Abstract
Relapsing fever is a rapidly progressive and severe septic disease caused by certain Borrelia spirochetes. The disease is divided into two forms, i.e., epidemic relapsing fever, caused by Borrelia recurrentis and transmitted by lice, and the endemic form, caused by several Borrelia species, such as B. duttonii, and transmitted by soft-bodied ticks. The spirochetes enter the bloodstream by the vector bite and live persistently in plasma even after the development of specific antibodies. This leads to fever relapses and high mortality and clearly indicates that the Borrelia organisms utilize effective immune evasion strategies. In this study, we show that the epidemic relapsing fever pathogen B. recurrentis and an endemic relapsing fever pathogen, B. duttonii, are serum resistant, i.e., resistant to complement in vitro. They acquire the host alternative complement pathway regulator factor H on their surfaces in a similar way to that of the less serum-resistant Lyme disease pathogen, B. burgdorferi sensu stricto. More importantly, the relapsing fever spirochetes specifically bind host C4b-binding protein, a major regulator of the antibody-mediated classical complement pathway. Both complement regulators retained their functional activities when bound to the surfaces of the spirochetes. In conclusion, this is the first report of complement evasion by Borrelia recurrentis and B. duttonii and the first report showing capture of C4b-binding protein by spirochetes.
Relapsing fever is transmitted via arthropod vectors. The human body louse (Pediculus humanus corporis) transmits Borrelia recurrentis, the causative organism of louse-borne relapsing fever (LBRF), while Ornithodoros ticks are vectors for the at least 15 different species of Borrelia that cause endemic or tick-borne relapsing fever (TBRF). While humans are the only known host for the LBRF spirochete, the TBRF-causing Borrelia species (with the exception of B. duttonii) have their reservoirs in small rodents and have been found in several locations worldwide. In North America, three species of Borrelia cause TBRF, and several outbreaks of the disease have been described (33). In East Africa, B. duttonii is the principal cause of TBRF.
Currently, epidemic LBRF is found in Africa (35). Crowding, poor hygiene, and poverty are risk factors for large outbreaks, such as the epidemics seen during World War II, when millions of soldiers and civilians were infected in Southern Europe. Relapsing fever spirochetes enter the bloodstream from the site of the insect or tick bite wound. This is soon followed by a massive spirochetemia, with spirochetes also invading through the endothelium. The first septic episode ends upon development of antibodies, but because relapsing fever spirochetes are able to change their variable outer surface proteins, the spirochetemia returns, causing one or more febrile relapses (5, 31). Clinical disease is severe, with mortality reaching 30 to 70% without antimicrobial treatment (11).
Antibody responses against relapsing fever Borrelia species are primarily directed against outer surface lipoproteins. Two major protein groups have been identified, namely, variable small proteins (approximately 22 kDa) and variable large proteins (approximately 38 kDa) (6). These proteins have been studied most thoroughly for B. hermsii (4, 5) and B. turicatae (27, 30). It is probable that all of the relapsing fever Borrelia species share the same antigenic variation scheme as that described in detail for these two species.
While spirochetes are present in blood, they must evade the immune defense systems. Before the acquired immune responses lead to the production of antibodies, the alternative pathway of complement operates as a major innate immune defense system against the invading organisms. In the presence of antibodies, complement acts as an effector system, mainly via the classical pathway (CP). Both pathways lead to coating of the target surface with C3b. Together with their cleavage products, such as iC3b, the C3b molecules opsonize the target for phagocytosis. Further activation can lead to the formation of lytic membrane attack complexes. To avoid overconsumption of the components of the complement cascade and to protect self cells from harmful attacks, complement activation must be tightly regulated. This is mediated by regulatory proteins in plasma and on cell surfaces.
The major fluid-phase regulators of complement are factor H (FH), for antibody-independent alternative pathway activation, and C4b-binding protein (C4BP), for antibody-dependent CP activation (7). These regulators accelerate decay of the C3 convertases (C3bBb and C4b2a, respectively) (15, 25, 36) and act as cofactors for the irreversible inactivation of C3b and C4b, respectively. As a net effect, these functions prevent complement-mediated destruction of the target in both the absence and presence of antibodies.
Acquisition of the host plasma complement regulator FH has been shown to be beneficial for complement evasion among other spirochetes, such as Borrelia burgdorferi sensu stricto and B. afzelii, which express at least two FH binding proteins (2, 16, 19, 20). Also, the relapsing fever agents B. hermsii and B. parkerii have been shown to bind FH, while no binding has been observed for B. turicatae (22). B. hermsii expresses a unique 20-kDa outer surface protein (FhbA) responsible for FH binding (17).
For this study, we have studied the only known agent of LBRF, B. recurrentis, and a TBRF agent, B. duttonii, to test the serum sensitivities of relapsing fever Borrelia species. We demonstrate the acquisition of the complement regulator FH by both types of Borrelia and describe a hitherto unidentified immune evasion mechanism for spirochetes by showing binding of C4BP to both types of relapsing fever Borrelia and, to some extent, also to the Lyme disease spirochete, B. burgdorferi sensu stricto. Binding occurs when Borrelia spirochetes are incubated with purified proteins or in whole plasma. Using factor I cofactor assays, we show that the surface-acquired FH and C4BP retain their functional activities on the surfaces of relapsing fever spirochetes. This indicates that the binding of FH and C4BP contributes to serum resistance of relapsing fever spirochetes and suggests that multiple simultaneous complement evasion strategies are used by these organisms in the pathogenesis of relapsing fever.
MATERIALS AND METHODS
Bacteria and culture conditions.
Four strains of Borrelia recurrentis (A1, A5, A6, and A17) and three strains of Borrelia duttonii (La, Ku, and Kw) were isolated from relapsing fever patients in Ethiopia and Tanzania, respectively (12, 13). Strains of B. burgdorferi sensu stricto (B31 and IA) and B. garinii (50/97) were kind gifts from Matti Viljanen (University of Turku, Finland). All strains were grown at +33°C in Barbour-Stoenner-Kelly (BSK-H) complete medium (Sigma, St. Louis, MO) and subcultured approximately twice a week. The passage number for the B. recurrentis strains was between 8 and 15; a high passage number was inevitable to enable purification of isolates and to gain enough material for the assays. For the B. duttonii strains, the passage number was between 10 and 30, and for the B. burgdorferi strains, it was below 10.
Serum sensitivity assay.
The sensitivity of spirochetes to human serum was analyzed by cultivating B. recurrentis A17, B. duttonii La, B. burgdorferi sensu stricto B31, and B. garinii 50/97 in 50% normal human serum (NHS) in BSK-H complete medium. Bacteria (1 × 107) were pipetted into 48- or 96-well microtiter plates (Nunc A/S, Roskilde, Denmark) in triplicate in BSK-H complete medium. Fresh nonimmune NHS was collected from a healthy laboratory person and added to the wells to achieve a final concentration of 50% in 200 μl. The same serum was used as a control after heat inactivation (30 min at +56°C) (HI-NHS). The presence of antibodies against the relapsing fever and Lyme disease Borrelia species in NHS was tested by using whole-cell lysates in a Western blot analysis. At 1-, 2-, 3-, 4-, 5-, and 24-h time points, 20-μl samples were added to 180 μl of BSK-H complete medium to analyze the viability of the Borrelia species after exposure to NHS. After removal of each sample, 20 μl of fresh 50% NHS (diluted in BSK-H medium) was added to prevent total consumption of complement in the mixture. The viability of the spirochetes within these secondary cultures was checked microscopically, using dark-field optics, after 48 h of cultivation at +33°C.
Serum absorption experiments.
Freshly harvested borrelia organisms (1 × 109) were washed four times with Veronal-buffered saline (VBS; 145 mM NaCl, 5 mM barbiturate, pH 7.4). The spirochetes were incubated in HI-NHS for 60 min at +37°C on a shaker (750 rpm) and washed five times with VBS. The last wash fraction was collected. Proteins bound to the surfaces of bacteria were eluted with 0.1 M glycine-HCl, pH 2.0, and supernatants were collected after centrifugation (8,000 × g, 10 min). Samples of the wash and eluate fractions were subjected to nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Nonspecific binding was blocked with 3% fat-free milk in phosphate-buffered saline (120 mM NaCl, 30 mM phosphate, pH 7.3) for 1 h at 22°C. The membranes were incubated with a sheep polyclonal anti-C4BP antibody (The Binding Site, Birmingham, United Kingdom) at a 1:2,000 dilution for 12 h at 4°C. After five washes with phosphate-buffered saline, a horseradish peroxidase-conjugated rabbit anti-sheep immunoglobulin G antibody (Jackson Immunoresearch Laboratories, Cambridgeshire, United Kingdom) was added at a dilution of 1:2,000, and the membranes were incubated at 22°C for 3 h. After the membranes were washed, the bound antibodies were detected by enhanced chemiluminescence.
Protein binding assays.
C4BP was purified from human plasma as described previously (14), and FH was purchased from Quidel (San Diego, CA) or Calbiochem (La Jolla, CA). C4BP, FH, and bovine serum albumin (BSA; Sigma, St. Louis, MO) were labeled with 125I by using the Iodogen technique (Pierce Chemical Corp., Rockford, IL) (32). Freshly harvested bacteria were washed three times with VBS diluted 1:3 containing 0.1% gelatin (1/3 GVBS). Approximately 5 × 107 bacteria/assay were incubated with the radiolabeled proteins (approximately 20,000 cpm/assay) in 1/3 GVBS for 30 min at 37°C on a shaker (750 rpm). Cell-associated and free radioactive proteins were separated by centrifuging the samples through 200-μl columns of 20% (wt/vol) sucrose in 1/3 GVBS. Radioactivities in the supernatant and pellet fractions were measured with a gamma counter, and the amounts of bound proteins were calculated as percentages of the total radioactivities in the corresponding pellets and supernatants. All experiments were performed in quadruplicate. Some radioactivity (1 to 3%) was found to be sedimented in the absence of bacteria, possibly due to protein aggregates. These values were considered background and were subtracted from the values shown. In an inhibition assay, nonlabeled C4BP was added to the reaction mixture prior to the addition of 125I-C4BP.
Cofactor assay for C4b and C3b inactivation.
The cofactor activities of the surface-attached C4BP and FH molecules for factor I were assayed by cleavage of 125I-C4b and 125I-C3b, respectively. The bacteria (1 × 109/assay) were washed three times with VBS and incubated with HI-NHS (at a 1:2 dilution) or purified C4BP (10 μg/ml) for 120 min on a shaker (700 rpm) at 37°C. Bacteria were washed three times with VBS, followed by the addition of factor I (50 ng/reaction) and either 125I-C4b or 125I-C3b (all from Calbiochem), and the mixtures were incubated for 60 min at 37°C. The bacteria were pelleted by centrifugation, and the supernatants were subjected to SDS-PAGE under reducing conditions. The gels were fixed with 5% acetic acid for 30 min and dried prior to autoradiography to detect the cleavage products of 125I-C4b and 125I-C3b. As a positive control, purified C4BP or FH (50 ng) was added to the reaction mixture. As negative controls, 125I-C4b and 125I-C3b were incubated in the presence of factor I alone.
RESULTS
Serum resistance of relapsing fever Borrelia spp.
To assess the serum sensitivities of B. recurrentis and B. duttonii, the spirochetes were incubated in nonimmune serum, and their viabilities were monitored microscopically and verified by cultivation. To enable comparison of the relapsing fever spirochetes with the previously analyzed Lyme disease spirochetes, serum-resistant B. burgdorferi sensu stricto and serum-sensitive B. garinii were included as controls (3, 8). The Borrelia spp. were incubated in the presence of 50% NHS, and fresh NHS was added to the cultures at 1, 2, 3, 4, and 5 h to ensure exposure of the spirochetes to active complement. Samples were taken prior to the NHS addition at each time point and after 24 h of incubation. The samples were subjected to dark-field microscopy and further cultivation for 48 h in NHS-free BSK-H complete medium. The microscopic and culture analyses gave similar results. B. garinii was killed within 4 h upon NHS exposure, and B. burgdorferi sensu stricto succumbed within 5 to 24 h of serum exposure, while both relapsing fever Borrelia spp. tolerated serum exposure for at least 24 h (Table 1). Based on these results, it seems that the relapsing fever spirochetes are more resistant to complement than are Lyme disease spirochetes. This is concordant with the well-known differences in the clinical courses of the diseases.
TABLE 1.
Comparison of survival of Borrelia burgdorferi and that of relapsing fever spirochetes in human seruma
| Time (h) | Survival of indicated strainb
|
|||
|---|---|---|---|---|
| B. burgdorferi sensu stricto B31 | B. garinii 50/97 | B. recurrentis A17 | B. duttonii LA | |
| 0 | + | + | + | + |
| 1 | + | + | + | + |
| 2 | + | + | + | + |
| 3 | + | + | + | + |
| 4 | + | + | + | + |
| 5 | + | − | + | + |
| 24 | − | − | + | + |
Spirochetes were incubated in 50% NHS in BSK-H complete medium, with fresh NHS added after each sample was taken. All strains grew well for the 24-h period in control wells where heat-inactivated NHS was added instead of NHS (data not shown).
+, the sample taken from NHS-free BSK-H complete medium at the indicated time point contained microscopically viable spirochetes after a 48-hour incubation; −, no viable spirochetes were detected after a 48-hour incubation in NHS-free culture medium.
Relapsing fever Borrelia spp. acquire both FH and C4BP from human serum.
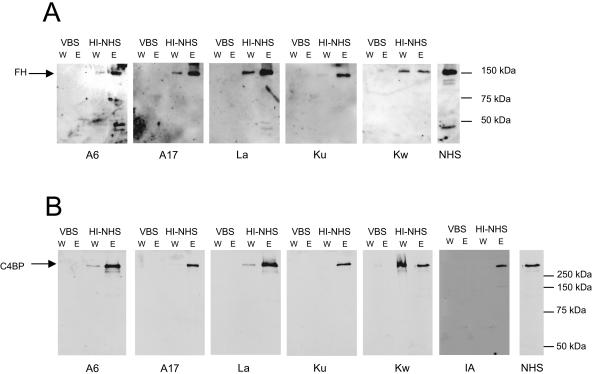
Previously, several species of Borrelia were shown to acquire host FH from serum. We analyzed whether the relapsing fever pathogens B. recurrentis and B. duttonii could bind FH during incubation in nonimmune, heat-inactivated NHS. After serum incubation and a thorough washing, the borrelia-bound material was eluted and subjected to Western blotting with an anti-FH antibody. All tested strains of B. recurrentis (A6 and A17) and B. duttonii (La, Ku, and Kw) were found to acquire FH during serum incubation (Fig. 1A). Since the relapsing fever Borrelia spp. are able to cause a septic relapsing fever with multiple fever relapses despite specific antibodies in serum, we examined if they also acquire the classical complement pathway regulator C4BP from human serum. Eluates taken following serum incubations were subjected to Western blotting with anti-C4BP antibody. All eluted fractions for B. recurrentis (A6 and A17) and B. duttonii (La, Ku, and Kw) contained C4BP (Fig. 1B). We also analyzed the acquisition of C4BP by B. burgdorferi sensu stricto (strain IA) and saw that this strain of Lyme disease spirochetes bound C4BP to the same extent. The anti-FH and anti-C4BP antibodies used failed to bind to any components of the elution fractions when the spirochetes had been incubated in VBS instead of NHS (Fig. 1).
FIG. 1.
Acquisition of FH (A) and C4BP (B) from human serum by B. recurrentis (strains A6 and A17), B. duttonii (strains La, Ku, and Kw), and B. burgdorferi sensu stricto (strain IA). The spirochetes (1 × 109) were incubated in either HI-NHS or VBS prior to washings and elution of the bound proteins. Aliquots of the last wash (W) and the eluate (E) fractions were subjected to nonreducing SDS-PAGE and analyzed with anti-FH (A) or anti-C4BP (B) antibody by Western blotting. Binding of the antibodies to proteins in HI-NHS samples (diluted 1:500) is shown as a positive control. The mobilities of the size markers are shown on the right.
Relapsing fever Borrelia spp. bind purified C4BP and FH.
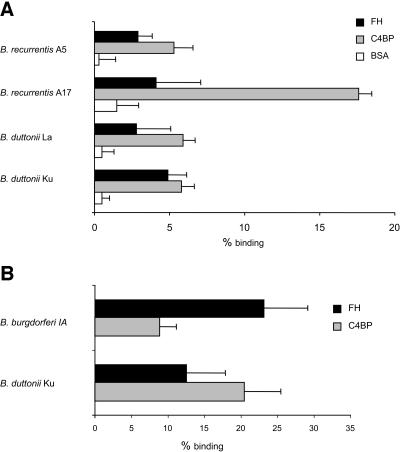
To test whether C4BP and FH bind to relapsing fever spirochetes in the absence of other serum components, we analyzed direct binding of purified C4BP to B. recurrentis and B. duttonii. C4BP, FH, and a control protein (BSA) were radiolabeled with 125I, and the labeled proteins were incubated with the spirochetes. The cell-associated proteins were separated from the unbound proteins by centrifuging the mixtures through sucrose columns. The labeled C4BP clearly bound (4.8 to 17%) to all strains studied, while BSA failed to bind to any of the strains (0.3 to 1.5%) (Fig. 2A). The B. recurrentis strain A17 showed a threefold higher binding percentage than the other strains. This indicates that the intensity of C4BP binding varies markedly between the different strains. FH bound to all the strains studied, but with a somewhat lower intensity than C4BP (Fig. 2A).
FIG. 2.
Binding of purified FH, C4BP, and BSA to B. recurrentis (strains A5 and A17) and B. duttonii (strains La and Ku) (A) and of FH and C4BP to B. duttonii (KU) and B. burgdorferi sensu stricto (IA) (B). The spirochetes were incubated with 125I-labeled FH, C4BP, or BSA (approximately 50,000 cpm each) for 30 min, and unbound protein was separated from borrelia-bound protein by centrifugation through 20% sucrose columns. The background was detected as sedimentation of the radioactivity in the absence of any bacteria (1 to 3%) and was subtracted from the raw data, resulting in the values shown. Means ± standard deviations of quadruplicate assays are shown.
To compare binding efficiencies between relapsing fever and Lyme disease spirochetes, we used B. duttonii strain KU and B. burgdorferi sensu stricto strain IA simultaneously in a direct binding assay (Fig. 2B). We noticed that FH bound more profoundly to B. burgdorferi than to B. duttonii (23.2% versus 12.6%) but that C4BP bound more efficiently to B. duttonii than to B. burgdorferi (20.5% versus 8.9%).
Specificity of binding of C4BP.
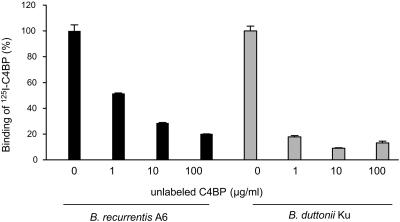
The specificity of C4BP binding to Borrelia spp. was studied by using 125I-labeled C4BP and increasing concentrations (0.1 μg/ml to 100 μg/ml) of unlabeled C4BP in a binding assay. Unlabeled C4BP was found to inhibit binding of 125I-labeled C4BP in a dose-dependent manner (Fig. 3). At a concentration of 100 μg/ml of cold C4BP, binding was inhibited by 70% for B. recurrentis (strain A6) and by 86% for B. duttonii (strain Ku).
FIG. 3.
Specificity of binding of 125I-labeled C4BP to B. recurrentis (strain A6) and B. duttonii (strain Ku). Spirochetes (5 × 107) were incubated with 125I-labeled C4BP for 30 min in the presence of the indicated amounts of unlabeled C4BP. Unbound protein was separated from the surface-bound protein by centrifuging the bacteria through 20% sucrose columns. The background was detected as sedimentation of the radioactivity in the absence of any bacteria and was subtracted from the raw data.
Cofactor activity of Borrelia-bound regulators.
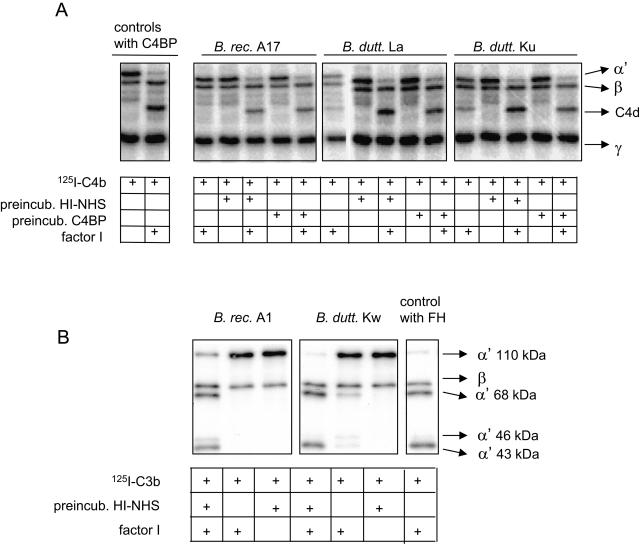
We next tested if the relapsing fever Borrelia-bound complement regulators FH and C4BP were still functional for acting as cofactors for the serum protease factor I in cleaving C3b and C4b, respectively. The B. recurrentis and B. duttonii spirochetes were first incubated in NHS or with purified C4BP, and after washing of the organisms, factor I was added together with 125I-labeled C3b or C4b. After incubation, the cleavage fragments of the radiolabeled proteins were detected by SDS-PAGE and autoradiography. Borrelia organisms that had been preincubated with NHS showed cofactor activity for cleavage of C3b (Fig. 4A). Similarly, Borrelia spp. preincubated with NHS or with purified C4BP possessed cofactor activity for the cleavage of 125I-labeled C4b (Fig. 4B). The C3b and C4b fragments that were generated by the Borrelia-bound cofactors and factor I were similar in their apparent molecular weights to the fragments generated by factor I in combination with the positive controls, purified FH and C4BP. The spirochetes preincubated in buffer alone or with factor I in the absence of C4BP or FH did not promote cleavage of C3b or C4b (Fig. 4). This indicates that the studied Borrelia spp. lack endogenous C3b or C4b cleaving activity or cofactor activity for the cleavage.
FIG. 4.
Cofactor activities of relapsing fever spirochete surface-bound C4BP (A) and FH (B). Bacteria (1 × 109) were incubated in HI-NHS or with purified C4BP (10 μg/ml) and washed, and factor I was added in combination with 125I-labeled C4b (A) or 125I-labeled C3b (B). Samples from supernatants were run in reducing SDS-PAGE gels, after which the gels were dried and visualized by autoradiography. As positive controls, 125I-labeled C4b (A) and 125I-labeled C3b (B) were incubated in the presence of factor I and purified C4BP and FH, respectively. The cleavage of 125I-labeled C4b is observed by the appearance of the C4d fragment, and that of 125I-labeled C3b is observed by the appearance of the α′ 68-kDa, 46-kDa, and 43-kDa fragments.
DISCUSSION
In this study, we have shown that the two most significant relapsing fever spirochetes for humans, B. recurrentis and B. duttonii, are more resistant to human serum than are Lyme disease spirochetes and that, in addition to the common FH-mediated complement evasion strategy of Borrelia spp., they also acquire host C4BP on their surfaces. Both surface-bound complement regulators are functionally active on relapsing fever spirochetes, indicating a possible explanation for the clinically observed extraordinary serum resistance of the bacteria and their evasion of antibody-mediated clearance from blood circulation.
B. recurrentis and B. duttonii remain clinically significant pathogens in their areas of endemicity. Furthermore, B. recurrentis retains its potential to cause local or larger epidemics under poor sanitary conditions. The isolates used in this study were obtained from patients from the areas where B. recurrentis and B. duttonii are currently endemic, and the spirochetes were of the lowest possible passage number available (13). There are no vaccines available against any of the relapsing fever Borrelia spp., although a vaccine would be highly desirable in the areas of endemicity. Difficult cultivation methods have hampered research on the molecular pathology caused by the relapsing fever Borrelia spp., and studies on, for example, the immune evasion mechanisms of these pathogens are scarce.
Most B. burgdorferi sensu stricto and B. afzelii strains studied bind human FH, and there seem to be at least two distinct groups of FH-binding proteins on these spirochetes (1-3, 16, 20, 21). Therefore, it is not surprising that relapsing fever Borrelia spp., such as B. hermsii, that cause a more severe septic disease have also been reported to bind FH (17, 22). Some other TBRF-causing species, such as B. turicatae, do not bind human FH (22). It remains to be studied if the ability of a strain or species to bind FH affects its capacity to cause certain pathologies in, for example, the central nervous system. The ability of different host species to bind FH could also play a role in the known species specificity of the different relapsing fever Borrelia spp.
In this study, we have shown that several isolates of B. recurrentis and B. duttonii bind human C4BP in addition to FH. C4BP binding has not been shown previously with any other spirochetes or related bacteria. Since the Lyme disease Borrelia strains cause chronic infections with formation of antibodies, it should also be beneficial for Lyme disease spirochetes to evade the classical complement pathway. Indeed, we observed that the studied strain (IA) of B. burgdorferi sensu stricto also bound C4BP (Fig. 1 and 2B), although apparently more weakly than the relapsing fever Borrelia spp.
During relapsing fever, the Borrelia spirochetes persist and multiply in the blood of patients for several days, or even weeks. The best-known immune evasion mechanism demonstrated for the relapsing fever Borrelia spp. is antigenic variation, i.e., the ability to change the major outer surface proteins in cycles, leading to persistence of the strain as a new “serotype” in the human host (5, 31). Antigenic variation leads to the change of only one type of surface protein, while the other surface structures remain unchanged. Since other antigenic structures exist on the bacteria, it is likely that some of the antibodies generated recognize these spirochetes. Therefore, the acquisition of C4BP on the bacterial surface could be essential for down-regulating antibody-dependent CP activation and the development of clinical fever relapses. The evasion of adaptive immunity by a combination of antigenic variation and C4BP acquisition might underlie the long bacteremic course and high mortality of the disease.
With the exception of certain primates, there is no animal model for B. recurrentis since humans are the only known host. A mouse model exists for the New World endemic relapsing fever Borrelia spp., and this has been utilized in previous studies on host-microbe interactions. The role of complement in the host defense against relapsing fever borrelia spirochetes was investigated by using a mouse model with B. hermsii where wild-type mice were compared with C3- and C5-deficient mice (9, 10). In those studies, no difference was observed in the abilities of the different mice to clear the infection, suggesting that complement is not effective against the spirochetes in vivo. The current study provides a good explanation for this, since at least B. recurrentis and B. duttonii are able to acquire both alternative and classical pathway regulators from host plasma in a way that allows the regulators to be functionally active. It remains to be studied, however, whether the relapsing fever Borrelia spp. bind mouse FH and C4BP. It is known that OspE of B. burgdorferi is able to bind mouse FH (34), and we assume that at least those species of relapsing fever borrelias that are able to cause severe disease in mice should be able to evade mouse complement. These species are therefore likely to bind mouse FH and C4BP. Interestingly, different species causing tick-borne relapsing fever have different wildlife hosts, and the reason for this species specificity is not fully understood. The role of complement regulator acquisition from different animal sera as an aspect of species specificity warrants future investigation.
The ability of B. recurrentis and B. duttonii to acquire both FH and C4BP is an exceptional phenomenon in the microbe-host relationship. There are reports that only three pathogens, Streptococcus pyogenes (group A streptococci) (18, 26), Neisseria gonorrhoeae (28, 29), and Candida albicans (23, 24), are able to bind both FH and C4BP. In the case of S. pyogenes, the receptor for both ligands is M protein, and most of the strains that bind FH do not seem to bind C4BP, and vice versa. In the case of N. gonorrhoeae, the receptors for FH and C4BP do not seem to be the same. In the case of C. albicans, the ligands have not yet been identified. FH and C4BP share a similar structural framework, since they consist of multiple short consensus repeat domains and share common binding features for heparin, C3b, and streptococcal M protein. It is known that Lyme disease Borrelia spirochetes possess at least two receptors for FH (OspE and CRASP-1 proteins). The receptors for FH and C4BP have not been identified for B. recurrentis and B. duttonii, but it is possible that multiple receptors exist or that one receptor binds both FH and C4BP. The novel finding that at least one strain of Lyme disease Borrelia is able to bind C4BP warrants further studies both to describe the extent of that ability in other strains and species and to search for the borrelial receptor for C4BP.
In conclusion, this study has demonstrated a novel immune evasion strategy for the relapsing fever Borrelia spp. It shows that two relapsing fever spirochetes, B. recurrentis and B. duttonii, acquire the human complement regulators FH and C4BP from human plasma, which is the pathophysiological niche of these organisms. The regulators bind to the bacterial surface in a way that retains their functional activities. It is likely that these properties help relapsing fever spirochetes to survive for prolonged periods in human blood and to cause severe disease.
Acknowledgments
We thank Marjatta Ahonen for excellent technical assistance.
This study was supported by the Finnish Cultural Foundation, The Academy of Finland (201506 and 202529), The Biomedicum Foundation, Maud Kuistila Foundation, The Sigrid Juselius Foundation, and The Helsinki University Central Hospital Funds.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alitalo, A., T. Meri, P. Comstedt, L. Jeffery, J. Tornberg, T. Strandin, H. Lankinen, S. Bergstrom, M. Cinco, S. R. Vuppala, D. R. Akins, and S. Meri. 2005. Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur. J. Immunol. 35:3043-3053. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo, A., T. Meri, H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 3.Alitalo, A., T. Meri, L. Ramo, T. S. Jokiranta, T. Heikkila, I. J. Seppala, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour, A. G., O. Barrera, and R. C. Judd. 1983. Structural analysis of the variable major proteins of Borrelia hermsii. J. Exp. Med. 158:2127-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G., C. J. Carter, and C. D. Sohaskey. 2000. Surface protein variation by expression site switching in the relapsing fever agent Borrelia hermsii. Infect. Immun. 68:7114-7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour, A. G., and B. I. Restrepo. 2000. Antigenic variation in vector-borne pathogens. Emerg. Infect. Dis. 6:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnum, S. R. 1991. C4b-binding protein, a regulatory protein of complement. Immunol. Res. 10:28-42. [DOI] [PubMed] [Google Scholar]
- 8.Breitner-Ruddock, S., R. Wurzner, J. Schulze, and V. Brade. 1997. Heterogeneity in the complement-dependent bacteriolysis within the species of Borrelia burgdorferi. Med. Microbiol. Immunol. (Berlin) 185:253-260. [DOI] [PubMed] [Google Scholar]
- 9.Connolly, S. E., and J. L. Benach. 2001. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J. Immunol. 167:3029-3032. [DOI] [PubMed] [Google Scholar]
- 10.Connolly, S. E., D. G. Thanassi, and J. L. Benach. 2004. Generation of a complement-independent bactericidal IgM against a relapsing fever borrelia. J. Immunol. 172:1191-1197. [DOI] [PubMed] [Google Scholar]
- 11.Cutler, S. J. 2001. Molecular biology of the relapsing fever borrelia, p. 2093-2113. In R. Sussman (ed.), Molecular medical microbiology. Academic Press, Oxford, United Kingdom.
- 12.Cutler, S. J., C. O. Akintunde, J. Moss, M. Fukunaga, K. Kurtenbach, A. Talbert, H. Zhang, D. J. Wright, and D. A. Warrell. 1999. Successful in vitro cultivation of Borrelia duttonii and its comparison with Borrelia recurrentis. Int. J. Syst. Bacteriol. 49:1793-1799. [DOI] [PubMed] [Google Scholar]
- 13.Cutler, S. J., D. Fekade, K. Hussein, K. A. Knox, A. Melka, K. Cann, A. R. Emilianus, D. A. Warrell, and D. J. Wright. 1994. Successful in-vitro cultivation of Borrelia recurrentis. Lancet 343:242. [DOI] [PubMed] [Google Scholar]
- 14.Dahlback, B. 1983. Purification of human C4b-binding protein and formation of its complex with vitamin K-dependent protein S. Biochem. J. 209:847-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gigli, I., J. Sorvillo, and L. Halbwachs-Mecarelli. 1985. Regulation and deregulation of the fluid-phase classical pathway C3 convertase. J. Immunol. 135:440-444. [PubMed] [Google Scholar]
- 16.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 17.Hovis, K. M., J. V. McDowell, L. Griffin, and R. T. Marconi. 2004. Identification and characterization of a linear-plasmid-encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 186:2612-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnsson, E., K. Berggard, H. Kotarsky, J. Hellwage, P. F. Zipfel, U. Sjobring, and G. Lindahl. 1998. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J. Immunol. 161:4894-4901. [PubMed] [Google Scholar]
- 19.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. M. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279:2421-2429. [DOI] [PubMed] [Google Scholar]
- 20.Kraiczy, P., C. Skerka, V. Brade, and P. F. Zipfel. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800-7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraiczy, P., C. Skerka, M. Kirschfink, P. F. Zipfel, and V. Brade. 2001. Mechanism of complement resistance of pathogenic Borrelia burgdorferi isolates. Int. Immunopharmacol. 1:393-401. [DOI] [PubMed] [Google Scholar]
- 22.McDowell, J. V., E. Tran, D. Hamilton, J. Wolfgang, K. Miller, and R. T. Marconi. 2003. Analysis of the ability of spirochete species associated with relapsing fever, avian borreliosis, and epizootic bovine abortion to bind factor H and cleave c3b. J. Clin. Microbiol. 41:3905-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meri, T., A. M. Blom, A. Hartmann, D. Lenk, S. Meri, and P. F. Zipfel. 2004. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect. Immun. 72:6633-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meri, T., A. Hartmann, D. Lenk, R. Eck, R. Wurzner, J. Hellwage, S. Meri, and P. F. Zipfel. 2002. The yeast Candida albicans binds complement regulators factor H and FHL-1. Infect. Immun. 70:5185-5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misasi, R., H. P. Huemer, W. Schwaeble, E. Solder, C. Larcher, and M. P. Dierich. 1989. Human complement factor H: an additional gene product of 43 kDa isolated from human plasma shows cofactor activity for the cleavage of the third component of complement. Eur. J. Immunol. 19:1765-1768. [DOI] [PubMed] [Google Scholar]
- 26.Morfeldt, E., K. Berggard, J. Persson, T. Drakenberg, E. Johnsson, E. Lindahl, S. Linse, and G. Lindahl. 2001. Isolated hypervariable regions derived from streptococcal M proteins specifically bind human C4b-binding protein: implications for antigenic variation. J. Immunol. 167:3870-3877. [DOI] [PubMed] [Google Scholar]
- 27.Pennington, P. M., D. Cadavid, and A. G. Barbour. 1999. Characterization of VspB of Borrelia turicatae, a major outer membrane protein expressed in blood and tissues of mice. Infect. Immun. 67:4637-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ram, S., M. Cullinane, A. M. Blom, S. Gulati, D. P. McQuillen, R. Boden, B. G. Monks, C. O'Connell, C. Elkins, M. K. Pangburn, B. Dahlback, and P. A. Rice. 2001. C4bp binding to porin mediates stable serum resistance of Neisseria gonorrhoeae. Int. Immunopharmacol. 1:423-432. [DOI] [PubMed] [Google Scholar]
- 29.Ram, S., D. P. McQuillen, S. Gulati, C. Elkins, M. K. Pangburn, and P. A. Rice. 1998. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J. Exp. Med. 188:671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ras, N. M., D. Postic, P. Ave, M. Huerre, and G. Baranton. 2000. Antigenic variation of Borrelia turicatae Vsp surface lipoproteins occurs in vitro and generates novel serotypes. Res. Microbiol. 151:5-12. [DOI] [PubMed] [Google Scholar]
- 31.Restrepo, B. I., C. J. Carter, and A. G. Barbour. 1994. Activation of a vmp pseudogene in Borrelia hermsii: an alternate mechanism of antigenic variation during relapsing fever. Mol. Microbiol. 13:287-299. [DOI] [PubMed] [Google Scholar]
- 32.Salacinski, P. R., C. McLean, J. E. Sykes, V. V. Clement-Jones, and P. J. Lowry. 1981. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3alpha,6alpha-diphenyl glycoluril (Iodogen). Anal. Biochem. 117:136-146. [DOI] [PubMed] [Google Scholar]
- 33.Schwan, T. G., P. F. Policastro, Z. Miller, R. L. Thompson, T. Damrow, and J. E. Keirans. 2003. Tick-borne relapsing fever caused by Borrelia hermsii, Montana. Emerg. Infect. Dis. 9:1151-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundnes, K. O., and A. T. Haimanot. 1993. Epidemic of louse-borne relapsing fever in Ethiopia. Lancet 342:1213-1215. (Erratum, 343:244, 1994.) [DOI] [PubMed] [Google Scholar]
- 36.Weiler, J. M., M. R. Daha, K. F. Austen, and D. T. Fearon. 1976. Control of the amplification convertase of complement by the plasma protein beta1H. Proc. Natl. Acad. Sci. USA 73:3268-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]