Abstract
Allergic bronchopulmonary mycosis (ABPM) is a hypersensitivity lung disease in which fungal colonization is accompanied by an allergic response to the fungus. Using a mouse model of ABPM caused by Cryptococcus neoformans infection of C57BL/6 mice, the goal of the present studies was to determine the effect of the CD4-depleting monoclonal antibody GK1.5 on the development of the allergic responses seen during active fungal infection. These results would provide insight into the role of CD4+ T cells in this disease. Our results show that GK1.5 treatment resulted in attenuation of pulmonary inflammation and eosinophilia in these animals. These mice also had reduced T2 cytokine production and no serum immunoglobulin E production. Absence of CD4+ T cells did not affect recruitment of CD8+ T cells to the site of infection; however, the numbers of CD19+ B cells were severely reduced in the lungs of CD4+ T-cell-depleted animals. We also examined changes in the pulmonary architecture and found that depletion of CD4+ T cells was associated with a significant reduction in mucus production and goblet cell metaplasia in these mice. Interestingly, attenuation of Th2 responses in CD4+ T-cell-depleted animals did not increase the fungal load in their lungs. We also compared development of ABPM in young and mature mice and did not find any differences at any of the time points. Overall, our results show that depletion of CD4+ T cells prevents the development of Th2 responses seen during ABPM.
Allergic bronchopulmonary mycosis (ABPM) is a hypersensitivity disease that results when fungal colonization of the lung is accompanied by an allergic response to the fungus. It is typically seen in atopic individuals such as asthmatics and patients with cystic fibrosis. This disease is characterized by several clinical, radiologic, immunologic, and pathological symptoms that range from mild asthma to end-stage fibrotic disease (9, 14, 26). Significant pulmonary pathology is associated with fungus-induced allergic and asthmatic lung disease, characterized by increased Th2 cytokine generation, immunoglobulin E (IgE) and IgG production, eosinophilia, airway hyperresponsiveness, and airway remodeling.
In humans, ABPM is caused largely by molds and includes significant involvement of the bronchi, where hyphae can be seen. To date, there is no mouse model that recapitulates all of the aspects of this disease. Aspergillus fumigatus, the most common cause of ABPM in humans, has not been reported to cause the same disease in mice. Allergic responses can be generated by repeated exposure to A. fumigatus antigen or conidia if the animal is primed, but it is impossible to establish A. fumigatus colonization in these mice similar to that seen in humans. In C57BL/6 mice, C. neoformans will induce an allergic response in the lungs and the pulmonary infection becomes chronic with the fungal load driving the allergic response (3, 16). This is a very similar response to ABPM in humans, with two notable exceptions: a relative lack of bronchial involvement (there is some, but it is extensively alveolar infection and inflammation) and the presence of cryptococcal polysaccharide. Cryptococcus neoformans does not cause ABPM in humans; however, by using this model, we can study the underlying immunologic regulation during concurrent fungal infection and development of allergic disease.
CD4+ T cells are known to be required for clearing C. neoformans from the lung. Deficiency of CD4+ T cells results in markedly enhanced morbidity and mortality in patients with cryptococcal pneumonia and disseminated cryptococcosis. Several laboratories have shown the relative importance of CD4+ T cells in generation of protective immunity to C. neoformans in CBA/J, BALB/c, and C.B-17 mice (18, 22, 31). In contrast to these mouse strains, C57BL/6 mice that are CD4+ T-cell competent develop chronic pulmonary infection with features of an allergic response upon C. neoformans infection. A previous study showed the requirement of CD4+ T cells in generation of allergic symptoms in an Aspergillus antigen-induced airway disease model (11). This study focuses on investigating the role of CD4+ T cells in the generation of allergic symptoms in the lungs and control of infection during C. neoformans-induced ABPM.
Age-related differences in clearance and development of delayed-type hypersensitivity responses to C. neoformans infection in C57BL/6 mice have been recently reported. These studies showed that 15-week-old C57BL/6 mice immunized with C. neoformans 184A (a weakly encapsulated strain) were more efficient in handling the infection and developed stronger delayed-type hypersensitivity responses compared to 7-week-old mice (5). Since we used C57BL/6 mice that were 8 to 12 weeks old for our studies, we wanted to make sure that none of the effects of CD4+ T-cell depletion were due to age differences in mice in different experiments. In order to eliminate this discrepancy, C57BL/6 mice that were young (<8 weeks old) or mature (>15 weeks old) were infected with 104 C. neoformans cells and assessed for the development of ABPM.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice (16 ± 2 g body weight) were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were 8 to 12 weeks of age at the time of infection. For age experiments, female C57BL/6 mice at 6 to 8 weeks (young) and 15 to 20 weeks (mature) were obtained from Harlan Sprague-Dawley, Inc. Mice were housed in sterilized cages covered with a filter top. Food and water were given ad libitum. The mice were maintained by the Unit for Laboratory Animal Medicine at the University of Michigan (Ann Arbor), in accordance with regulations approved by the University of Michigan Committee on the Use and Care of Animals.
Cryptococcus neoformans.
C. neoformans strain 52D was obtained from the American Type Culture Collection (ATCC 24067). For infection, yeast cells were grown to stationary phase (48 to 72 h) at 34°C in Sabouraud dextrose broth (1% neopeptone, 2% dextrose; Difco, Detroit, Mich.) on a shaker. The cultures were then washed in nonpyrogenic saline (NPS), counted on a hemocytometer, and diluted to 3.3 × 105 CFU/ml in sterile nonpyrogenic saline.
Intratracheal inoculation of C. neoformans.
Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (6.8 mg/kg; Lloyd Laboratories, Shenandoah, IA) and restrained on a small surgical board. A small incision was made through the skin over the trachea, and the underlying tissue was separated. A 30-gauge needle was attached to a 1-ml tuberculin syringe filled with diluted C. neoformans culture. The needle was inserted into the trachea, and 30 μl of inoculum (104 CFU) was dispensed into the lungs. The needle was removed, and the skin was closed with cyanoacrylate adhesive. The mice recovered with minimal visible trauma.
Induction of CD4+ T-cell deficiency.
Mice were treated with 300 μg of anti-CD4 monoclonal antibody (MAb) GK1.5 intraperitoneally on the day prior to C. neoformans inoculation and boosted with 100 μg of MAb at days 7 and 14. Antibody was prepared from ascites by dilution in NPS and filtering through a 0.45-μm syringe filter. The efficiency of T-cell depletion was assessed by flow cytometric analysis using anti-CD4 Ab RM 4-4, which binds to a region of CD4 distinct from GK1.5. Depletion was >98% for CD4+ T cells in the lungs as well as spleen. This antibody (GK1.5) also tolerizes the mouse against an anti-rat Ig response because an anti-rat Ig response requires CD4 T-cell help. Thus, isotype-matched rat Ig would not be a good control because injection would lead to a mouse anti-rat Ig response, potentially resulting in Ab-antigen complexes and immune deviation or inflammation. This is why we chose to use vehicle alone (NPS) as the control treatment.
Lung leukocyte isolation.
Individual lungs were excised, minced, and enzymatically digested for 30 min in 15 ml of digestion buffer (RPMI, 5% fetal calf serum, antibiotics, 1 mg/ml collagenase, 30 μg/ml DNase). The cell suspension and undigested fragments were further dispersed by being drawn up and down 20 times through the bore of a 10-ml syringe. After erythrocyte lysis using NH4Cl buffer (0.83% NH4Cl, 0.1% KHCO3, 0.037% Na2 EDTA, pH 7.4), cells were washed, resuspended in complete medium, and centrifuged for 30 min at 2,000 × g in the presence of 20% Percoll (Sigma-Aldrich) to separate leukocytes from cell debris and epithelial cells. The isolated leukocytes were repelleted and resuspended in complete medium. Total lung leukocyte numbers were assessed in the presence of trypan blue using a hemocytometer.
Lung leukocyte subsets.
Macrophages, neutrophils, and eosinophils were visually counted in Wright-Giemsa-stained samples of lung cell suspensions cytospun onto glass slides (Shandon Cytospin, Pittsburgh, PA). For Wright-Giemsa staining, the slides were fixed for 2 min with a one-step, methanol-based Wright-Giemsa stain (Harleco; EM Diagnostics, Gibbstown, NJ) followed by steps 2 and 3 of the Diff-Quik whole-blood stain kit (Diff-Quik, Baxter Scientific, Miami, FL). A total of 200 to 300 cells were counted from randomly chosen high-power microscope fields for each sample. The percentage of a leukocyte subset was multiplied by the total number of leukocytes to give the absolute number of that type of leukocyte in the sample.
Numbers of B, CD4, and CD8 T cells were determined by flow cytometry. Lung leukocytes (5 × 105) were incubated for 30 min on ice in a total volume of 120 μl of staining buffer (FA buffer; Difco), 0.1% NaN3, and 1% fetal calf serum. Each sample was incubated with 1 μg of the respective fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled monoclonal antibody (PharMingen, San Diego, CA), or isotype-matched rat IgG. The samples were washed in staining buffer and fixed in 4% paraformaldehyde (Sigma) in buffered saline. Stained samples were stored in the dark at 4°C until analyzed on a flow cytometer (Coulter Corp., Hialeah, FL). The percentage of a lymphocyte subset was multiplied by the total number of leukocytes to give the absolute number of that type of lymphocyte in the sample.
Hydroxyproline assay.
Total lung collagen levels were determined using a previously described assay (19). Briefly, a 1-ml sample of lung homogenate was added to 1 ml of 12 N HCl for a minimum of 8 h at 120°C. To a 5-μl sample of the digested lung, 5 μl of citrate/acetate buffer (5% citric acid, 7.2% sodium acetate, 3.4% sodium hydroxide, 1.2% glacial acetic acid, pH 6.0) and 100 μl of Chloramine-T solution (282 mg Chloramine-T, 2 ml of n-propanol, 2 ml of distilled water, 16 ml of citrate/acetate buffer) were added. The resulting sample was then incubated at room temperature for 20 min before 100 μl of Ehrlich's solution (Aldrich, Milwaukee, WI) was added. These samples were incubated for 20 min at 65°C, and cooled samples were read at 550 nm in a Beckman DU 640 spectrophotometer. Hydroxyproline concentrations were calculated from a standard curve of hydroxyproline (0 to 400 μg/ml).
Histology.
Lungs were fixed by inflation with 10% neutral buffered formalin. After paraffin embedding, 5-μm sections were cut and stained with hematoxylin and eosin, periodic acid-Schiff stain (PAS; to stain mucus and mucus-secreting goblet cells), or Masson's trichrome (collagen deposition stains blue).
Total serum IgE.
Blood was obtained by ocular bleeding of the mice, and serum was separated using serum separator tubes (Becton Dickinson, Paramus, NJ). Serum samples were then assayed using an IgE-specific sandwich enzyme-linked immunosorbent assay (ELISA) (BD-PharMingen, San Diego, CA).
Cytokine production.
Lung leukocytes were isolated by enzymatic digestion, and the cultures were normalized for cell numbers, since there was a significantly reduced number of cells in anti-CD4 Ab-treated mice. Equal numbers of leukocytes (5 × 106/ml) from both groups were cultured with exogenous heat-killed C. neoformans (at ratio of 2:1 heat-killed cryptococcus cells to leukocytes) for 24 h to maximize cytokine production. Culture supernatants were collected at approximately 24 h and assayed for interleukin-4 (IL-4), IL-5, IL-13, IL-10, gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) production by sandwich ELISA using the manufacturer's instructions supplied with the cytokine-specific kits (BD-PharMingen, San Diego, CA; and R&D, Minneapolis, MN).
Statistical analysis.
All values are means ± standard error of the mean unless otherwise indicated. Differences between two means were evaluated using Student's t test (assuming unequal variance where dictated by F test), with P < 0.05 considered to be statistically significant.
RESULTS
Role of CD4+ T cells in pulmonary inflammation during ABPM.
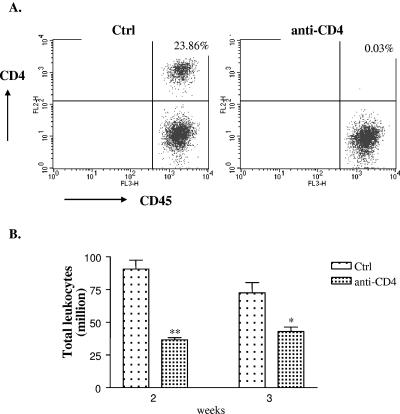
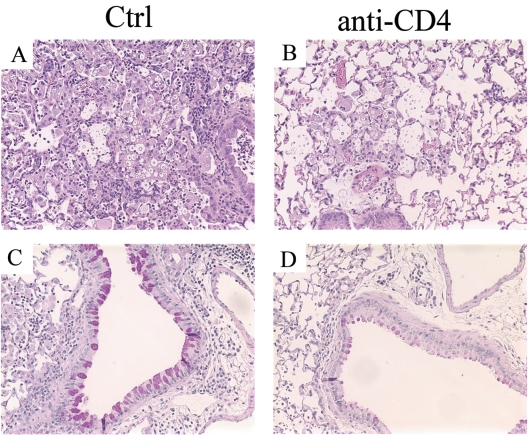
Mice were rendered CD4+ T-cell deficient by injecting them intraperitoneally with the anti-CD4 MAb GK1.5. The efficacy of CD4+ T-cell depletion was >98% in the lungs (Fig. 1A) as well as spleen (data not shown). In humans, CD4 is primarily present on T-helper/inducer lymphocytes and in low density on the surface of monocytes (36). In mice, CD4 (L3T4) is expressed on most thymocytes, a subpopulation of mature T lymphocytes, and a subset of NK-T cells (4). There are no reports of CD4 expression on monocytes in mice. Thus, anti-CD4 treatment of mice should not affect monocytes/macrophages. To examine if CD4+ T cells were required for generation of pulmonary inflammation, mice were harvested at weeks 2 and 3 postinfection and lung leukocytes were isolated by enzymatic digestion. Depletion of CD4+ T cells resulted in more than a 50% reduction in the number of leukocytes present in the lungs in comparison to control animals at week 2 postinfection (Fig. 1B). Similar results were obtained from mice harvested at week 3 postinfection. Histology of lung sections stained with hematoxylin and eosin demonstrated a dramatic difference between the two groups. Control mice had considerable inflammation, large macrophages with C. neoformans inside them, and a few extracellular cryptococci (Fig. 2A). In comparison, anti-CD4 Ab-treated animals had much less inflammation, including fewer macrophages and eosinophils (Fig. 2B). These data demonstrate that CD4+ T cells are required for generation of pulmonary inflammation during ABPM.
FIG. 1.
(A) Lung leukocytes were isolated from mice that were either left untreated (Ctrl) or were treated (anti-CD4) with anti-CD4 Ab GK1.5 followed by C. neoformans infection for 2 weeks. Cells were stained with CD45 Ab and CD4 Ab (RM4-5) and were analyzed by flow cytometry. Treatment of mice with anti-CD4 MAb resulted in >98% CD4+ T-cell depletion, confirming the efficacy of antibody depletion. (B) Leukocytes were isolated from mice at weeks 2 and 3 postinfection as described in Materials and Methods, and total leukocytes were counted with a hemocytometer. Anti-CD4 Ab-treated mice had a significant reduction in total leukocyte numbers compared to the untreated group. Data are representative of two independent experiments. n ≥ 6 mice/group/time point. **, P < 0.005; *, P < 0.05.
FIG. 2.
Photomicrographs of lung sections from (A and C) untreated (Ctrl) or (B and D) anti-CD4 Ab-treated mice infected for 3 weeks. The lung sections were stained with (A and B) hematoxylin and eosin or (C and D) PAS and examined under a microscope (×200). Lungs from untreated mice had severe inflammation, and cryptococcal cells were seen mainly in the macrophages. In contrast, the anti-CD4 group had significantly less inflammation and a larger number of cryptococcal cells were seen in the extracellular matrix. PAS-stained sections show bright pink staining in the untreated group versus dull pink staining in the airway epithelial cells from the anti-CD4 Ab-treated group.
Role of CD4+ T cells in goblet cell metaplasia and mucus production during ABPM.
We next investigated if CD4+ T cells had a role in the development of characteristic histological changes associated with the allergic phenotype during ABPM. Histological sections of lungs from control and anti-CD4 Ab-treated mice at week 3 postinfection were stained with PAS, which stains mucus pink. Airway epithelial cells from control mice stained pink, showing mucus hypersecretion in response to C. neoformans infection (Fig. 2C). In contrast, airway epithelial cells from anti-CD4 Ab-treated mice had a dramatic decrease in PAS staining (Fig. 2D). Thus, CD4+ T cells are required for goblet cell metaplasia and mucus production seen during ABPM.
Role of CD4+ T cells in the recruitment of granulocytes to the lungs.
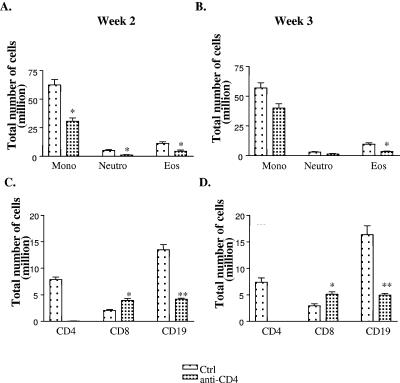
To determine the cell type or types whose recruitment was affected in the absence of CD4+ T cells, we examined the composition of the leukocyte infiltrate. Granulocytes from total lung digests were identified by Wright-Giemsa staining. At week 2 postinfection, anti-CD4 Ab-treated mice showed ∼50% reduction in the number of mononuclear cells (62.5 ± 4 in control versus 30.7 ± 3 in the anti-CD4 Ab-treated group) and ∼60% reduction in the number of eosinophils (11.3 ± 1 in control versus 4.5 ± 1 in anti-CD4 Ab-treated group) (Fig. 3A). A similar pattern was observed in mice harvested at week 3 postinfection (Fig. 3B). Clearly, CD4+ T cells are required for maximal recruitment of eosinophils, neutrophils, and mononuclear cells in response to infection.
FIG. 3.
(A and B) Isolated lung leukocytes from untreated mice (Ctrl) or anti-CD4 Ab-treated (anti-CD4) C. neoformans-infected mice for (A and C) 2 and (B and D) 3 weeks were spun onto a glass slide and stained with Wright-Giemsa stain. Granulocytes were identified, and absolute numbers of granulocytes were calculated. *, P < 0.05 (n = 3 mice/group/time point). (C and D) Lung leukocytes were stained with CD45 and CD4 or CD8 or CD19 Abs. Cells were analyzed by flow cytometry. The percentages of CD4+, CD8+, or CD19+ cells were multiplied by absolute cell numbers to get total number of cells. The results are the mean of two independent experiments with n ≥ 6 mice/group. Mono, mononuclear cells; Neutro, neutrophils; Eos, eosinophils. *, P < 0.05; **, P < 0.005.
Role of CD4+ T cells in the recruitment of lymphocytes to the lungs.
To analyze how the absence of CD4+ T cells affected recruitment of other lymphocytes, we conducted flow cytometry analysis on leukocytes isolated from lung digest. Cells in the lymphocyte and CD45+ gate were used for further analysis. Although control mice had a considerable number of CD4+ T cells (∼7 to 8 million) recruited to the lungs upon infection, we could not find any CD4+ T cells in the anti-CD4 Ab-treated infected mice. The number of CD8+ T cells was higher in anti-CD4 Ab-treated mice (2.8 ± 0.1 in the control group versus 3.9 ± 0.4 in anti-CD4 Ab-treated group), suggesting that CD4+ T cells were not required for recruitment of CD8+ lymphocytes to the site of infection at weeks 2 and 3 (Fig. 3C). Recruitment of B cells, on the other hand, was dramatically reduced in the absence of CD4+ T cells (13.5 ± 0.9 in the control group versus 4.1 ± 0.2 in the anti-CD4 Ab-treated group). This finding indicates an important role of CD4+ T cells in recruitment and/or proliferation of B cells at the site of infection. Taken together, these results demonstrate a role for CD4+ T cells in recruitment of B lymphocytes (but not CD8+ T cells) to the lungs during ABPM.
Cytokine production in the absence of CD4+ T cells.
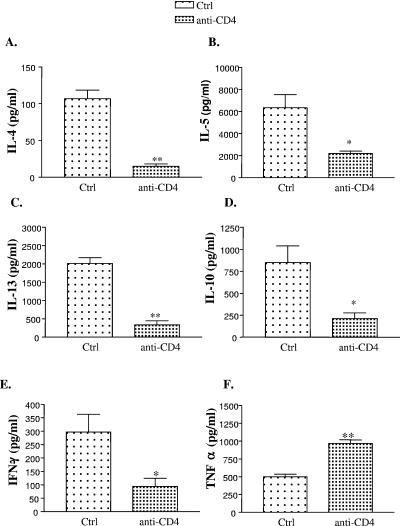
We next examined the effect of CD4+ T-cell deficiency on T2 cytokine production. Absence of CD4+ T cells resulted in a significant decrease in the production of IL-4, IL-5, IL-13, and IL-10 by lung leukocytes compared to that in control mice (Fig. 4A, B, C, and D). Similar results were obtained at week 3 postinfection (data not shown). Reduced levels of IL-5 in cultures were consistent with decreased eosinophilia seen in the lungs of anti-CD4 Ab-treated animals. In addition to T2 cytokines, we also measured production of IFN-γ by lung leukocytes. Interestingly, anti-CD4 Ab-treated animals demonstrated a decrease in IFN-γ production compared to control animals (Fig. 4E). In contrast, levels of TNF-α increased in anti-CD4 Ab-treated compared to control animals suggesting that cell viability was not an issue in these cultures (Fig. 4F). Together, these results demonstrate that CD4+ T cells are required for maximal production of IL-4, IL-5, IL-13, and IFN-γ by lung leukocytes during infection.
FIG. 4.
Lung leukocytes from week 2 infected untreated (Ctrl) or anti-CD4 Ab-treated mice were cultured for 24 h in the presence of heat-killed C. neoformans as a source of exogenous stimulation. Cytokines (A) IL-4, (B) IL-5, (C) IL-13, (D) IL-10, (E) IFN-γ, and (F) TNF-α were measured in the supernatants by ELISA. The results are the mean of two independent experiments (n ≥ 7 mice/group). *, P < 0.05; **, P < 0.005.
Role of CD4+ T cells in production of serum IgE.
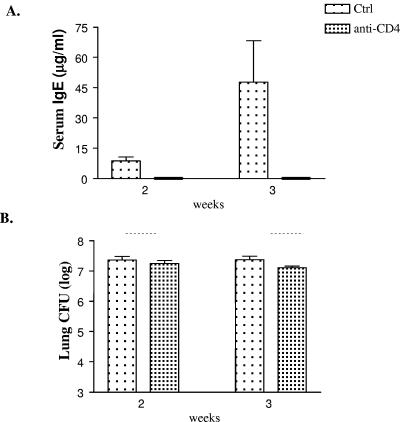
Production of serum IgE is a hallmark of ABPM. We next investigated if CD4+ T cells were required for increased production of IgE. Blood was collected from both control and anti-CD4 Ab-treated mice, and serum was assayed for total IgE by ELISA. Serum IgE levels increased in control mice from undetectable levels (uninfected mice) and continued at elevated levels at week 3. In contrast, serum IgE levels did not increase following infection in anti-CD4 Ab-treated mice at weeks 2 and 3 (Fig. 5A). An undetectable level of IgE in this experimental group is consistent with reduction in the levels of IL-4, which is required for isotype switching. Hence, CD4+ T cells are required for production of IgE during ABPM.
FIG. 5.
(A) Serum was obtained from untreated (Ctrl) and anti-CD4 Ab-treated mice infected for 2 or 3 weeks, and total serum IgE levels were determined by ELISA. IgE levels in anti-CD4 Ab-treated animals remained undetected at both time points. n ≥ 3 mice/group/time point. (B) Lungs were harvested at weeks 2 and 3 postinfection from untreated and anti-CD4 Ab-treated C. neoformans-infected mice, and CFU were determined. Data are representative of two independent experiments. n ≥ 6 mice/group/time point. There was no difference in lung CFU levels between the two groups.
Role of CD4+ T cells in pulmonary fungal clearance.
Since absence of CD4+ T cells leads to decreased T2 inflammation in the lungs, we next asked whether the absence of CD4+ T cells altered C. neoformans load in the lungs. C. neoformans-infected C57BL/6 mice treated with or without anti-CD4 Ab were harvested at weeks 2 and 3, and the CFU were determined. Lung CFU increased by 4 logs in untreated animals (control). Apparently, anti-CD4 Ab-treated animals also had a similar fungal burden in their lungs. Loss of CD4+ T cells neither increased nor decreased lung CFU at weeks 2 and 3 (Fig. 5B). Dissemination to extrapulmonary sites such as lung-associated lymph nodes (LALN) was minimal, and the levels of dissemination were similar in both groups (data not shown). These studies demonstrate that during ABPM, there is a CD4+ T-cell-independent mechanism that controls the pulmonary burden of C. neoformans, resulting in chronic levels of infection that remain largely localized to lungs.
ABPM in young versus mature mice.
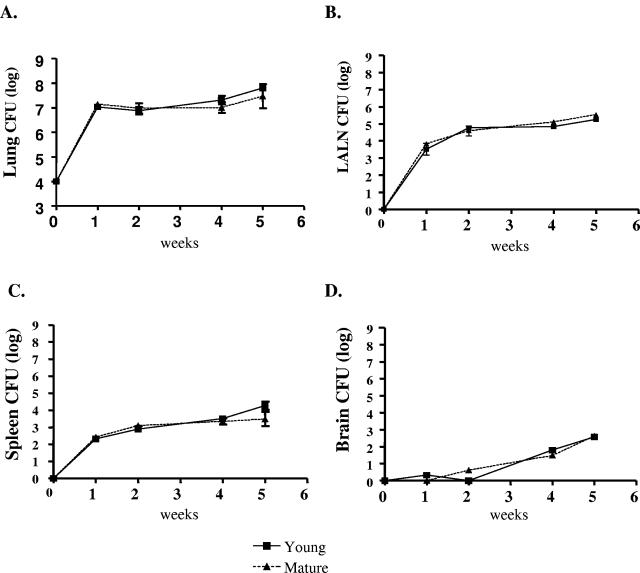
To determine whether the age of mice influenced clearance of C. neoformans during ABPM, young (8 to 10 weeks old) and mature (≥15 weeks old) mice were challenged intratracheally with C. neoformans and fungal burden was quantified. Mice were harvested, and CFU from lungs, LALN, spleen, and brain were measured at weeks 1, 2, 4, and 5 postinfection. Dissemination was observed from lungs to LALN (105), spleen (104), and brain (103). However, we found no differences in the extent or kinetics of fungal colonization between young and mature mice (Fig. 6). We next examined if age of the mice would influence generation of pulmonary inflammation in response to C. neoformans infection. Lung leukocyte differentials revealed a similar extent of inflammation and eosinophilia between young and mature mice (Table 1).
FIG. 6.
CFU were assayed from (A) lung, (B) LALN, (C) spleen, and (D) brain of infected young and mature mice at various time points as described in Materials and Methods. Day 0 corresponds to the initial inoculum at that site. There was no difference in the fungal clearance from pulmonary or extrapulmonary sites between young and mature mice. Data are expressed as means ± standard error (n = 6 to 8 mice/group/time point from two experiments).
TABLE 1.
Leukocyte differentials for young versus mature mice
| Cell type | No. of leukocytes (106) at wka:
|
||
|---|---|---|---|
| 0 | 1 | 2 | |
| Total | |||
| Young | 15.6 ± 4.4 | 37.3 ± 11.0 | 120.8 ± 27.9 |
| Mature | 13.2 ± 1.1 | 38.7 ± 6.2 | 111 ± 18.0 |
| Mononuclear cells | |||
| Young | 14.7 ± 4.2 | 26.6 ± 6.5 | 82.7 ± 20.3 |
| Mature | 12.0 ± 1.5 | 27.8 ± 3.8 | 73.5 ± 12.6 |
| Neutrophils | |||
| Young | 0.59 ± 0.22 | 5.3 ± 1.9 | 13.9 ± 5.4 |
| Mature | 0.63 ± 0.04 | 5.9 ± 1.7 | 11.2 ± 3.5 |
| Eosinophils | |||
| Young | 0.18 ± 0.01 | 5.2 ± 3.6 | 23.8 ± 7.0 |
| Mature | 0.28 ± 0.11 | 4.8 ± 1.2 | 26.5 ± 9.7 |
Lung leukocytes were isolated from uninfected (week 0) or infected young and mature mice at weeks 1 and 2 postinfection. Cells were spun onto a glass slides, stained with Wright-Giemsa stain, and analyzed using a microscope. The absolute number of leukocyte subsets was calculated by multiplying the percentage by the total number of cells. Data are expressed as means ± standard error (n ≥ 4 mice/group/time point) and are representative of two independent experiments. Leukocyte numbers from young and mature mice were not statistically different from one another at any time point.
Serum IgE levels in C. neoformans-infected young versus mature mice.
Levels of IgE in the serum of uninfected and infected groups of mice were measured by ELISA. Serum IgE levels in uninfected young and mature mice averaged 2 to 4 μg/ml. Upon infection, levels started to increase at week 1 and continued to increase at weeks 2, 3, and 4 (Table 2). By week 4, serum IgE levels were elevated to almost 20-fold compared to baseline levels. No significant differences were observed between young and mature mice. Together, these results demonstrate similar levels of IgE production in young and mature mice.
TABLE 2.
Serum IgE production by young versus mature mice
| Mouse type | Serum IgE production (μg/ml) at wka:
|
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 4 | |
| Young | 4.2 ± 3.1 | 7.0 ± 2.4 | 29.4 ± 16.7* | 69.9 ± 38.2* |
| Mature | 2.1 ± 1.1 | 3.6 ± 2.7 | 32.3 ± 12.5† | 55.7 ± 29.9† |
Total IgE was measured using ELISA as described in Materials and Methods. Data are expressed as means ± standard error (n ≥ 4/group/time point). *, P < 0.05 when young infected mice are compared with young uninfected mice; †, P < 0.05 when mature infected mice are compared with mature uninfected mice. Serum IgE production levels between young and mature mice were not statistically different at any time point.
Generation of T1/T2 cytokines in young and mature mice at the site of infection.
We next examined T1/T2 cytokine production at the site of infection in young versus mature mice. Briefly, lung leukocytes from uninfected and C. neoformans-infected young and mature mice were isolated at weeks 1 and 2 postinfection and cultured overnight in the absence of exogenous heat-killed C. neoformans. Lung leukocytes from both young and mature mice produced similar and significant levels of IL-4, IL-5, IL-10, IFN-γ, and TNF-α (Table 3).
TABLE 3.
Cytokine production by lung leukocytes from young and mature mice
| Cytokine and mouse type | Cytokine production (pg/ml) at wka:
|
||
|---|---|---|---|
| 0 | 1 | 2 | |
| IL-4 | |||
| Young | 17.7 ± 8.0 | 63.5 ± 30.9* | 54.0 ± 45.4* |
| Mature | 9.5 ± 0.7 | 60.1 ± 18.1† | 58.0 ± 27.0 |
| IL-5 | |||
| Young | 2,859.5 ± 41.7 | 4,336.0 ± 866.6* | 3,478.6 ± 1,140.1 |
| Mature | 2,466.0 ± 1,142.6 | 3,054.7 ± 575.4 | 4,482.8 ± 1,519.3 |
| IL-10 | |||
| Young | 72.1 ± 29.6 | 298.2 ± 85.6** | 213.8 ± 36.0* |
| Mature | 56.1 ± 28.0 | 268.8 ± 131.8† | 207.6 ± 32.0† |
| IFN-γ | |||
| Young | 39.4 ± 7.8 | 326.0 ± 67.0** | 555.8 ± 121.0** |
| Mature | 50.1 ± 31.0 | 357.2 ± 57.8†† | 5,050.4 ± 90.5†† |
| TNF-α | |||
| Young | 826.3 ± 6.2 | 1,364.0 ± 274.8 | 1,198.0 ± 462.8 |
| Mature | 498.1 ± 75.1 | 1,319.2 ± 364.0 | 936.8 ± 129.5 |
Lung leukocytes were cultured for 24 h, and supernatants were collected. Cytokines IL-4, IL-5, IL-10, IFN-γ, and TNF-α were measured from uninfected (week 0) or infected mice by ELISAs. Data are expressed as means ± standard error (n ≥ 4 mice/group/time point). * and **, P < 0.05 and P < 0.005, respectively, when young infected mice are compared with young uninfected mice; † and ††, P < 0.05 and P < 0.005, respectively, when mature infected mice are compared with mature uninfected mice. Both groups of mice show similar induction of cytokines upon infection, and there were no significant differences between the two groups.
Pulmonary fibrosis, mucus production, and goblet cell metaplasia in young versus mature mice.
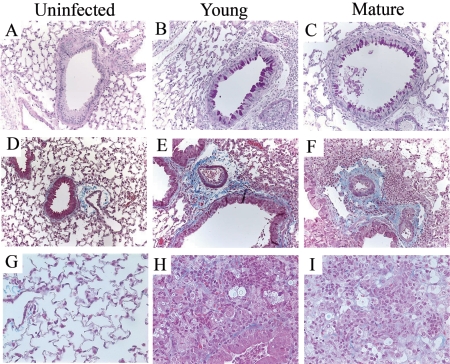
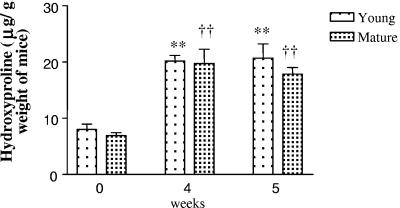
We next analyzed mice challenged with C. neoformans for symptoms of airway remodeling, such as mucus production, goblet cell hyperplasia, and pulmonary fibrosis. Whole-lung sections from uninfected and infected young and mature mice were stained with PAS, which stains mucus pink. Both young and mature C. neoformans-infected mice showed airway epithelial cell hypertrophy, goblet cell metaplasia, and hyperproduction of acidic mucus (Fig. 7B and C). Uninfected young (Fig. 7A) or mature (data not shown) mice, however, did not show any of these symptoms. To assess fibrosis in the lungs of these animals, tissue sections were stained with Masson trichrome, which is used to reveal subepithelial deposition of collagen. Increased collagen staining was seen around airways (Fig. 7E and F) as well as in the lung matrix (Fig. 7H and I) of infected mice. To further confirm these findings, total hydroxyproline levels were measured in whole-lung samples from both young and mature mice before and after C. neoformans challenge. Mice were harvested at weeks 4 and 5 postinfection, since fibrotic changes are associated with the latter stage of the disease. Hydroxyproline levels were approximately 7 to 8 μg/g body weight of mice in uninfected animals. Upon C. neoformans infection, both groups showed significantly greater levels (twofold) of hydroxyproline compared to their respective baseline levels. However, there was no difference between the two groups (Fig. 8). Overall, these results demonstrate that C. neoformans infection in C57BL/6 mice is associated with markedly altered pulmonary architecture.
FIG. 7.
Uninfected (A, D, and G), young (B, E, and H), and mature (C, F, and I) mice at week 4 postinfection. (A, B, and C) Lung sections were stained with PAS. Magnification, ×200. Hyperproduction of mucus (stained dark pink) is readily apparent in airways from C. neoformans-infected mice but not uninfected mice. Also note the hypertrophy of epithelial cells lining the airways in infected animals. (D to I) Lung sections stained with trichrome, which stains collagen blue. Magnification: D, E, and F, ×200; G, H, and I, ×400. Both groups (young and mature) of infected mice had much thicker collagen lining around the airways compared to uninfected mice. In addition, infected animals also had collagen in the lung matrix, as seen at higher magnification. “Uninfected” denotes uninfected young mice. There was no difference in trichrome and PAS staining of young uninfected and mature uninfected mice (data not shown).
FIG. 8.
Hydroxyproline levels were measured in lung homogenates from young and mature mice before and after C. neoformans challenge. Data are expressed as means ± standard error (n ≥ 3 mice/group/time point). **, P < 0.005 when young infected mice are compared with young uninfected mice; ††, P < 0.005 when mature infected mice are compared with mature uninfected mice. Hydroxyproline levels were not different between young and mature mice at the time points studied.
DISCUSSION
The present study demonstrates a crucial role for CD4+ T cells in the manifestation of allergic responses seen during ABPM. CD4+ T cells have classically been known to be important in allergic airway diseases and asthma, by producing key cytokines, including IL-4, IL-5, and IL-13 (17, 29, 35). Here we show that in a murine model of fungus-induced hypersensitivity disease, absence of CD4+ T cells reduces pulmonary inflammation, eosinophilia, and serum IgE production in C. neoformans-infected C57BL/6 mice but does not alter the chronicity of pulmonary infection.
A majority of T-cell clones isolated from patients with ABPM follow a typical Th2 profile (28). In fact upon stimulation, they were found to secrete cytokines IL-4, IL-5, IL-10, and IL-13 (34). IL-5 enhances activation, survival, and recruitment of eosinophils. On the other hand, IL-4 and IL-13 are instrumental in IgE switching of B cells and activation of mast cells. Of these cytokines, IL-4 seems to play a key role in the manifestation of the disease. In murine models of fungal allergen-induced airway disease, absence of IL-4 results in suppressed T2 responses and pronounced T1 responses (25, 27). Mice treated with anti-IL-4 or anti-IL-4 receptor antibodies and IL-4−/− mice fail to develop most but not all airway changes (8, 10, 12). Phenotypes seen in the absence of IL-4 reflect the contribution of IL-13 in the pathogenesis; IL-13 shares a common receptor chain with IL-4 and can mediate its effects independently of IL-4 (15). Induction of T1 responses, such as administration of IL-12 in this model, negatively regulates T2 cytokine production and inhibits responses associated with ongoing antigen-induced pulmonary inflammation and airway hyperreactivity (13). Together, these studies advocate the importance of T2 cytokine induction in the initiation of this disease.
Previous studies from our laboratory demonstrated the importance of IL-4 in the generation of ABPM (16). IL-4−/− mice do not generate allergic responses in response to C. neoformans infection. In addition, these mice are also resistant to C. neoformans and begin to clear the fungi from their lungs as soon as week 2 postinfection. So, why is it that IL-4−/− mice are able to clear the infection, whereas CD4+ T-cell-deficient mice (which produce little IL-4 in lungs) are unable to do so? It is likely that proinflammatory cytokines like IFN-γ or TNF-α are important in controlling the infection. In fact, we have recently shown that IFN-γ does have a role in regulating chronicity of infection in the presence of IL-4 (3). In IL-4−/− mice, IFN-γ levels remain similar to those of WT animals (16); however, in anti-CD4+ T-cell-depleted animals, levels of IFN-γ are reduced (Fig. 4), which might explain why CD4+ T-cell-deficient animals are unable to clear the infection.
Changes associated with airway remodeling such as mucus production and goblet cell metaplasia were significantly reduced in anti-CD4 MAb-treated animals compared to control animals (Fig. 2C and D). IL-13 is likely key in promoting these changes. In A. fumigatus-induced ABPM, targeted overexpression of IL-13 in mice promotes severe inflammatory and airway remodeling responses reminiscent of asthma and allergic airway disease, such as goblet cell hyperplasia, mucus hypersecretion, and subepithelial fibrosis (37). Neutralization of IL-13 in these mice significantly attenuated all these features (6). Similarly, in our studies, the reduction of IL-13 that accompanies depletion of CD4+ T cells correlates well with attenuation of these symptoms, suggesting that CD4+ T cells promote structural changes via IL-13 in the pulmonary architecture in response to C. neoformans during ABPM.
In this study, we see complete abrogation of IgE in the absence of CD4+ T cells (Fig. 5A). In humans, increased IgE is a hallmark of hypersensitivity during ABPM and levels of IgE fluctuate with the severity of ABPM. Serum IgE is significantly elevated in patients with ABPM compared to healthy patients and patients with asthma (9). IgE-mediated activation of mast cells in the bronchial airways leads to bronchial obstruction and asthma. We have previously shown that IL-4−/− mice are unable to generate any IgE in response to C. neoformans infection (16). Together, these results reiterate the importance of CD4+ T cells in the production of IL-4, which is required for IgE production from B cells (by class switching).
Reduction of IL-5 in the absence of CD4+ T cells is consistent with decreased eosinophilia seen in the lungs of these animals (Fig. 3A and B) and suggests an important role for CD4+ T cells in induction of pulmonary eosinophilia via IL-5. IL-5 plays a critical role in recruitment, differentiation, and activation of eosinophils. Development of chronic pulmonary eosinophilia to C. neoformans infection is genetically determined and is generally associated with the inability of the animal to clear the infection (20). Exposure of C57BL/6 mice to C. neoformans induces a strong inflammatory response in these animals, which mainly comprises macrophages, eosinophils, and some neutrophils. Eosinophils can cause significant tissue destruction by release of mediators at the site of inflammation, including reactive oxygen metabolites, major basic proteins, and eosinophil cationic proteins.
In mice that can clear C. neoformans, CD4+ T cells play an important role in host defense against C. neoformans (21). Depletion of CD4+ T cells during pulmonary C. neoformans infection in BALB/c, CBA/J, or C.B-17 mice markedly lowers the ability of these mice to recruit macrophages to the site of infection and results in impaired clearance, increased dissemination to the brain, and increased lethality (22, 31). In these situations, CD4+ T cells are generally associated with production of proinflammatory cytokines like IFN-γ and TNF-α (1, 33). In C57BL/6 mice, however, CD4+ T cells play a key role in production of T2 cytokines and depletion of these cells does not influence the fungal burden in the lungs, suggesting a limited role of CD4+ T cells in protection in this scenario (Fig. 5B).
In terms of age, we did not see a difference in the generation of pulmonary immune responses between 8- and 15-week-old C57BL/6 mice to C. neoformans infection. Age-related differences have been reported to C. neoformans infection in several mouse models in the past. In one study, intravenous injection of C. neoformans strain 52D rendered young CD1 mice more susceptible to infection and increased mortality compared to that in older mice (30). Another study showed that both young and aged C57BL/6 × A/J F1 (AB6F1) mice were equally resistant to intravenous injection of the 184A strain of C. neoformans (2). A more recent study demonstrated that immunized mature (>15 weeks age) C57BL/6 mice were more resistant to intratracheal challenge of the 184A strain of C. neoformans than younger mice (<6 weeks age) (5). Together, these studies demonstrate that the route of infection and strain of Cryptococcus influence the type of immune response generated in the host. We, however, did not observe any differences in the generation of immune responses between young (6 to 8 weeks old) and mature (15 to 18 weeks old) mice. These mice showed similar kinetics of fungal infection in both pulmonary and extrapulmonary sites. Cytokine responses, serum IgE production, and fibrosis were observed that were similar in both groups of mice. Thus, the age of the mice does not influence development of immune responses in this murine model of ABPM.
It is clear from our studies that development of ABPM is profoundly dependent on the generation of T2 responses, including production of IL-4, IL-5, and IL-13. Neutralization of these cytokine mediators in experimental models significantly reduces generation of allergic responses (6, 7, 27). CD4+ T cells play a vital role in production of these cytokines either directly or indirectly. Although the amounts of IL-5, IL-10, and IL-13 were significantly reduced in the absence of CD4+ T cells (Fig. 6), there was, however, low-level production of these cytokines, suggesting that there is a non-CD4+ T-cell source of these cytokines as well. As for T1 cytokines, production of proinflammatory cytokine IFN-γ was also reduced in CD4+ T-cell-depleted animals. T1 and T2 cytokines are mutually inhibitory for the differentiation and effector function of the reciprocal phenotype (32); however, in WT C57BL/6 mice, both IL-4 and IFN-γ seem to coexist. Both IL-4 and IFN-γ maintain a dynamic balance that leads to development of a steady-state chronic infection in these animals. It is likely that regulatory T cells have a role in maintaining this balance. In the absence of CD4+ T cells, both of the cytokines are reduced, suggesting an important role for CD4+ T cells in maintaining this balance. A role for CD4+ CD25+ regulatory T cells in suppressing allergen-induced airway inflammation in vivo has been demonstrated recently. In these studies, depletion of the CD4+ CD25+ T-cell population resulted in increased airway eosinophilia following ovalbumin peptide inhalation (23, 24). Clearly, in our model with heterogeneous CD4+ T-cell populations, the overriding function of CD4+ T cells seems to be inflammation. Understanding the role of regulatory T cells in this model is a subject of future investigation. Taken together, these experiments indicate that CD4+ T cells are necessary to mediate several aspects of the allergic response, including eosinophilic inflammation and lung pathology, but in an allergic setting CD4+ T cells do not play a role in controlling the fungal burden in the lungs.
Acknowledgments
Support for these studies was provided by grants from the National Institutes of Health (R01-HL65912 and R01-AI059201 to G.B.H. and R01-HL051082 to G.B.T.) and the Office of Veterans’ Affairs (VA Merit to G.B.T.).
Editor: A. Casadevall
REFERENCES
- 1.Aguirre, K., E. A. Havell, G. W. Gibson, and L. L. Johnson. 1995. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect. Immun. 63:1725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre, K. M., G. W. Gibson, and L. L. Johnson. 1998. Decreased resistance to primary intravenous Cryptococcus neoformans infection in aged mice despite adequate resistance to intravenous rechallenge. Infect. Immun. 66:4018-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 174:6346-6356. [DOI] [PubMed] [Google Scholar]
- 4.Bendelac, A. 1995. Mouse NK1+ T cells. Curr. Opin. Immunol. 7:367-374. [DOI] [PubMed] [Google Scholar]
- 5.Blackstock, R., and J. W. Murphy. 2004. Age-related resistance of C57BL/6 mice to Cryptococcus neoformans is dependent on maturation of NKT cells. Infect. Immun. 72:5175-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blease, K., C. Jakubzick, J. M. Schuh, B. H. Joshi, R. K. Puri, and C. M. Hogaboam. 2001. IL-13 fusion cytotoxin ameliorates chronic fungal-induced allergic airway disease in mice. J. Immunol. 167:6583-6592. [DOI] [PubMed] [Google Scholar]
- 7.Blease, K., C. Jakubzick, J. Westwick, N. Lukacs, S. L. Kunkel, and C. M. Hogaboam. 2001. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J. Immunol. 166:5219-5224. [DOI] [PubMed] [Google Scholar]
- 8.Brusselle, G., J. Kips, G. Joos, H. Bluethmann, and R. Pauwels. 1995. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4-deficient mice. Am. J. Respir. Cell Mol. Biol. 12:254-259. [DOI] [PubMed] [Google Scholar]
- 9.Cockrill, B. A., and C. A. Hales. 1999. Allergic bronchopulmonary aspergillosis. Annu. Rev. Med. 50:303-316. [DOI] [PubMed] [Google Scholar]
- 10.Corry, D. B., H. G. Folkesson, M. L. Warnock, D. J. Erle, M. A. Matthay, J. P. Wiener-Kronish, and R. M. Locksley. 1996. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 183:109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corry, D. B., G. Grunig, H. Hadeiba, V. P. Kurup, M. L. Warnock, D. Sheppard, D. M. Rennick, and R. M. Locksley. 1998. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol. Med. 4:344-355. [PMC free article] [PubMed] [Google Scholar]
- 12.Gavett, S. H., D. J. O'Hearn, C. L. Karp, E. A. Patel, B. H. Schofield, F. D. Finkelman, and M. Wills-Karp. 1997. Interleukin-4 receptor blockade prevents airway responses induced by antigen challenge in mice. Am. J. Physiol. 272:L253-L261. [DOI] [PubMed] [Google Scholar]
- 13.Gavett, S. H., D. J. O'Hearn, X. Li, S. K. Huang, F. D. Finkelman, and M. Wills-Karp. 1995. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J. Exp. Med. 182:1527-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberger, P. A. 2002. Allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. 110:685-692. [DOI] [PubMed] [Google Scholar]
- 15.Grunig, G., M. Warnock, A. E. Wakil, R. Venkayya, F. Brombacher, D. M. Rennick, D. Sheppard, M. Mohrs, D. D. Donaldson, R. M. Locksley, and D. B. Corry. 1998. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282:2261-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez, Y., S. Arora, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J. Immunol. 174:1027-1036. [DOI] [PubMed] [Google Scholar]
- 17.Herrick, C. A., and K. Bottomly. 2003. To respond or not to respond: T cells in allergic asthma. Nat. Rev. Immunol. 3:405-412. [DOI] [PubMed] [Google Scholar]
- 18.Hill, J. O., and K. M. Aguirre. 1994. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J. Immunol. 152:2344-2350. [PubMed] [Google Scholar]
- 19.Hogaboam, C. M., K. Blease, B. Mehrad, M. L. Steinhauser, T. J. Standiford, S. L. Kunkel, and N. W. Lukacs. 2000. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am. J. Pathol. 156:723-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 160:2393-2400. [PubMed] [Google Scholar]
- 21.Huffnagle, G. B., M. F. Lipscomb, J. A. Lovchik, K. A. Hoag, and N. E. Street. 1994. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J. Leukoc. Biol. 55:35-42. [DOI] [PubMed] [Google Scholar]
- 22.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J. Exp. Med. 173:793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaffar, Z., T. Sivakuru, and K. Roberts. 2004. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J. Immunol. 172:3842-3849. [DOI] [PubMed] [Google Scholar]
- 24.Kearley, J., J. E. Barker, D. S. Robinson, and C. M. Lloyd. 2005. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 202:1539-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurup, V. P., H. Y. Choi, P. S. Murali, J. Q. Xia, R. L. Coffman, and J. N. Fink. 1999. Immune responses to Aspergillus antigen in IL-4−/− mice and the effect of eosinophil ablation. Allergy 54:420-427. [DOI] [PubMed] [Google Scholar]
- 26.Kurup, V. P., and G. Grunig. 2002. Animal models of allergic bronchopulmonary aspergillosis. Mycopathologia 153:165-177. [DOI] [PubMed] [Google Scholar]
- 27.Kurup, V. P., P. S. Murali, J. Guo, H. Choi, B. Banerjee, J. N. Fink, and R. L. Coffman. 1997. Anti-interleukin (IL)-4 and -IL-5 antibodies downregulate IgE and eosinophilia in mice exposed to Aspergillus antigens. Allergy 52:1215-1221. [DOI] [PubMed] [Google Scholar]
- 28.Kurup, V. P., B. W. Seymour, H. Choi, and R. L. Coffman. 1994. Particulate Aspergillus fumigatus antigens elicit a TH2 response in BALB/c mice. J. Allergy Clin. Immunol. 93:1013-1020. [DOI] [PubMed] [Google Scholar]
- 29.Larche, M., D. S. Robinson, and A. B. Kay. 2003. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 111:450-464. [DOI] [PubMed] [Google Scholar]
- 30.Lortholary, O., L. Improvisi, C. Fitting, J. M. Cavaillon, and F. Dromer. 2002. Influence of gender and age on course of infection and cytokine responses in mice with disseminated Cryptococcus neoformans infection. Clin. Microbiol. Infect. 8:31-37. [DOI] [PubMed] [Google Scholar]
- 31.Mody, C. H., M. F. Lipscomb, N. E. Street, and G. B. Toews. 1990. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J. Immunol. 144:1472-1477. [PubMed] [Google Scholar]
- 32.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 33.Olszewski, M. A., G. B. Huffnagle, T. R. Traynor, R. A. McDonald, D. N. Cook, and G. B. Toews. 2001. Regulatory effects of macrophage inflammatory protein 1α/CCL3 on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect. Immun. 69:6256-6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson, D. S., Q. Hamid, S. Ying, A. Tsicopoulos, J. Barkans, A. M. Bentley, C. Corrigan, S. R. Durham, and A. B. Kay. 1992. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326:298-304. [DOI] [PubMed] [Google Scholar]
- 35.Romagnani, S. 2001. T-cell responses in allergy and asthma. Curr. Opin. Allergy Clin. Immunol. 1:73-78. [DOI] [PubMed] [Google Scholar]
- 36.Wood, G. S., N. L. Warner, and R. A. Warnke. 1983. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J. Immunol. 131:212-216. [PubMed] [Google Scholar]
- 37.Zhu, Z., R. J. Homer, Z. Wang, Q. Chen, G. P. Geba, J. Wang, Y. Zhang, and J. A. Elias. 1999. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 103:779-788. [DOI] [PMC free article] [PubMed] [Google Scholar]