramatic inhibitory effect of MaxiK channel blockade on release of TNF-α from macrophages stimulated by various stimuli shows the involvement of the functional MaxiK channel in cell activation. Since channel activation is induced by ligands of different TLRs and the IL-1 receptor, it is reasonable to propose that the intracellular signaling domain, referred to as the Toll/interleukin-1 receptor (TIR) domain, is the common starting point for MaxiK activation.
FIG. 2.
MaxiK function is required for the inflammatory response to bacterial virulence factors and to interleukin-1. Shown is TNF-α production from human blood macrophages (2 · 105 cells per well in a 96-well dish) after 4 h of stimulation with either LPS (1 ng/ml), PG (1 μg/ml), LP (100 ng/ml), LTA (10 μg/ml), or IL-1 (10 ng/ml) in the absence or presence of the MaxiK blocker paxilline (20 μM) or the K+ channel blocker TEA (20 mM). The cytokine response to each stimulus in the absence of a channel blocker was set at 100%, and the corresponding data were normalized to these values. The absolute amounts of TNF-α in the supernatants of the stimulated cells are given above the rows. Data shown are means ± standard deviations from triplicate samples of one donor and are representative of three independent experiments with cells from different donors.
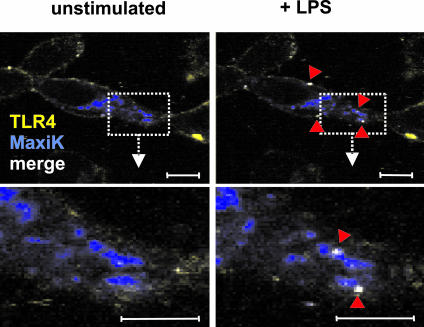
Activation of MaxiK by TLRs and IL-1 receptor ligands implies a physical association of the ion channel with the respective receptor complex. This would require a spatial proximity of the proteins. We established a HEK293 cell line stably expressing human TLR4 and MD2 protein as well as the pore-forming alpha subunit of MaxiK as a green fluorescent protein (GFP) fusion protein. The clones derived responded to stimulation with LPS, and patch-clamp experiments demonstrated that the MaxiK-GFP fusion protein showed characteristics similar to those of MaxiK in primary macrophages and could be activated by stimulation with LPS (data not shown). We investigated the distribution of receptor proteins by confocal microscopy with an inverted LSM510 META microscope (Carl Zeiss, Jena, Germany). Briefly, HEK293-TLR4/MD2-MaxiKGFP cells were cultured on microchamber culture slides and stained with an anti-TLR4 antibody (HTA125; Imgenex) and an Alexa 647-conjugated goat anti-mouse secondary antibody (Molecular Probes, Oregon). For stimulation experiments, tracks were scanned sequentially every minute before and after stimulation of cells, and confocal images were analyzed using LSM Image Browser software, version 3.2 (Carl Zeiss). In unstimulated cells, MaxiK and TLR4 appear to localize in different membrane compartments (Fig. 3, left). In contrast, after stimulation with LPS, distinct patches where MaxiK and TLR4 signal colocalized could be observed on the cell membranes of the same cells (Fig. 3, right). Our data indicate that cell stimulation by LPS induces translocation of MaxiK and TLR4 to similar cellular compartments. Whether this colocalization is accompanied by formation of a complex will have to be elucidated in further investigations.
FIG. 3.
Colocalization of MaxiK and TLR4 determined by confocal microscopy. HEK293-TLR4/MD-2-MaxiKGFP cells were subjected to time lapse experiments before (left) and 3 min after (right) stimulation with 10 ng/ml LPS. Cells were placed on a temperature-adjustable microscope stage and equilibrated to 37°C. In unstimulated cells, TLR4 (yellow) and MaxiK (blue) localize to different compartments. Upon stimulation with LPS, distinct areas where TLR4 and MaxiK colocalize can be observed (white pixels in the image). Lower panels are enlargements of boxed areas. Bars, 10 μM. Results shown are from one experiment representative of three independent experiments.
Acknowledgments
We acknowledge the excellent technical assistance of C. Hamann and S. Groth and thank K. Marienfeld for cell sorting. The synthetic lipopeptide (Pam)3Cys-Ser-(Lys)4 was a kind gift of K.-H. Wiesmüller (EMC Microcollections GmbH, Tübingen, Germany). We are grateful to Douglas Golenbock, Kensuke Miyake, and Martin Wallner for kindly providing us with mammalian expression vectors coding for human TLR4, MD2, and MaxiKGFP, respectively.
This work was funded by DFG grants (SFB 367 project B8 and Emmy-Noether grant SCHR 621/2-1).
Editor: J. T. Barbieri
REFERENCES
- 1.Akashi, S., S. Saitoh, Y. Wakabayashi, T. Kikuchi, N. Takamura, Y. Nagai, Y. Kusumoto, K. Fukase, S. Kusumoto, Y. Adachi, A. Kosugi, and K. Miyake. 2003. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198:1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Blunck, R., O. Scheel, M. Müller, K. Brandenburg, U. Seitzer, and U. Seydel. 2001. New insights into endotoxin-induced activation of macrophages: involvement of a K+ channel in transmembrane signaling. J. Immunol. 166:1009-1015. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature 420:885-891. [DOI] [PubMed] [Google Scholar]
- 5.da Silva, C. J., K. Soldau, U. Christen, P. S. Tobias, and R. J. Ulevitch. 2001. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. Transfer from CD14 to TLR4 and MD-2. J. Biol. Chem. 276:21129-21135. [DOI] [PubMed] [Google Scholar]
- 6.Galanos, C., O. Lüderitz, E. T. Rietschel, O. Westphal, H. Brade, L. Brade, M. Freudenberg, U. Schade, M. Imoto, H. Yoshimura, and T. Shiba. 1985. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur. J. Biochem. 148:1-5. [DOI] [PubMed] [Google Scholar]
- 6a.Glasbey, C. A., and R. J. Martin. 1988. The distribution of numbers of open channels in multi-channel patches. J. Neurosci. Methods 24:283-287. [DOI] [PubMed] [Google Scholar]
- 7.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal, R. S., and R. Dziarski. 1994. Isolation of peptidoglycan and soluble peptidoglycan fragments. Methods Enzymol. 235:253-285. [DOI] [PubMed] [Google Scholar]
- 10.Schromm, A. B., E. Lien, P. Henneke, J. C. Chow, A. Yoshimura, H. Heine, E. Latz, B. G. Monks, D. A. Schwartz, K. Miyake, and D. T. Golenbock. 2001. Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J. Exp. Med. 194:79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seydel, U., O. Scheel, M. Müller, K. Brandenburg, and R. Blunck. 2001. A K+ channel is involved in LPS signaling. J. Endotoxin Res. 7:243-247. [PubMed] [Google Scholar]
- 12.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]