Abstract
Adjuvant-induced survival of T cells after antigen activation correlates with increased expression of Bcl-3. Bcl-3 is an NF-κB/IκB family member and has been implicated in transcriptional regulation in several cell types. We tested the ability of mice deficient in Bcl-3 (Bcl-3 KO) to exhibit T-cell adjuvant-induced survival after challenge with the superantigen staphylococcal enterotoxin B (SEB), using lipopolysaccharide (LPS) as a natural adjuvant. These studies showed that Bcl-3 is required for secondary gamma interferon (IFN-γ) production by CD8 T cells but not for adjuvant-induced survival effects. Specifically, wild-type and Bcl-3 KO mice exhibited comparable long-term increases in the Vβ8+ T-cell populations, indicating no lack of survival in response to adjuvant stimulation in the Bcl-3 KO activated T cells. Ectopic expression of the Bcl-3-related molecules IκBα, IκBβ, and IκBɛ in SEB-activated T cells increased survival during in vitro culture in the absence of adjuvant, suggesting that these IκB molecules could exert a survival function in antigen-activated T cells in place of Bcl-3. However, Vβ8+ CD8 T cells from SEB- plus LPS-treated Bcl-3 KO mice produced less IFN-γ upon in vitro restimulation than Vβ8+ CD8 T cells from wild-type mice. Therefore, Bcl-3 plays a unique role in the regulation of IFN-γ production in this model system.
The mechanisms by which adjuvants permit some T cells to escape death during clonal contraction remain to be fully elucidated. B7/CD28-mediated signaling is frequently invoked as being the primary means by which adjuvants exert survival effects. However, it is the initial clonal burst that is the CD28-dependent phase of the T-cell response (16, 36), not the survival during the ensuing clonal contraction. Proliferative bursts are accompanied by short-term increases in Bcl-2 and Bcl-x expression levels (13, 22, 23); however, the transcription of these survival factors is rapidly curtailed by increasing reactive oxygen species generated as by-products of activation (13). Therefore, adjuvants must cause other survival factors to be induced in activated T cells to increase survival throughout clonal contraction. Bcl-3 was previously identified as a candidate survival factor for T cells activated under conditions of productive immunization (22). Gene array analysis of the mRNA populations obtained from T cells that were activated with superantigen either alone or in the presence of two separate adjuvants showed that at least 23 different transcripts, including that of Bcl-3, were significantly increased by either adjuvant (22, 23). Similar adjuvant- and cytokine-mediated increases in Bcl-3 mRNA have been demonstrated in vivo after antigen plus adjuvant treatment of OT-1 mice and in vitro when cells were activated with antigen and interleukin-12 (IL-12) (30, 34). Moreover, IL-12-dependent survival was defective in Bcl-3 knockout (KO) T cells after in vitro stimulation (34). Bcl-3 KO mice were also unable to control infection with Toxoplasma gondii, a model pathogen for studying Th1-dependent immunity (9). Therefore, Bcl-3 plays an important role in supporting T-cell responses, although it remains unclear whether Bcl-3 is needed primarily for T-cell survival or differentiation or both.
Bcl-3 is an atypical IκB member of the NF-κB family of transcription factors that was originally named for its involvement in chronic lymphoblastic leukemia (3). It was later shown that translocations of the bcl3 gene near the immunoglobulin heavy chain constant region result in increased transcription of an otherwise unmutated gene product (19, 20), which is consistent with the idea that the normal function of Bcl-3 is to enhance cellular survival and/or division. Transgenic expression of Bcl-3 in B cells was associated with lymphoaccumulation rather than overt oncogenicity (26), and enforced expression of Bcl-3 in T cells did not drive proliferation in vitro (22, 24), further suggesting that Bcl-3 functions primarily as a survival factor in these contexts.
Defects in B- and T-cell responses have been demonstrated in Bcl-3 KO mice, including decreased T-cell-dependent immunoglobulin class switching, defective generation of influenza-specific antibodies, and susceptibility to a variety of pathogens, such as T. gondii, Listeria monocytogenes, and Streptococcus pneumonia (9, 28, 31). Bcl-3 KO mice develop normally but have decreased numbers of B cells and germinal centers (9). They are also defective in their ability to populate peripheral lymph nodes in an anterior-to-posterior manner, which is similar to a defect in NF-κB2 KO mice that has been attributed to stromal cell chemokine production (4). Thus, Bcl-3 plays multiple roles in various immune cell types. To understand how adjuvant effects boost T-cell clonal expansion, we concentrated our efforts on identifying defects in the T-cell responses of Bcl-3 KO mice.
We investigated the effect of Bcl-3 deficiency on T-cell survival and the ability of activated T cells to produce cytokine after activation. Visualization of activated T cells in vivo was performed by injecting wild-type or Bcl-3 KO mice with the model antigen staphylococcal enterotoxin B (SEB) in the absence or presence of a natural adjuvant, lipopolysaccharide (LPS). SEB is a superantigen that, when given in purified form, drives abortive proliferation of Vβ8 T-cell receptor-positive (TCR+) T cells in H-2b mice, and LPS has been used previously as an adjuvant to drive Th1 differentiation and cytotoxic T lymphocyte effector gene expression as well as to increase survival of activated T cells (18, 22). A useful attribute of superantigens is that they stimulate T cells in a major histocompatibility complex-dependent manner without requiring proteolytic processing and loading into major histocompatibility complex. By giving SEB in the presence and absence of LPS, we were therefore able to focus on the postprocessing effects of adjuvants.
Our experiments showed that net clonal expansion of T cells was not diminished in Bcl-3 KO mice. Bcl-3 KO T cells activated with SEB plus LPS had a significantly bigger burst size 3 days after treatment than the wild-type T cells. Because there was no quantifiable difference in cell number or percentage at the 7-day time point, more Bcl-3 KO T cells must have died during clonal contraction than wild-type T cells, although the difference was modest. Tests of other IκB family molecules showed that they could also prolong survival of activated T cells, suggesting that they are redundant to Bcl-3 in this function.
T cells that had been activated and survived the clonal contraction from Bcl-3 KO mice were not anergic or unresponsive upon encountering antigen during restimulation cultures in vitro. However, the ability of Bcl-3 KO CD8 T cells to produce gamma interferon (IFN-γ) upon secondary stimulation was markedly reduced. These results indicate that Bcl-3 expression is required for continued production of IFN-γ by CD8 T cells upon restimulation and provides insight as to how Bcl-3 functions as an important intracellular mediator of microbial resistance (9). The results also show that Bcl-3 may not be uniquely required for survival of T cells after adjuvant activation and that other members of the IκB protein family are capable of prolonging the survival of antigen-activated T cells. The redundant nature of the IκB family members to elicit survival of T cells activated in the presence of adjuvant further suggests that Bcl-3 acts as a member of a critical survival signaling pathway.
MATERIALS AND METHODS
Animals.
The Bcl-3 KO mice were initially obtained from the NIAID Emerging Models Program (administered by Taconic Farms, Germantown, NY). Four to six-week-old C57BL/6 mice (Taconic Farms) were used as wild-type controls. VβDO mice that express Vβ8 chain of the DO11.10 TCR as a transgene were also used in some survival assays (8). Mice were bred and housed in a specific-pathogen-free barrier facility at the Institute for Cellular Therapeutics, University of Louisville, Louisville, Kentucky, and cared for according to specific University of Louisville and National Institutes of Health animal care guidelines.
Reagents.
Superantigen, SEB, was purchased from Toxin Technology, Inc. (Sarasota, FL), and LPS (from Salmonella enterica serovar Typhosa), phorbol 12-myristate 13-acetate, ionomycin, and brefeldin A were obtained from Sigma Aldrich (St. Louis, MO). Monoclonal antibodies (MAb) used in flow analysis were purchased from BD Pharmingen (San Diego, CA). MAb specific for CD19 (1D3; rat immunoglobulin G2a [IgG2a])-fluorescein isothiocyanate, CD4 (GK1.5; rat IgG2b)-allophycocyanin (APC), CD8 (53-6.7; rat IgG2b)-peridinin chlorophyll protein, and Vβ8 TCR (F23.1; mouse IgG2a) or Vβ6 TCR (RR4-7; rat IgG2b)-fluorescein isothiocyanate were used to characterize and enumerate cells after SEB treatment. Intracellular cytokine staining was performed with MAb against IFN-γ (XMG1.4; rat IgG1)-phycoerythrin (PE), and anti-IL-4 (11B11; rat IgG1)-PE MAb was also used in fluorescence-activated cell sorter (FACS) analysis after in vivo culture. 5,6-Carboxyflourescein diacetate, succinimidyl ester (CFSE) was purchased from Molecular Probes, Inc. (Eugene, OR).
Survival of Vβ8+ T cells after 3 and 7 days of in vivo activation with SEB.
The ability of T cells to survive after antigen exposure was tested by treating age-matched Bcl-3 KO and C57BL/6 mice with 50 μg SEB and 10 μg LPS or with SEB alone. All treatments were through intravenous injection. Seven days after treatment, lymph nodes and spleens were harvested from naive controls and treated mice. The percentage of Vβ8 TCR+ CD4 and CD8 T cells was quantified by flow cytometry using a FACSCaliber flow cytometer (Becton Dickinson, San Diego, CA) and CellQuest software (Becton Dickinson), and the numbers of cells were calculated using this percentage multiplied by the total cell numbers per organ. Cell numbers were measured after treating diluted cell suspensions with Zap-oglobin II lytic reagent (Coulter Corp., Fullerton, CA), and nuclei were counted using a Coulter Z2 particle counter (Coulter Corp.).
Intracellular cytokine staining.
Spleen cells obtained after treatment with SEB alone or in combination with LPS were cultured in complete tissue culture media (RPMI 1640 [Invitrogen, Carlsbad, CA] supplemented with 10% fetal bovine serum [Valley Biomedical, Inc., Winchester, VA], 1 mM sodium pyruvate [Invitrogen], 100 units/ml penicillin, 100 μg/ml streptomycin [Invitrogen], and 2 mM l-glutamine [Invitrogen]) in the absence and presence of 100 ng/ml SEB for a total of 48 h. During the final 6 h of culture, phorbol 12-myristate 13-acetate (50 ng/ml), ionomycin (500 ng/ml), and brefeldin A (10 μg/ml) were added to the culture. Cells were washed in FACS buffer, consisting of Hank's balanced salt solution (Invitrogen, Carlsbad, CA), 1% heat-inactivated fetal bovine serum (Invitrogen) and 0.02% NaN3 (Sigma Aldrich), and stained for CD4 and Vβ8 or CD8 and Vβ8 for 30 min at 4°C. Cells were then washed and fixed in 4% formaldehyde (Polysciences, Warrington, PA) for 15 min at 37°C, followed by ice-cold methanol at 4°C overnight. The methanol was washed away, and the cells were permeabilized with 1% Triton X-100 (Roche Diagnostic Corp., Indianapolis, IN) in FACS buffer. PE-conjugated anti-IFN-γ MAb diluted in Triton X-100-FACS buffer was added to the cells for 30 min, followed by washing in Triton X-100-FACS buffer.
CFSE dilution to determine cell cycling.
Spleen cells from 7-day-treated mice were suspended to 40 × 106 cells/ml in Hank's balanced salt solution and then mixed with an equal volume of prewarmed 5 μM CFSE for 10 min at 37°C. The stained cells were then washed two times and resuspended in complete tissue culture media in the absence or presence of 100 ng/ml SEB at 37°C and 5% CO2. At various times after culture, cells were harvested and stained with anti-CD4-APC, anti-CD8-peridinin chlorophyll protein, and anti-Vβ8 TCR-PE MAb for flow cytometry. Average numbers of cell divisions were calculated as described elsewhere (33).
Retroviral transduction of activated cells and 20-h in vitro survival assay.
Transgenic VβDO mice were used as a source of cycling T cells to increase the frequency of retroviral transduction. Sixty-six hours after injection of SEB (50 μg/mouse), lymph nodes were harvested and cells were infected with retrovirus containing the parental retroviral vector murine stem cell virus-internal ribosome entry site-Thy1.1 (MiT) or MiT vectors also encoding Bcl-3, IκBα, IκBβ, IκBɛ, or Bcl-2, as previously described (24). Infected cells were then cultured for 20 h in complete tissue culture medium in the absence of growth factors. Survival was quantified by measuring the percentage of infected (Thy1.1+) cells present in the “live gate,” as determined by light scatter properties of the cells. This method has previously been shown to accurately detect live versus dead lymphocytes (10, 32). To standardize the survival analysis, increasing amounts of the different retrovirus preparations were used to infect the cells, and comparisons were made between cultures that had similar proportions of activated T cells infected (2 to 5%), as assessed by Thy1.1 expression.
Statistical analysis.
The SPSS statistical software package for Windows, version 13.0, was used to run two-tailed paired sample tests on the data presented. A P value of <0.05 was considered significant.
RESULTS
Bcl-3 knockout mice show adjuvant-mediated survival of activated T cells that is similar to that of wild-type mice.
The correlation of increased or forced Bcl-3 expression with better survival of adjuvant-stimulated antigen-activated T cells (22, 23) prompted us to determine whether T cells from Bcl-3 KO mice would be defective in adjuvant-induced survival. Wild-type B6 and Bcl-3 KO mice were treated in vivo with SEB in the absence or presence of LPS for 3 or 7 days. The numbers and percentages of responding Vβ8+ TCR cells in the T-cell populations were then evaluated in spleens from treated mice and compared to untreated controls (Fig. 1). Three days after SEB exposure (peak expansion), the inclusion of LPS as an adjuvant resulted in a significantly increased clonal burst size of both CD4 and CD8 Vβ8+ T cells in Bcl-3 KO mice compared to similarly treated wild-type mice (Fig. 1A). This result indicates that Bcl-3 is likely to be playing an inhibitory, not an enhancing, role during prepeak T-cell clonal expansion in normal mice. Although there is a significant increase in the number of Vβ8 T cells in Bcl-3 KO mice on day 3, there is not a significant increase in the percentage of CD4 or CD8 T cells expressing Vβ8+ TCR. SEB can bind and activate T cells expressing other Vβ regions, and increases in these T cells may explain this apparent discrepancy. In fact, the increase in Vβ8 T cells only constitutes approximately 60% of the increase in CD4 and CD8 T cells obtained from day 3 activated SEB- plus LPS-treated mice, either wild-type or Bcl-3 KO. However, the numbers of nonactivated Vβ6+ T cells from these same mice did not change at either time point regardless of treatment or genotype (data not shown).
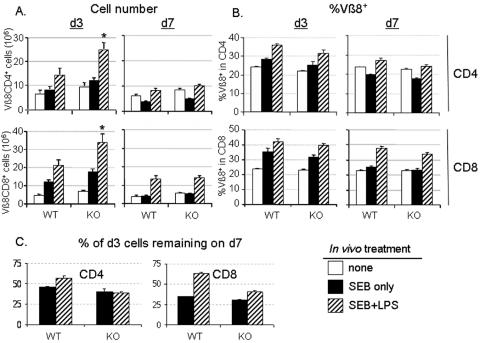
FIG. 1.
Increased clonal expansion of T cells in Bcl-3 KO mice is followed by normal adjuvant-mediated survival after in vivo treatment with SEB plus LPS. In vivo survival of activated T cells in wild-type (WT) and Bcl-3 KO (KO) mice was assessed by treating groups of mice with SEB in the absence or presence of LPS. Three (d3) and seven (d7) days after treatment, the number (A) and percentage (B) of Vβ8 TCR+ CD4 and CD8 T cells was determined via FACS analysis. Data depicted are averages ± standard deviations of results from 8 to 14 individual animals from 3 to 7 individual experiments. The data for the untreated cohort of animals (n = 12) were included in the graphs for both day 3 and day 7 after treatment for comparison. Asterisks indicate P values of <0.05 when comparing the numbers of Vβ8+ cells obtained from wild-type B6 and Bcl-3 KO mice at the peak of clonal expansion. (C) Decreased postpeak LPS-dependent survival observed in Bcl-3 KO T cells compared to wild-type B6 T cells. Shown are the percentages of day 3 Vβ8 cells that remained on d7.
At the day 7 time point, Bcl-3 KO animals showed no difference in net T-cell clonal expansion in the absence or presence of LPS. Specifically, the number of Vβ8+ CD4 T cells from SEB alone-treated mice was decreased at day 7 compared to untreated controls, whereas the SEB- plus LPS-treated mice had a net gain of Vβ8+ CD4 T-cell numbers and percentages in both wild-type and Bcl-3 KO mice (Fig. 1A and B). Although mice treated with SEB alone had numbers and percentages of Vβ8+ CD8 T cells equivalent to those of untreated mice, SEB plus LPS treatment resulted in marked increases in the number and percentage of Vβ8+ CD8 T cells (Fig. 1A and B) in both wild-type and Bcl-3 KO mice. These data indicate that net peripheral deletion as well as adjuvant-assisted clonal expansion is unimpaired in superantigen-treated Bcl-3 KO mice.
On day 3 after SEB plus LPS treatment, there were more Vβ8+ T cells in the Bcl-3 KO mice than in wild-type mice; however, the Vβ8+ T-cell numbers were similar between strains on day 7. This is suggestive of a more rapid rate of death in the activated Bcl-3 KO T cells during clonal contraction than in wild-type T cells. Comparison of net versus peak clonal expansion undergone in Bcl-3 KO versus wild-type mice shows that the Vβ8+ CD4 T cells that were enumerated on day 7 in the SEB- plus LPS-treated mice represented only 39% of the peak day 3 levels in Bcl-3 KO mice, which was much less than the corresponding 57% in the wild-type mice (Fig. 1C). Likewise, the day 7 Vβ8+ CD8 T-cell populations represented only 41% of the peak numbers obtained from Bcl-3 KO mice compared to 63% of the wild-type Vβ8+ CD8 T cells (Fig. 1C). This apparent defect in full survival of activated Bcl-3 KO T cells may either be caused directly by loss of Bcl-3 or indirectly by negative feedback mechanisms that are stronger when the peak burst size is greater.
Possible role for other IκB molecules in rescuing Bcl-3 KO T cells from cell death following activation.
It has been demonstrated that the ectopic expression of Bcl-3 in CD4 T cells activated with antigen alone is sufficient to rescue the cells from death to similar extents as those seen when exogenous adjuvants are used (22, 24). Other members of the IκB family have also been shown to influence lymphocyte survival (11). To assess whether other proteins in the IκB family can confer T-cell survival after activation in the absence of adjuvant, SEB was injected into mice that express the β chain of the DO11.10 TCR as a transgene. Because this β chain includes Vβ8, all T cells in these mice are responsive to SEB (8). Lymph node cells were harvested 66 h after SEB treatment and infected with retrovirus containing parental retroviral vector MiT or MiT-based vectors encoding Bcl-3, IκBα, IκBβ, IκBɛ, or Bcl-2. The cells were then cultured for 20 h in the absence of growth factors to assess whether the expression of these molecules could confer a survival advantage to the activated T cells. The viability of the infected cells was determined by measuring the percentage of Thy1.1-expressing cells present in the live gate during FACS analysis (24). As shown in Fig. 2A, CD4 T cells infected with the Bcl-3-encoding vector averaged a 1.5-fold increase in survival after 20 h compared to the cells infected with parental MiT-expressing retrovirus. This value was equivalent to that of Bcl-2-encoding vectors (1.44-fold), which indicated that Bcl-3 expression in antigen-activated T cells rescued the majority of the activated cells that could have been rescued by retroviral transfer of a survival gene (Fig. 2A). Rescue of the activated CD4 T cells via expression was also achieved through expression of either IκBα, IκBβ, or IκBɛ, with increased survival being most consistently observed after expression of IκBɛ (P < 0.001 compared to MiT vector).
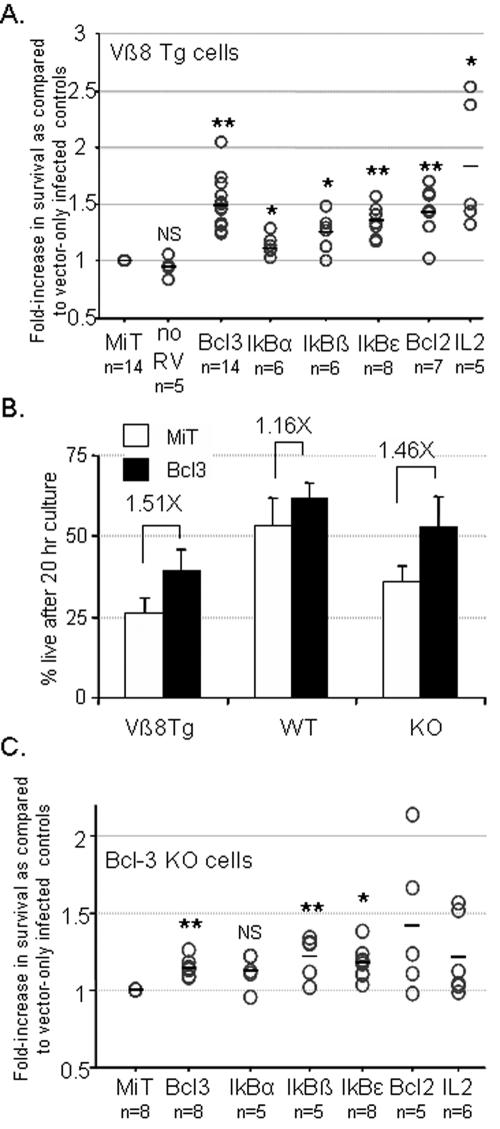
FIG. 2.
Survival of activated T cells is conferred by expression of different IκB family members. (A) Vβ8 transgenic mice (Vβ8 Tg) were treated in vivo with SEB for 66 h, and lymph node cells were harvested and infected with retrovirus containing the parental MiT vector or MiT vectors also encoding various IκB family molecules (Bcl-3, IκBα, IκBβ, or IκBɛ) or Bcl-2. After infection, cells were cultured for 20 h in minimal medium without growth factors. Control cultures that had received no retrovirus were also cultured in the absence (no RV) or presence of 20 ng/ml recombinant IL-2 (IL2). Cells were stained for CD4, Vβ8 TCR, and Thy1.1 expression, and viability was assessed by back-gating these cells to determine viability. Data are expressed as relative increases in the viability of T cells with forced expression of the indicated protein compared to viability of vector control-infected cells. Circles and lines represent individual values and their means, respectively, of experimental data sets obtained from 4 to 9 separate retrovirus transduction experiments. (B) Percent viability of SEB-activated cells from Vβ8 Tg, C57BL/6 (WT), or Bcl-3 KO (KO) mice infected with either MiT parental control or MiT-expressing Bcl-3 virus after 20 h of culture under growth factor withdrawal conditions. The Vβ8 Tg and KO cells infected with MiT retrovirus were decreased in survival compared to the WT cells, allowing increased survival in the activated cells infected with the MiT-Bcl-3 virus. Numbers indicate relative increases in survival of the Bcl-3 cultures compared to MiT cultures. (C) T cells from Bcl-3 KO mice were infected as for panel A after 48 h of in vivo activation with SEB. Single asterisks indicate P values of <0.05 compared to MiT controls in 2-tailed paired sample tests using SPSS for Windows statistical software package, version 13. The double asterisks indicate P values of <0.01, and N.S. indicates no significance in the difference compared to the MiT controls.
As shown in Fig. 2B, wild-type T cells activated in vivo with SEB survive well during the 20-h growth factor withdrawal assay (>50% survival), and therefore, there is little opportunity for the retrovirally expressed proteins to rescue the cells during this assay. There was a tendency toward increased survival through Bcl-3 expression; however, this increase is not significant. The Bcl-3 KO T cells survive less well than wild-type cells in this assay (Fig. 2B) and are therefore capable of rescue. Expression of Bcl-3 in activated Bcl-3 KO T cells increased survival in the 20-h growth factor withdrawal assay compared to cells infected with control virus (MiT) to levels similar to that observed in the Vβ8 transgenic (Tg) T cells (1.46- versus 1.51-fold, respectively). Since the wild-type (WT) CD4 cells do not die well in our in vitro assay, we decided to assess the ability of the IκB molecules to rescue activated Bcl-3-deficient T cells from death during 20-h growth factor withdrawal in vitro (Fig. 2C). Vβ8+ T cells from SEB-activated Bcl-3 KO mice were infected with retrovirus expressing the various IκB molecules. As in the Vβ8 Tg T cells, retroviral expression of IκBβ or IκBɛ increased the survival of infected cells after 20 h of culture in the absence of growth factor (Fig. 2C), but no consistent increase was observed with IκBα expression. The increase in survival through expression of either IκBβ or IκBɛ constructs in the activated CD4 T cells was comparable to what was observed with the Bcl-3 constructs. Therefore, although Bcl-3 was sufficient to moderately increase the survival of antigen-activated T cells, forced expression of other molecules in the IκB protein family also led to better survival in Bcl-3-deficient T cells. Thus, a redundant mechanism mediated by this family of molecules likely acts in the absence of Bcl-3 to ensure a survival advantage to T cells activated in the presence of adjuvant.
Proliferation of Vβ8+ T cells remains intact in Bcl-3 KO mice after in vivo treatment with SEB in the absence and presence of LPS.
In spite of the evidence of in vivo adjuvant-induced survival of activated T cells from Bcl-3 KO mice, previous reports have shown that no lasting T-cell response occurs during infection with T. gondii or influenza (9, 22). Although SEB-activated T cells from Bcl-3 KO mice were rescued from deletion by the coadministration of LPS, the surviving Bcl-3 KO T cells could be refractory to further stimulation. Alternatively, the surviving Bcl-3 KO T cells could be deficient in effector functions upon subsequent stimulation compared to wild-type T cells. To determine which, if either, of these possibilities was likely to be true, we tested the ability of the T cells that had survived after in vivo stimulation with SEB with or without LPS to either proliferate or to produce IFN-γ after restimulation with SEB during in vitro culture. Spleen cells from wild-type and Bcl-3 KO mice were harvested 7 days after SEB with or without LPS treatment and also from untreated controls. To test the proliferative response in culture, the harvested cells were labeled with CFSE and cultured in the absence or presence of SEB for 4 days, after which the CFSE cell division profiles of Vβ8+ T cells were determined via flow cytometry. The Bcl-3 KO T cells that survived 7 days after in vivo treatment with SEB plus LPS entered the cell cycle to the same extent (Fig. 3A) and averaged a number of cell divisions (Fig. 3B) similar to that of wild-type T cells. This was true of both CD4 and CD8 Vβ8+ T cells during in vitro culture (Fig. 3). Limited proliferation was seen upon culture in the absence of SEB, but this was not influenced by Bcl-3 because it occurred to the same extent in both wild-type and KO cell populations (Fig. 3A and B). Although a large percentage of the cells divide, this effect appears to be short lived because few of the cells undergo more than one division during the culture in medium alone. It is possible that this residual cell cycle activity is due to adjuvant-induced retention of at least some superantigen on surviving antigen-presenting cells.
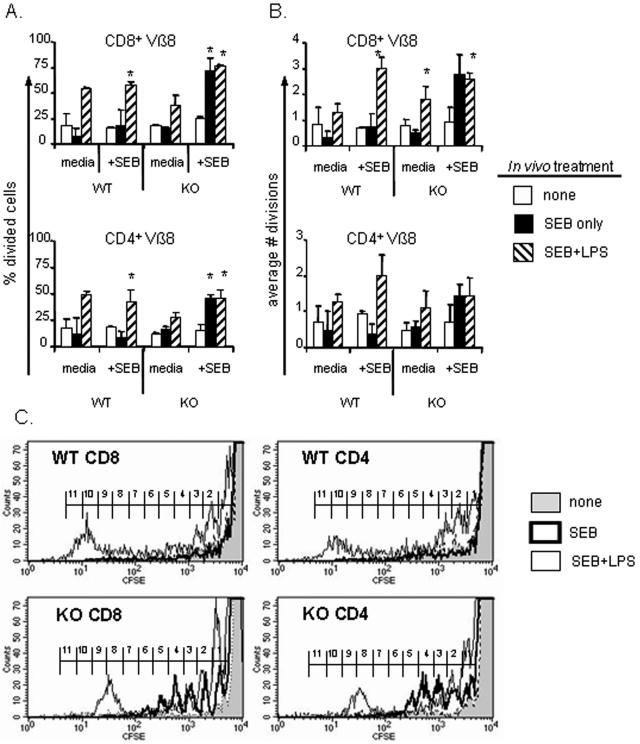
FIG. 3.
Bcl-3 KO T cells do not become anergic after stimulation with SEB. Spleen cells were harvested 7 days after in vivo activation with SEB alone or with LPS and from untreated control mice, labeled with CFSE, and cultured in the absence (media) or presence of 100 ng/ml SEB (+SEB). Cultures were harvested after 4 days, and the cells were stained for Vβ8 TCR, CD4, and CD8 and analyzed for the percentage of surviving cells that had divided while in culture (A) and the average number of cell divisions undergone in each culture (B). Asterisks indicate P values of <0.05 compared to cells from naive controls in 2-tailed paired sample tests. Results shown are averages ± standard deviations of four individuals per group obtained from two separate experiments. (C) Examples of the CFSE division profiles for Vβ8+ CD8 or CD4 cells from WT or KO cells cultured with SEB for 4 days. Gray: untreated cells; dark line: SEB only; thin line: SEB plus LPS in vivo. Brackets indicate the bins for determining the number of cell divisions.
The Bcl-3 KO T cells obtained from mice initially treated with SEB alone proliferated significantly more upon restimulation than the wild-type cells given the same in vivo treatment. Both the proportion of cells that had divided and the number of divisions undergone were approximately three- to fivefold greater in the CD4 and CD8 Bcl-3 KO T cells, reaching values that were strikingly similar to those from the adjuvant-treated groups. In contrast, T cells from the SEB-treated wild-type mice were refractory to restimulation. These results show that Bcl-3 KO T cells did not show an abnormal tendency to anergy and, if anything, they were better able to proliferate even when activation occurred without adjuvant.
The surviving Bcl-3 KO CD8 T cells produce less LPS-dependent IFN-γ during in vitro restimulation cultures.
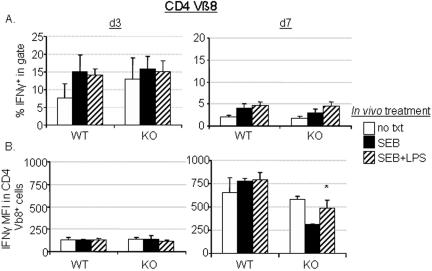
To determine if effector pathways could be defective in Bcl-3 KO mice, IFN-γ production was analyzed. IFN-γ is especially important in mediating a range of cellular immune effects which include antibody isotype switching, cytotoxic T lymphocyte activity, and resistance to intracellular pathogens, all of which have been shown to be defective or diminished in Bcl-3 KO mice (9, 31). Therefore, we tested the surviving T cells from mice treated with SEB with or without LPS to determine the effect, if any, that Bcl-3 deficiency had on the ability of T cells to produce IFN-γ following restimulation. Spleen cells harvested from Bcl-3 KO and wild-type mice 3 or 7 days after treatment were cultured with SEB for a total of 48 h, at which time the level of IFN-γ-producing cells was assessed. As shown in Fig. 4A, CD8 T cells from Bcl-3 KO mice were not responsive to LPS at the level of adjuvant-dependent IFN-γ production. That is, wild-type CD8 T cells showed increased IFN-γ production upon restimulation if SEB plus LPS, but not SEB alone, had been used during the initial activation in vivo. The response of Bcl-3 KO CD8 T cells that had been primed with SEB plus LPS was the same as that of SEB-primed mice (Fig. 4A, right), indicating that the IFN-γ-enhancing adjuvant effect of LPS was impaired in CD8 T cells that were unable to express Bcl-3. Therefore, Bcl-3 was uniquely required for LPS-dependent increase in IFN-γ production in restimulated CD8 T cells.
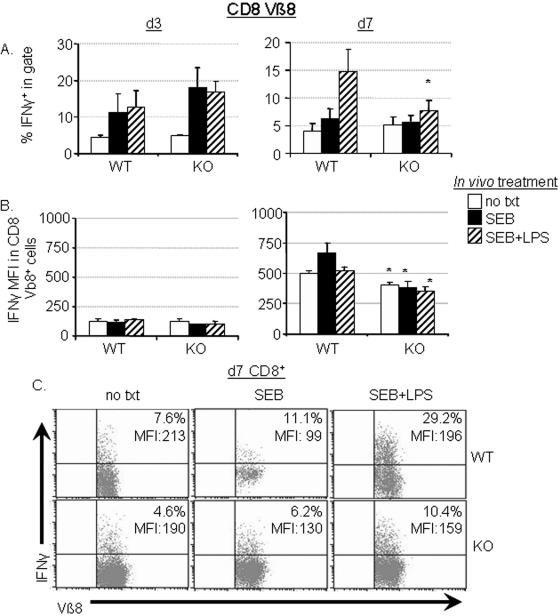
FIG. 4.
Bcl-3 KO CD8 T cells lack adjuvant-dependent IFN-γ recall responses. (A) CD8 T cells from untreated control mice (no txt) or from mice treated in vivo for 3 days (d3; left panels) or 7 days (d7; right panels) with SEB with or without LPS were assessed for their ability to express IFN-γ in response to in vitro restimulation for 48 h with 100 ng/ml SEB. (B) The IFN-γ-specific MFI of CD8+ Vβ8 TCR+ cells cultured as described for panel A. Data for panel A are means ± standard errors of the means (SEM) of 5 to 6 (day 3) or 10 to 12 (day 7) individual mice. Data in panel B are means ± SEM from three individual splenic cultures from one of either two (day 3) or four (day 7) similar experiments performed. Asterisks denote P values of <0.05 when comparing values for Bcl-3 KO cultures to wild-type cultures. (C) Representative dot plots of IFN-γ-stained cells from the CD8+-gated cells from each group of mice on day 7 are shown. The percentage of IFN-γ+ cells in the CD8+ Vβ8 TCR+ population and the values for IFN-γ-specific MFI are listed in the upper right corners. Each plot represents a culture from an individual mouse treated as described from one representative experiment of four performed with two treated animals per group. This experiment was distinct from those shown in panels A and B.
In addition, no LPS-dependent increase in the IFN-γ-specific mean fluorescence intensity (MFI) was noted in the CD8+ Vβ8+ cells from Bcl-3 KO mice on day 7 after treatment when compared to untreated KO CD8+ Vβ8+ cells (Fig. 4B and C). A slight, but significant, decrease in the IFN-γ MFI was detected in Bcl-3 KO cells obtained after 7 days of in vivo treatment in SEB plus LPS compared to wild-type cells (Fig. 4B, right). These data show that a Bcl-3-dependent mechanism is important for the retention of the ability to produce increased levels of IFN-γ by CD8 T cells upon exposure to the same antigen that is usually observed in antigen- plus adjuvant-experienced T cells.
The defective LPS responsiveness of Bcl-3 KO cells to restimulation was complex, however, because their IFN-γ production immediately after priming was strong (Fig. 4A, left). The frequency of IFN-γ-producing Vβ8+ CD8 T cells was, in fact, higher than that of wild-type cultures, which could possibly be an indirect result of the larger burst size that was observed at this same day 3 time point (Fig. 1A). Whatever the cause, the fact that IFN-γ production was robust indicates that Bcl-3 is not fundamentally required for IFN-γ transcription under all conditions but may instead play a regulatory role that influences adjuvant responsiveness.
When Vβ8+ CD4 T cells from the same cultures were assessed for IFN-γ production, the percentage of IFN-γ+ cells was greater in cells from both wild-type and Bcl-3 KO mice treated with SEB alone or plus LPS at both the day 3 and day 7 time points (Fig. 5A). Although the IFN-γ MFI in the CD4 T cells from untreated wild-type and Bcl-3 KO mice are similar, CD4 T cells from SEB- plus LPS-treated Bcl-3 KO mice had a significantly lower IFN-γ MFI than those from similarly treated wild-type mice on day 7 after treatment (Fig. 5B), suggesting that, although the proportion of activated CD4 cells producing IFN-γ was not affected in Bcl-3 KO mice, IFN-γ production in Bcl-3 KO CD4 cells was lower. We also stained CD4 T cells from SEB-restimulated cultures for IL-4, but it was not detected on a consistent basis (data not shown). Together, these data indicate that Bcl-3 is an important factor in maintaining the IFN-γ-secreting effector function of previously activated CD8 T cells, but no significant Bcl-3-dependent changes were noted in the cytokine expression in CD4 T cells.
FIG. 5.
IFN-γ production by Bcl-3 KO CD4 T cells is unimpaired. As described in the legend to Fig. 4, CD4 T cells from untreated control mice (no txt) or from mice treated in vivo for 3 days and 7 days with SEB alone or SEB plus LPS were assessed for their ability to express IFN-γ in response to in vitro restimulation for 48 h with 100 ng/ml SEB. Percentages of IFN-γ+ T cells were increased in Vβ8 TCR+ CD4 populations from WT mice when harvested on day 3 and from WT and KO mice when harvested on day 7 (A). The MFI of the IFN-γ stain in the positive population is also shown for day 3 and day 7 (B). Data for panel A are means ± standard errors of the means of 5 to 10 individual spleen cell cultures performed between 3 to 5 separate experiments. Data in panel B are means ± standard deviations from three individual splenic cultures from one of two (day 3) or four (day 7) similar experiments. Asterisks denote P values of <0.05 when comparing values for Bcl-3 KO cultures to those for wild-type cultures. Cultures are the same as those used for Fig. 4.
DISCUSSION
Through the course of this study, we consistently observed that the in vivo treatment of Bcl-3 KO mice with SEB plus adjuvant yielded increased cell numbers compared to SEB treatment alone. The numerative increases, however, were not accompanied by increases in IFN-γ production by CD8 T cells. This pattern indicates that the susceptibility to infection of Bcl-3 KO mice is more likely explained by inadequate T-cell effector functions than insufficient numbers of surviving pathogen-specific T cells. Although the Bcl-3 KO mice do not have an absolute defect in IFN-γ production, the decrease in IFN-γ-expressing CD8 T cells is the most striking and reproducible difference observed between the in vivo-activated Bcl-3 KO and wild-type T cells. The frequency of cells producing IFN-γ upon restimulation was markedly decreased in the LPS-treated Bcl-3 KO CD8 T cells, as was the amount of IFN-γ produced per cell. Zhou et al. have shown that the IFN-γ gene is associated with hyperacetylated histone 4 in CD8 T-cell cultures primed and maintained under Tc1 conditions (IL-12, anti-IL-4 MAb) and that hyperacetylation leads to increased production of IFN-γ compared to CD8 T cells cultured in neutral conditions (39). This study further showed that histone deacetylase (HDAC) inhibitors increased IFN-γ production under conditions of neutral stimulation. Bcl-3 has been shown to either enhance or antagonize the activity of different HDAC molecules, depending on the cell type (14, 29, 37). Therefore, Bcl-3-dependent antagonism of HDAC activity could explain why IFN-γ production is decreased but not totally eliminated in the Bcl-3-deficient CD8 cells during restimulation cultures.
The decrease in CD8-specific IFN-γ response upon restimulation of Bcl-3 KO spleen populations indicates that there could be a defect in memory CD8 T-cell formation. However, we have not noted a difference in the memory phenotype of the total T-cell populations comparing Bcl-3 KO cells to wild-type cells in either the spleen or bone marrow (data not shown). Superantigen activation in the presence of LPS is perhaps not the most useful model to assay the development or maintenance of memory T cells for two reasons. First, thymic production eventually replenishes the periphery with naive Vβ8+ T cells, and second, Vβ8 T cells represent a very large percentage of the total T-cell repertoire, from 25% in untreated mice to 35 to 40% in the SEB- plus LPS-treated mice (Fig. 1). It has been recently reported that the development of memory T-cell populations is dramatically influenced by the initial precursor frequency of the antigen-specific T cells (17). This report shows that the antigen-presenting cell-to-responding T-cell ratio is critical to the development of stable effector memory T cells, presumably because competition for presented antigen limits divisions. Therefore, more appropriate studies are required to assess the effect of Bcl-3 deficiency on generation of T-cell memory.
Our data showing that maximal IFN-γ production by antigen-experienced CD8 T cells is dependent on Bcl-3 may conflict with those of Corn et al. (7). In their recent report, they showed Bcl-3-dependent Th2 differentiation during in vitro culture, not Th1, as would be expected in light of our results. Moreover, Bcl-3 KO CD4 cells showed normal Th1 polarization in their in vitro study (7). However, lineage-specific T-cell differences in the requirements for IFN-γ production have been previously demonstrated. At the promoter level, IFN-γ transcription in CD4 cells can be driven by two separate promoter elements, proximal (−70 to −47 bp) and distal (−92 to −72), while CD8 cells only utilize the distal element (1). CD4 cells depend on Stat4 activity to produce IFN-γ after TCR engagement, but CD8 cells do not (5). Another difference is that CD4 cells require T-bet to maintain histone hypermethylation of the IFN-γ promoter region in differentiated Th1 cells (2), whereas CD8 cells can use either T-bet or eomesodermin (27). Therefore, CD4 and CD8 cells do not utilize the same mechanisms to regulate IFN-γ expression.
Although our CD8 IFN-γ data seem different from those of Corn et al., we have similar results in our in vitro studies of the surviving CD4 cells from SEB- plus LPS-treated Bcl-3 KO mice. No significant differences in the percentage of IFN-γ+ cells were observed in the CD4 populations from Bcl-3 KO mice compared to wild-type mice. We observed a slight but significant decrease in the IFN-γ-specific MFI of Bcl-3 KO CD4 cells, and they reported a nonsignificant decrease in MFI compared to wild-type CD4 cells (7). The differences between data reported by Corn et al. and ours may therefore be explained by relatively modest changes in experimental method. For example, we used superantigen in vivo to activate T cells, while they used peptide in vitro. Further experimentation will show whether our results are truly in conflict or whether Bcl-3 simply has different functions in CD4 versus CD8 cells.
The finding that Bcl-3 is not required to rescue adjuvant-activated T cells from clonal contraction was initially surprising because Bcl-3 expression in activated T cells is directly correlated with survival in the same type of model system (22, 23). Bcl-3 transcripts are markedly increased in antigen- plus adjuvant-activated T cells compared to T cells activated with antigen alone. Further, the ectopic expression of Bcl-3, or even a minimal domain of Bcl-3 (24), in antigen-activated T cells confers a survival advantage without adjuvant exposure both in vivo and in vitro. Valenzuela et al. also showed that the IL-12-mediated increase in activated CD8 T-cell survival is dependent on Bcl-3 expression (34). Therefore, multiple lines of evidence have shown that Bcl-3 is sufficient to ensure survival of activated T cells. Although we observed no difference in the net clonal expansion (day 7) with adjuvant in the Bcl-3 KO mice, there is an apparent deficit in adjuvant-induced survival in the T cells that is discernible when the day 7 cell numbers are compared to peak clonal expansion cell numbers (day 3; Fig. 1). Moreover, the Bcl-3 KO T cells exhibit a decrease in survival after peak expansion when cultured ex vivo under growth factor withdrawal conditions compared to wild-type T cells (Fig. 2B). Together, these data indicate that Bcl-3 is an adjuvant-induced survival factor, but its modes of action are more complicated than initially expected.
Our data also show that Bcl-3 may not be absolutely required for survival because it is redundant to other similar factors. Goudeau et al. have demonstrated that control of NF-κB activity via other IκB molecules is required for T- and B-cell survival in vivo (11). This report and others also demonstrated reciprocal increases in IκBɛ expression in IκBα KO mice. Additionally, IκBα and IκBβ were increased in various tissues in the IκBɛ-deficient mice (21, 38); thus, the mild phenotype observed in IκBɛ KO mice suggests that compensatory functions exist within the IκB family (21). Transcript levels of other IκB family molecules were also increased in the gene array screens comparing antigen- plus adjuvant-activated T cells to antigen-only-activated T cells (23). Therefore, the redundancy built into the IκB molecule family supports the possibility that multiple members of the family could function in place of Bcl-3 to direct survival of adjuvant-activated T cells. Indeed, our results demonstrated the ability of forced expression of IκB molecules to rescue antigen-only-activated T cells from death, similar to Bcl-3 expression. A recent report by Mittal et al. has shown that the preferential decrease of NF-κB p65 from the nucleus of activated T cells accompanied by the retention of c-Rel in the nucleus correlated with the kinetics of T-cell death after activation. Our laboratory has shown previously that expression of c-Rel in activated T cells leads to increased cell death (24). Mittal et al. suggest that the high affinity of IκB molecules for p65 leads to its nuclear export and a decrease in expression of the p65-associated antiapoptotic proteins. They further suggest that increased expression of IκB molecules could lead to a delay in cell death by increasing the amount of IκB available to bind c-Rel, thereby decreasing the expression of proapoptotic proteins (25). It is possible that the survival effect we found when expressing various IκB family members is attributable to their ability to remove c-Rel from the nucleus.
Because T-cell activation in the absence of adjuvants can lead to functional anergy or tolerance of T cells (15, 35, 36) and because Bcl-3 is associated with survival and effector function of T cells when stimulated in the presence of adjuvants and/or IL-12 (12, 22, 23, 34), we also assessed the ability of the cells that had survived the day 7 treatment with SEB in the absence and presence of LPS to proliferate during in vitro restimulation with SEB. A lack of response in vitro would have indicated the emergence of nonresponsive cells in the surviving population, even in the face of normal levels of survival after activation. However, the surviving Bcl-3 KO T cells showed no defect in proliferation detected upon in vitro restimulation. Similar percentages of cells entered the cell cycle, and these cells went through nearly the same average number of cell divisions as the wild-type T cells. Therefore, Bcl-3 KO T cells do not become anergic in this model system.
One difference in the proliferation patterns observed was that the cells from Bcl-3 KO mice that had received SEB alone proliferated much more than their wild-type counterparts. In fact, the Bcl-3 KO cells stimulated with SEB alone proliferated almost exactly as much as wild-type cells stimulated with SEB plus LPS. This LPS-independent proliferation was observed in both CD4 and CD8 T-cell populations. Proliferation in the cultures from wild-type mice that received only SEB was decreased slightly compared to untreated wild-type mice and was markedly lower than that exhibited by wild-type cells that had seen SEB plus LPS in vivo, indicating establishment of a tolerant population that survived in wild-type mice after receiving only SEB. Although we cannot, at present, determine why more SEB-surviving cells proliferated in the Bcl-3 KO cultures, we can conclude that SEB treatment of Bcl-3 KO mice in the absence of adjuvant does not result in induction of tolerance in the surviving T-cell population.
With these results, we have demonstrated that, although Bcl-3 may be sufficient to mediate survival of antigen-activated T cells, it is not always required for adjuvant-induced survival of activated T cells, as was true for IL-12-increased survival (34). It is likely that other IκB family members act in a redundant manner in the absence of Bcl-3 to ensure survival of T cells that have been activated in the presence of adjuvant. Most importantly, Bcl-3 is required for the LPS-associated increase of IFN-γ by previously activated CD8 T cells during an effector recall assay.
Acknowledgments
This work was supported by USPHS grants AI51377 and AI059023, the Commonwealth of Kentucky Research Challenge Trust Fund, the Kentucky Lung Cancer Research Program, and the Jewish Hospital Foundation.
We thank Ulrich Siebenlist for his gift of Bcl-3 KO breeders, Carolyn Casella and Bruce Thompson for helpful suggestions and comments regarding this project, and the staff of the Research Resource Facility at the University of Louisville for animal care.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Aune, T. M., L. A. Penix, M. R. Rincon, and R. A. Flavell. 1997. Differential transcription directed by discrete gamma interferon promoter elements in naive and memory (effector) CD4 T cells and CD8 T cells. Mol. Cell. Biol. 17:199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avni, O., D. Lee, F. Macian, S. J. Szabo, L. H. Glimcher, and A. Rao. 2002. TH cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat. Immunol. 3:643-651. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield, C. D., D. C. Arthur, G. Frizzera, E. G. Levine, B. A. Peterson, and K. J. Gajl-Peczalska. 1983. Nonrandom chromosome abnormalities in lymphoma. Cancer Res. 43:2975-2984. [PubMed] [Google Scholar]
- 4.Carragher, D., R. Johal, A. Button, A. White, A. Eliopoulos, E. Jenkinson, G. Anderson, and J. Caamano. 2004. A stroma-derived defect in NF-kappaB2-/- mice causes impaired lymph node development and lymphocyte recruitment. J. Immunol. 173:2271-2279. [DOI] [PubMed] [Google Scholar]
- 5.Carter, L. L., and K. M. Murphy. 1999. Lineage-specific requirement for signal transducer and activator of transcription (Stat)4 in interferon gamma production from CD4+ versus CD8+ T cells. J. Exp. Med. 189:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Corn, R. A., C. Hunter, H. C. Liou, U. Siebenlist, and M. R. Boothby. 2005. Opposing roles for RelB and Bcl-3 in regulation of T-Box expressed in T Cells, GATA-3, and Th effector differentiation. J. Immunol. 175:2102-2110. [DOI] [PubMed] [Google Scholar]
- 8.Fenton, R. G., P. Marrack, J. W. Kappler, O. Kanagawa, and J. G. Seidman. 1988. Isotypic exclusion of gamma delta T cell receptors in transgenic mice bearing a rearranged beta-chain gene. Science 241:1089-1092. [DOI] [PubMed] [Google Scholar]
- 9.Franzoso, G., L. Carlson, T. Scharton-Kersten, E. W. Shores, S. Epstein, A. Grinberg, T. Tran, E. Shacter, A. Leonardi, M. Anver, P. Love, A. Sher, and U. Siebenlist. 1997. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity 6:479-490. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Angelats, M., C. D. Bortner, and J. A. Cidlowski. 2000. Cell volume regulation in immune cell apoptosis. Cell Tissue Res. 301:33-42. [DOI] [PubMed] [Google Scholar]
- 11.Goudeau, B., F. Huetz, S. Samson, J. P. Di Santo, A. Cumano, A. Beg, A. Israel, and S. Memet. 2003. IkappaBalpha/IkappaBepsilon deficiency reveals that a critical NF-kappaB dosage is required for lymphocyte survival. Proc. Natl. Acad. Sci. USA 100:15800-15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundstrom, S., P. Anderson, P. Scheipers, and A. Sundstedt. 2004. Bcl-3 and NFkappaB p50-p50 homodimers act as transcriptional repressors in tolerant CD4+ T cells. J. Biol. Chem. 279:8460-8468. [DOI] [PubMed] [Google Scholar]
- 13.Hildeman, D. A., T. Mitchell, B. Aronow, S. Wojciechowski, J. Kappler, and P. Marrack. 2003. Control of Bcl-2 expression by reactive oxygen species. Proc. Natl. Acad. Sci. USA 100:15035-15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamaluddin, M., S. Choudhary, S. Wang, A. Casola, R. Huda, R. P. Garofalo, S. Ray, and A. R. Brasier. 2005. Respiratory syncytial virus-inducible BCL-3 expression antagonizes the STAT/IRF and NF-κB signaling pathways by inducing histone deacetylase 1 recruitment to the interleukin-8 promoter. J. Virol. 79:15302-15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney, E. R., K. A. Pape, D. Y. Loh, and M. K. Jenkins. 1994. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1:327-339. [DOI] [PubMed] [Google Scholar]
- 16.Khoruts, A., A. Mondino, K. A. Pape, S. L. Reiner, and M. K. Jenkins. 1998. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J. Exp. Med. 187:225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzo, A. L., K. D. Klonowski, B. A. Le, P. Borrow, D. F. Tough, and L. Lefrancois. 2005. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 6:793-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maxwell, J. R., C. Ruby, N. I. Kerkvliet, and A. T. Vella. 2002. Contrasting the roles of costimulation and the natural adjuvant lipopolysaccharide during the induction of T cell immunity. J. Immunol. 168:4372-4381. [DOI] [PubMed] [Google Scholar]
- 19.McKeithan, T. W., J. D. Rowley, T. B. Shows, and M. O. Diaz. 1987. Cloning of the chromosome translocation breakpoint junction of the t(14;19) in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 84:9257-9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeithan, T. W., G. S. Takimoto, H. Ohno, V. S. Bjorling, R. Morgan, B. K. Hecht, I. Dube, A. A. Sandberg, and J. D. Rowley. 1997. BCL3 rearrangements and t(14;19) in chronic lymphocytic leukemia and other B-cell malignancies: a molecular and cytogenetic study. Genes Chromosomes Cancer 20:64-72. [PubMed] [Google Scholar]
- 21.Memet, S., D. Laouini, J. C. Epinat, S. T. Whiteside, B. Goudeau, D. Philpott, S. Kayal, P. J. Sansonetti, P. Berche, J. Kanellopoulos, and A. Israel. 1999. IkappaBepsilon-deficient mice: reduction of one T cell precursor subspecies and enhanced Ig isotype switching and cytokine synthesis. J. Immunol. 163:5994-6005. [PubMed] [Google Scholar]
- 22.Mitchell, T. C., D. Hildeman, R. M. Kedl, T. K. Teague, B. C. Schaefer, J. White, Y. Zhu, J. Kappler, and P. Marrack. 2001. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat. Immunol. 2:397-402. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell, T. C., T. K. Teague, D. A. Hildeman, J. Bender, W. A. Rees, R. M. Kedl, B. Swanson, J. W. Kappler, and P. Marrack. 2002. Stronger correlation of bcl-3 than bcl-2, bcl-xL, costimulation, or antioxidants with adjuvant-induced T cell survival. Ann. N. Y. Acad. Sci. 975:114-131. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell, T. C., B. S. Thompson, J. O. Trent, and C. R. Casella. 2002. A short domain within Bcl-3 is responsible for its lymphocyte survival activity. Ann. N. Y. Acad. Sci. 975:132-147. [DOI] [PubMed] [Google Scholar]
- 25.Mittal, A., S. Papa, G. Franzoso, and R. Sen. 2006. NF-kappaB-dependent regulation of the timing of activation-induced cell death of T lymphocytes. J. Immunol. 176:2183-2189. [DOI] [PubMed] [Google Scholar]
- 26.Ong, S. T., M. L. Hackbarth, L. C. Degenstein, D. A. Baunoch, J. Anastasi, and T. W. McKeithan. 1998. Lymphadenopathy, splenomegaly, and altered immunoglobulin production in BCL3 transgenic mice. Oncogene 16:2333-2343. [DOI] [PubMed] [Google Scholar]
- 27.Pearce, E. L., A. C. Mullen, G. A. Martins, C. M. Krawczyk, A. S. Hutchins, V. P. Zediak, M. Banica, C. B. DiCioccio, D. A. Gross, C. A. Mao, H. Shen, N. Cereb, S. Y. Yang, T. Lindsten, J. Rossant, C. A. Hunter, and S. L. Reiner. 2003. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302:1041-1043. [DOI] [PubMed] [Google Scholar]
- 28.Riemann, M., R. Endres, S. Liptay, K. Pfeffer, and R. M. Schmid. 2005. The IkappaB protein Bcl-3 negatively regulates transcription of the IL-10 gene in macrophages. J. Immunol. 175:3560-3568. [DOI] [PubMed] [Google Scholar]
- 29.Rocha, S., A. M. Martin, D. W. Meek, and N. D. Perkins. 2003. p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-kappaB subunit with histone deacetylase 1. Mol. Cell Biol. 23:4713-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salem, M. L., A. N. Kadima, D. J. Cole, and W. E. Gillanders. 2005. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J. Immunother. 28:220-228. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz, E. M., P. Krimpenfort, A. Berns, and I. M. Verma. 1997. Immunological defects in mice with a targeted disruption in Bcl-3. Genes Dev. 11:187-197. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta, S., P. M. Chilton, and T. C. Mitchell. 2005. Adjuvant-induced survival signaling in clonally expanded T cells is associated with transient increases in pAKT levels and sustained uptake of glucose. Immunobiology 210:647-659. [DOI] [PubMed] [Google Scholar]
- 33.Thompson, B. S., and T. C. Mitchell. 2004. Measurement of daughter cell accumulation during lymphocyte proliferation in vivo. J Immunol. Methods 295:79-87. [DOI] [PubMed] [Google Scholar]
- 34.Valenzuela, J. O., C. D. Hammerbeck, and M. F. Mescher. 2005. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J. Immunol. 174:600-604. [DOI] [PubMed] [Google Scholar]
- 35.Vella, A. T., J. E. McCormack, P. S. Linsley, J. W. Kappler, and P. Marrack. 1995. Lipopolysaccharide interferes with the induction of peripheral T cell death. Immunity 2:261-270. [DOI] [PubMed] [Google Scholar]
- 36.Vella, A. T., T. Mitchell, B. Groth, P. S. Linsley, J. M. Green, C. B. Thompson, J. W. Kappler, and P. Marrack. 1997. CD28 engagement and proinflammatory cytokines contribute to T cell expansion and long-term survival in vivo. J. Immunol. 158:4714-4720. [PubMed] [Google Scholar]
- 37.Wessells, J., M. Baer, H. A. Young, E. Claudio, K. Brown, U. Siebenlist, and P. F. Johnson. 2004. BCL-3 and NF-kappaB p50 attenuate lipopolysaccharide-induced inflammatory responses in macrophages. J. Biol. Chem. 279:49995-50003. [DOI] [PubMed] [Google Scholar]
- 38.Whiteside, S. T., J. C. Epinat, N. R. Rice, and A. Israel. 1997. I kappa B epsilon, a novel member of the I kappa B family, controls RelA and c-Rel NF-kappa B activity. EMBO J. 16:1413-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou, W., S. Chang, and T. M. Aune. 2004. Long-range histone acetylation of the Ifng gene is an essential feature of T cell differentiation. Proc. Natl. Acad. Sci. USA 101:2440-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]