Abstract
Porphyromonas gingivalis, a gram-negative anaerobic bacterium, is a recognized periodontopathogen. It exhibits a high degree of aerotolerance and is able to survive in host cells, indicating that efficient oxidative stress protection mechanisms must be present in this organism. Manganese homeostasis plays a major role in oxidative stress protection in a variety of organisms; however, the transport and role of this metal in P. gingivalis is not well understood. Analysis of the genome of P. gingivalis W83 revealed the presence of two genes encoding homologs of a ferrous iron transport protein, FeoB1 and FeoB2. FeoB2 has been implicated in manganese accumulation in P. gingivalis. We sought to determine the role of the FeoB2 protein in metal transport as well as its contribution to resistance to oxygen radicals. Quantitative reverse transcriptase PCR analyses demonstrated that expression of feoB2 is induced in the presence of oxygen. The role of FeoB2 was investigated using an isogenic mutant strain deficient in the putative transporter. We characterized the FeoB2-mediated metal transport using 55Fe2+ and 54Mn2+. The FeoB2-deficient mutant had dramatically reduced rates of manganese uptake (0.028 pmol/min/107 bacteria) compared with the parental strain (0.33 pmol/min/107 bacteria) (after 20 min of uptake using 50 nM of 54Mn2+). The iron uptake rates, however, were higher in the mutant strain (0.75 pmol/min/107 bacteria) than in the wild type (0.39 pmol/min/107 bacteria). Interestingly, reduced survival rates were also noted for the mutant strain after exposure to H2O2 and to atmospheric oxygen compared to the parental strain cultured under the same conditions. In addition, in vitro infection of host cells with the wild type, the FeoB2-deficient mutant, and the same-site revertant revealed that the mutant had a significantly decreased capability for intracellular survival in the host cells compared to the wild-type strain. Our results demonstrate that feoB2 encodes a major manganese transporter required for protection of the bacterium from oxidative stress generated by atmospheric oxygen and H2O2. Furthermore, we show that FeoB2 and acquisition of manganese are required for intracellular survival of P. gingivalis in host cells.
Reactive oxygen species (ROS), including the superoxide anion (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (HO·), are generated by the incomplete reduction of oxygen. A variety of living cells, including bacterial and eukaryotic cells, produce ROS (45). ROS are much more reactive than molecular oxygen and can cause severe damage to nucleic acids, cell membranes, and proteins (15). Virtually all organisms have evolved complex defense and repair mechanisms to protect them from the damaging effects of ROS (15). Although the mechanisms are especially important for strict anaerobes and “nanaerobes” (defined as demonstrating growth inhibition by 5 μM of dissolved oxygen), little is known about ROS protection in these organisms.
Porphyromonas gingivalis is a gram-negative, nanaerobic bacterium (3) whose role in the onset and progression of periodontal disease has been established (14, 31). The organism normally grows without oxygen (or in the presence of a minimal concentration of oxygen) and is found in anaerobic periodontal pockets. However, to reach the periodontal pocket, it is transferred through different sites in the oral cavity (saliva, tongue, and buccal mucosa) where it is exposed to oxygen (10, 32, 53). Furthermore, P. gingivalis has been demonstrated to invade and survive within eukaryotic cells where it is exposed to intracellular ROS (30, 37, 43). Lastly, reactive oxygen species are generated by macrophages and neutrophils upon encounter with bacterial cells (5). Thus, in order to survive oxygen exposure, P. gingivalis has had to develop efficient oxidative stress protection mechanisms. Although important for P. gingivalis virulence, the mechanisms of oxygen tolerance are not well known in this organism.
Manganese homeostasis plays a major role in oxidative stress protection of a variety of bacteria (21, 26, 46, 47, 57); however, the effect of manganese on the protection of P. gingivalis from oxidative stress has not been investigated. Furthermore, the mechanisms involved in transport of the metal in P. gingivalis are not well defined. FeoB protein was initially identified and characterized as a cytoplasmic membrane ferrous iron transporter in Escherichia coli K-12 (23, 50). Currently, genes encoding homologs of the FeoB protein are present in a number of bacteria. Also, FeoB proteins of several bacteria were demonstrated to be required for high-affinity ferrous-iron uptake (23, 41, 50, 54). Interestingly, in the P. gingivalis W83 genome two gene products exhibited sequence similarity to the FeoB of E. coli K-12. FeoB1, encoded by PG1138, is 844 amino acids in length and shows 52.6% similarity and 39.2% identity to the E. coli FeoB. FeoB2, encoded by PG0930, is 725 amino acids in length and exhibits 40% similarity and 31% identity with the E. coli FeoB. While P. gingivalis FeoB1 was shown to transport iron, FeoB2 has been implicated in manganese accumulation (11). Surprisingly, the protein designated FeoB2 had only a minor contribution to the protection of P. gingivalis from oxidative stress. In the present study, we show that FeoB2 is a major manganese transporter protein in P. gingivalis and plays a role in oxidative stress protection and survival of the bacteria in host cells.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain | Plasmid | Description | Reference or source |
|---|---|---|---|
| P. gingivalis | |||
| W83 | Parental strain | 33 | |
| V2814 | Emr; FeoB2-defective mutant | This study | |
| V2831 | Complemented strain of feoB2 mutant | This study | |
| E. coli | |||
| One Shot Top 10 | Chemically competent cell | Invitrogen | |
| pCR 2.1 | Invitrogen | ||
| V2198 | pVA2198 | Carries 2-kb ermF-ermAM cassette between KpnI and BamHI | 16 |
| V2762 | pVA2762 | Apr; pCR 2.1 containing the 2.2-kb feoB2 fragment PCR amplified from P. gingivalis W83 genomic DNA | This study |
| V2760 | pVA2760 | Apr Emr; pVA2762 with the ermF-ermB cassette inserted at the NruI site of feoB2 | This study |
Media and growth conditions.
Porphyromonas gingivalis W83 was grown and maintained as described previously (33). Briefly, P. gingivalis cultures were prepared in an anaerobic atmosphere composed of 10% H2, 10% CO2, and 80% N2 at 37°C. Blood agar plates (TSA II, 5% sheep blood; BBL, Cockeysville, MD) were used for maintenance of P. gingivalis. Brain heart infusion broth (BHI; Difco Laboratories, Detroit, MI) supplemented with hemin (5 μg/ml; Sigma, St. Louis, MO), yeast extract (5 mg/ml), cysteine (1 mg/ml; Sigma, St. Louis, MO), and vitamin K3 (1 μg/ml; Sigma, St. Louis, MO) were used for preparation of liquid cultures of P. gingivalis.
For growth and metal transport studies, P. gingivalis cultures were prepared using BHI that was depleted of divalent and transition metals by treatment with Chelex 100 chelating ion-exchange resin (Bio-Rad, Hercules, CA). MgSO4, CaCl2, ZnCl2 (100 μM for each metal), and protoporphyrin IX (5 μg/ml) were then supplemented into Chelex-treated BHI.
Growth studies were performed as described below using Chelex-treated BHI.
Transport studies were carried out as described below using mycoplasma broth (BBL, Cockeysville, MD) also treated with Chelex to limit the availability of metal ions.
Escherichia coli was grown at 37°C in Luria-Bertani (LB) medium or on LB agar. Clindamycin (0.5 μg/ml), erythromycin (300 μg/ml), and carbenicillin (50 μg/ml) were used as required for selection and maintenance of strains.
Analysis of bacterial growth.
Growth studies were performed with bacterial inocula at an optical density at 660 nm (OD660) of 0.15 using Chelex-treated BHI supplemented with various concentrations of manganese (25 μM, 200 nM). Medium without manganese served as a negative control. Bacterial cultures were incubated anaerobically and in the presence of 6% oxygen. In addition, a further set of cultures was incubated in the presence of 50 μM hydrogen peroxide. Bacterial cell densities were monitored spectrophotometrically at various time intervals.
Exposure of P. gingivalis W83 cultures to oxidative stress and metal chelators for quantitative RNA analysis.
Anaerobic overnight cultures (OD660, 1.3 to 1.5) were diluted in an anaerobic chamber with anaerobic growth medium (BHI) to an OD660 of 0.2 and incubated anaerobically to an OD660 of 0.4. The culture was divided into four aliquots that were then incubated under various conditions: (i) anaerobically, (ii) aerobically with shaking, (iii) anaerobically with the addition of 200 μM hydrogen peroxide, or (iv) anaerobically with the addition of 5 mM EDTA. Cells were harvested by centrifugation, and RNA was isolated using the TRIzol Max bacterial RNA isolation kit (Invitrogen, San Diego, CA).
Quantitative RT-PCR analysis.
Quantitative RT-PCR was carried out using the TaqMan One-Step PCR master mix kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. DNA-free total RNA isolated from bacterial cultures grown as described above served as a template. Probe (5′-ACACGGGCTACGGGTTTCCTGCC-3′) and primers (forward, 5′-AGGTATTCTCCATCCTTTGCTGAA-3′; reverse, 5′-GAGGAATCCGCTCAACCAATC-3′) were used to study FeoB2 expression. A calibration curve was constructed using serial fivefold dilutions of 100 ng total RNA. Experimental samples were tested in triplicate under the conditions recommended by the manufacturer using 20 ng of total RNA (except for 16S rRNA, where we used 0.2 ng of total RNA). Reverse transcriptase was omitted in samples serving as negative controls. The experiments were performed in the ABI Prism 7900 sequence detection system (Applied Biosystems, Foster City, CA) with the thermal profile consisting of 1 cycle of 30 min at 48°C to synthesize cDNA, 1 cycle of 10 min at 95°C, and 40 cycles each consisting of 15s at 95°C and 1 min at 60°C. The threshold cycle was determined to provide the optimal standard-curve values (0.98 to 1.0). To control for RNA quantity, a probe specific for 16S rRNA was used. As a control for constitutive expression of mRNA, we used PG1357, which encodes a major outer membrane protein.
Construction of FeoB2-deficient mutant.
A DNA fragment containing the feoB2 gene (PG0930) disrupted by the ermF-ermB (ermB previously named ermAM) (40) cassette (inserted into the NruI site located at 300 bp of feoB2) was used to electroporate P. gingivalis W83 as described previously (16). A double-crossover mutant strain containing an insertionally inactivated feoB2 gene was selected using BHI plates supplemented with clindamycin. Genomic DNA from the parental and mutant strains was isolated and analyzed by PCR (forward primer, 5′-CCGAAGGTGCATTCTCGTATG-3′ located at 215 to 235 bp of feoB2; reverse primer, 5′-CAGTACCGATACTTGTCACTC-3′ located at 612 to 632 bp of feoB2) and Southern blotting to confirm the predicted disruption of the feoB2 gene by the antibiotic resistance cassette. The feoB2 mutant of P. gingivalis W83 was designated V2814.
Generation of same-site revertant.
A 2-kb DNA fragment carrying the intact feoB2 gene from P. gingivalis W83 was used to electroporate V2814 (P. gingivalis W83 feoB2 mutant). Immediately after electroporation, 1 ml of prereduced medium was added directly to the cuvette. Following anaerobic incubation at 37°C for 18 h, 1 mM hydrogen peroxide was added, and the cultures were incubated at 37°C anaerobically for an additional 40 min (since feoB2 mutant cells are extremely sensitive to hydrogen peroxide, this additional step was used to select revertants). Two hundred microliters of the mixtures was spread on BHI agar plates. The resulting colonies were tested for abolished clindamycin resistance on BHI plates supplemented with clindamycin (0.5 μg/ml). The intact feoB2 in the clindamycin-sensitive colonies was confirmed by PCR.
Metal transport assay.
P. gingivalis cultures were started from colonies grown on TSA II blood agar plates. Strains were grown anaerobically at 37°C for 48 h in enriched BHI until turbid growth was observed. One hundred microliters of this culture was used to prepare 3 ml of overnight culture in Chelex-treated BHI medium supplemented with Mg2+, Ca2+, Zn2+, and protoporphyrin IX (prepared as described above). This culture was passaged twice into Chelex-treated BHI medium using a 1:10 dilution. Cells were then harvested by centrifugation at ambient temperature and suspended to an OD660 of 0.7 in Chelex-treated mycoplasma broth. Uptake studies were performed with 55Fe2+ (103.46 mCi/mg) and 54Mn2+ (86.7 mCi/mg) (Perkin-Elmer/NEN, Boston, MA).
(i) Iron uptake study.
The MultiScreen assay system (Millipore, Bedford, MA) was used for uptake studies. The 96-well plate was prewetted with 150 μl of Chelex-treated mycoplasma broth to prevent nonspecific binding of iron to the filter membranes. Fifty microliters (2 × 107 cells) of bacterial cells was then added to selected wells. A stock solution of 55FeCl2 (0.1 μCi/μl) was prepared immediately before the assay. 55FeCl2 (1.0 μCi) was then added to selected wells of the plate. The final concentration of 55FeCl2 in each sample was 0.9 μM. Triplicate samples were prepared for each time point. The plates were incubated anaerobically at 37°C for 1 min, 5 min, 10 min, and 20 min. As a control for energy-independent binding and uptake, cells incubated anaerobically for 20 min with the proton ionophore carbonyl cyanide m-chlorophenylhydrazone (CCCP; 210 μg/ml) were used for the assay. Liquid was then removed by vacuum aspiration. The plates were washed three times with Tris-buffered saline (125 mM NaCl, 25 mM Tris [pH 8.0]) to remove any free 55Fe2+. Filters were air dried and isolated with a MultiScreen punch (Millipore, Bedford, MA). The filters were collected in scintillation vials, and 2 ml of BioSafe II scintillation cocktail (Research Products International, Inc., Mount Prospect, IL) was added to each vial. The samples were counted in a Beckman LS6500 liquid scintillation counter (Beckman Coulter, Fullerton, CA). 55Fe2+ uptake was measured by detection of beta radiation in channel 350 (25, 36). Active binding and uptake were determined by subtracting counts-per-minute values obtained from experiments performed using cells treated with CCCP from those obtained using untreated cells.
(ii) Manganese uptake assay.
This assay was carried out as described above (iron uptake assay) with some modifications. Manganese stock solution (0.01 μCi/μl) was prepared immediately before the assay. Solution (0.1 μCi) was added to selected wells at a final 54Mn2+ concentration of 0.05 μM. The plates were incubated at room temperature for periods ranging from 1 min to 20 min. Parallel uptake experiments were carried out using cells that were kept on ice as a control for energy-independent binding. Radioactivity was determined as described above, using channel 850 to detect gamma emission (25).
Sensitivity of P. gingivalis to air with and without hydrogen peroxide.
Overnight P. gingivalis cultures were diluted with enriched BHI to an OD660 of 0.3 and grown anaerobically at 37°C until they reached an OD660 of 0.5. The mid-logarithmic cultures were divided into three groups. The first was incubated anaerobically at 37°C and served as a control. The second was grown aerobically at 37°C with 0.5 mM hydrogen peroxide and agitation (200 rpm). The third was also incubated aerobically with agitation, but without hydrogen peroxide. Culture samples were withdrawn at various time intervals and placed in an anaerobic chamber, and serial dilutions were made using anaerobic BHI. Appropriate dilutions were plated on blood agar plates; the plates were then incubated anaerobically at 37°C for 7 days.
Infection of host cells with P. gingivalis.
Assays were carried out as described by Ueshima et al., with some modifications (52). Human umbilical vein endothelial cells (HUVECs) were maintained in endothelial growth medium (EGM; Cambrex Corporation, Walkersville, MD) at 37°C in 95% air-5% CO2 under standard conditions. For experiments, cells were seeded at 2.5 × 104 cells/well in 12-well tissue culture plates. P. gingivalis cells from mid-logarithmic growth phase (OD660, 0.5) were harvested, washed twice with phosphate-buffered saline (PBS), and suspended in oxygen-depleted serum-free EGM. Bacterial cells were added to host cell-containing wells at a multiplicity of infection of 100 (30). Plates were incubated anaerobically at 37°C for 30 min. After washing in EGM, extracellular bacteria were killed by incubation of the infected cells with medium containing 300 μg/ml of gentamicin and 400 μg/ml of metronidazole for periods of time ranging from 1 h to 4 h. The infected cells were washed in PBS and suspended in 500 μl of anaerobic BHI medium. Following three freeze-thaw cycles, an additional 500 μl of enriched anaerobic BHI was added to selected wells of the culture plate. The total 1 ml of the mixture was then mixed with 3 ml of enriched anaerobic BHI, and 200 μl of the diluted mixture was plated on blood agar plates and incubated anaerobically at 37°C for 11 days. The ability of the bacteria to survive in host cells was determined by counting the colonies formed.
Confocal laser scanning microscopy.
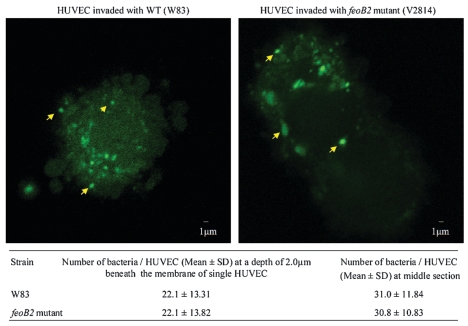
Confocal imaging was performed as described previously with some modifications (52). Briefly, cells from overnight cultures of P. gingivalis W83 were harvested, washed twice in PBS, and resuspended in PBS. The cells were then labeled by the addition of 2,7-bis-(2-carboxyethyl)-5 (and 6-)-carboxyfluorescein (BCECF) to a final concentration of 5 μM and incubated anaerobically at 37°C for 30 min. Cells were harvested and suspended in EGM. HUVECs were prepared on glass coverslips and infected with labeled P. gingivalis as described above. After specific time periods, HUVECs were fixed in 10% formaldehyde in PBS at an ambient temperature for 15 min, washed in PBS, and treated with PBS containing 50 mM NH4Cl and 0.3% Tween 20 for 10 min at ambient temperature. Following PBS washes, coverslips were mounted in Antifade (Molecular Probes, Eugene, OR) and examined using a Carl Zeiss LSM 510 Meta confocal imaging microscope with a Zeiss PlanApo 63×/1.4 oil objective. The gain settings and intermediate magnifications were optimized for the first image and then kept constant. Serial sections were obtained at 0.5-μm intervals for single HUVECs. The number of intracellular bacteria was quantified on the image of the central section and the top 2-μm section beneath the cell membrane.
RESULTS
Effect of manganese and feoB2 disruption on anaerobic and aerobic growth.
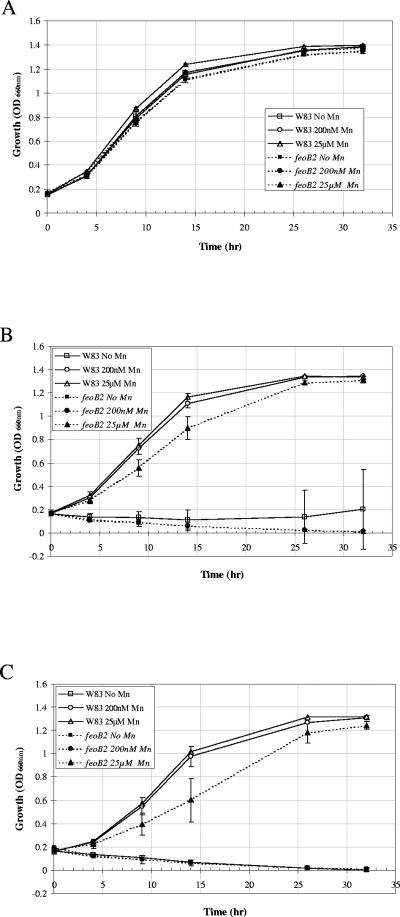
Both anaerobic and aerobic growth of the parental strain, P. gingivalis W83, and the feoB2 mutant were compared in the presence and absence of manganese. As shown in Fig. 1A, manganese had no effect on the growth of P. gingivalis W83 in anaerobic conditions. Also, no differences were found between the growth of the parent and the feoB2 mutant; both the shapes of the growth curves and the cell densities after 33 h are similar. These results indicate that manganese is not required for growth of P. gingivalis in anaerobic conditions. In addition, insertion of the ermF-ermB cassette into the genome of P. gingivalis W83 did not change the ability of the strain to grow under anaerobic conditions. More importantly, they demonstrate that FeoB2 is not required for anaerobic growth of the bacteria. However, manganese-dependent differences in P. gingivalis W83 growth were noted when the strain was incubated in the presence of 6% O2. No growth was observed in both the parental and the mutant strains in the absence of manganese (Fig. 1B). Addition of 200 nM Mn2+ to the culture medium restored the growth of the parental strain, thus indicating that manganese is required for aerobic growth of P. gingivalis. The observation that the growth of the mutant strain was not restored in the presence of 200 nM Mn2+ suggested that manganese was not being transported at such low concentrations and that the FeoB2 protein may be a high-affinity manganese transporter. Growth of the mutant strain was partially restored in the presence of 25 μM of manganese, suggesting that other lower-affinity manganese transporters may be functional in P. gingivalis. However, the 30% and 20% reductions in the growth rate of the mutant strain after 9 h and 14 h of incubation, respectively, indicated FeoB2 is required for optimal growth of P. gingivalis in the presence of oxygen, even at high manganese concentrations.
FIG. 1.
Growth curves for P. gingivalis strains. Overnight cultures were diluted in an anaerobic chamber to an OD660 of 0.15 with Chelex-treated anaerobic medium (A) or saturated with 6% oxygen (B and C) growth medium. Cultures were incubated in the presence or absence of manganese anaerobically (A), aerobically (in the presence of 6% oxygen) (B), and anaerobically in the presence of peroxide (100 μM) (C) at 37°C. Growth was monitored as the increase in OD660 versus time. Error bars indicate standard deviations for triplicate samples. feoB2, feoB2 mutant.
We also investigated the role of manganese and FeoB2 on the growth of P. gingivalis in the presence of peroxide. As shown in Fig. 1C, addition of 25 μM of Mn2+ to the culture medium rescued the growth of both strains, suggesting that this metal plays a role in protection of P. gingivalis from peroxide stress. The 30% and 40% reductions in growth of the mutant strain after 9 h and 14 h of incubation, respectively, indicated that the FeoB2 protein is required for optimal growth of the bacteria in the presence of peroxide with the high manganese concentrations. Furthermore, whereas the parental strain grew in the presence of 200 nM of Mn2+, no growth was observed in the mutant strain, indicating that FeoB2 is also indispensable for protection of the bacteria from peroxide in the presence of low manganese concentrations (Fig. 1C).
FeoB2 is a major manganese transporter in P. gingivalis.
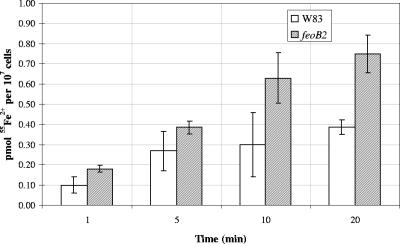
To investigate the role of the P. gingivalis FeoB2 protein in divalent metal cation transport, the feoB2 mutant V2814 and wild-type W83 were examined for their ability to acquire 55Fe2+ and 54Mn2+. Iron uptake in P. gingivalis occurred very rapidly and, whereas saturation was not reached in the mutant strain, the parental W83 strain showed saturation after 5 min of incubation (Fig. 2). Both mutant and wild-type strains exhibited significant iron uptake after 20 min of incubation (Fig. 2). Interestingly, the iron uptake level was 1.5- to 2-fold higher in the mutant strain throughout the experiment than in the parental strain, indicating that FeoB2 is not a major iron transporter in P. gingivalis.
FIG. 2.
Iron uptake assay. 55Fe2+ uptake was measured in picomoles per 107 cells using a range of extracellular radiolabeled iron concentrations and various incubation periods. Active uptake was calculated by subtracting the values of experiments performed in the presence of CCCP from the values of experiments performed in the absence of the protonophore. Error bars indicate standard deviations from triplicate samples. feoB2, feoB2 mutant.
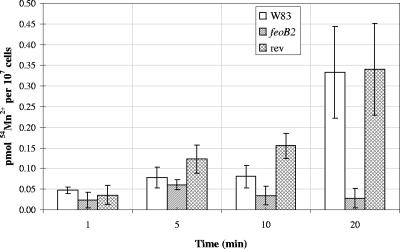
Next, we investigated the involvement of FeoB2 in manganese transport. As shown in Fig. 3, uptake of manganese was accomplished in P. gingivalis W83 using 50 nM of extracellular 54Mn2+. These data indicate that a high-affinity manganese transporter is present in the bacteria. Interestingly, the feoB2 mutant exhibited a decrease in the uptake of 54Mn2+ throughout the course of the experiment compared to the wild-type strain (approximately 12-fold reduction observed after 20 min of incubation) (Fig. 3). To further confirm that feoB2 mutation played a role in the reduction of manganese uptake, we examined a same-site revertant (V2831) for its ability to transport manganese. As shown in Fig. 3, this strain transports manganese similarly to the parental strain. Thus, we conclude that FeoB2 is a major manganese transporter in P. gingivalis.
FIG. 3.
Manganese uptake assay. 54Mn2+ uptake was measured in picomoles per 107 cells using 50 nM radiolabeled manganese over a 20-min incubation period. Active uptake was calculated by subtracting the values of experiments performed on ice (4°C) from the values of experiments performed at 37°C. Error bars indicate standard deviations from triplicate samples. feoB2, feoB2 mutant; rev, same-site revertant.
Induction of feoB2 transcription in response to oxidative stress.
The effects of oxygen, hydrogen peroxide, and divalent metals on expression of feoB2 are shown in Table 2. In the presence of oxygen, elevated expression of feoB2 was observed. Expression was time dependent, and 1.28-fold, 2.52-fold, and 5.48-fold increases were observed after 5 min, 15 min, and 30 min, respectively, of exposure of P. gingivalis cultures to atmospheric oxygen. Elevated expression of feoB2 was also observed in the presence of hydrogen peroxide, with 1.47-fold and 2.32-fold increases in the feoB2 transcript after 5 min and 15 min, respectively, of peroxide challenge. Addition of EDTA (a chelator of divalent ions) as well as dipyridyl (a chelator of iron) to the culture medium did not significantly alter the expression of feoB2. Thus, we conclude that in P. gingivalis W83, expression of feoB2 is regulated by oxygen and hydrogen peroxide but it is not metal dependent.
TABLE 2.
Effects of various growth conditions on feoB2 expression
| Conditions | feoB2 expressiona | 16S rRNA expressionb | feoB2/16S ratiob | Fold changec |
|---|---|---|---|---|
| Anaerobic incubation (−O2) | 14.4 ± 0.682 | 0.22 ± 0.011 | 65.45 | 1 |
| Exposed to air for 5 min | 16.7 ± 0.215 | 0.20 ± 0.024 | 83.50 | 1.28 |
| Exposed to air for 15 min | 57.8 ± 4.187 | 0.35 ± 0.011 | 165.14 | 2.52 |
| Exposed to air for 30 min | 100.4 ± 10.27 | 0.28 ± 0.068 | 358.57 | 5.48 |
| Anaerobic incubation with 200 μM H2O2 for 5 min | 21.1 ± 2.605 | 0.22 ± 0.030 | 95.91 | 1.47 |
| Anaerobic incubation with 200 μM H2O2 for 15 min | 22.8 ± 1.917 | 0.15 ± 0.01 | 152.00 | 2.32 |
| Anaerobic incubation with 5 mM EDTA for 15 min | 10.0 ± 0.557 | 0.13 ± 0.006 | 76.92 | 1.17 |
Expression rates were determined by real-time RT-PCR using fivefold serial dilutions of 100 ng of total RNA. Means and standard deviations of experiments performed in triplicate are shown.
Expression rates were normalized using 16S as an internal standard and are designated as feoB2/16S ratios.
The normalized feoB2 expression rate that was derived from anaerobically incubated cells (−O2) serves as the control and its fold change is designated as 1. Fold changes for feoB2 expression under different growth conditions were determined by dividing their corresponding normalized values by that of the control (−O2).
feoB2 mutation imparts increased sensitivity to oxidants.
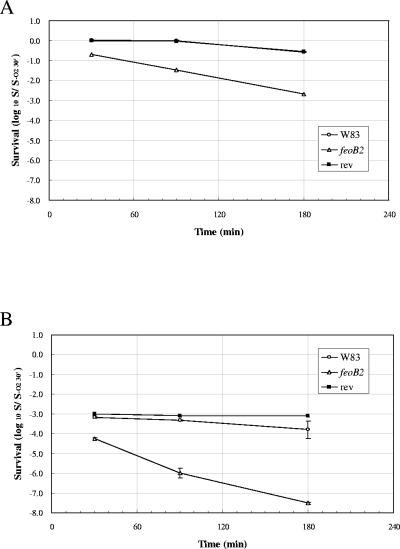
The growth kinetics of the mutant strain incubated anaerobically were similar to those of the wild-type bacteria (Fig. 1). Therefore, in order to determine whether FeoB2 contributes to the high aerotolerance observed in P. gingivalis, we incubated the mutant and the wild-type strains under aerobic conditions. While no viability loss was observed in the wild-type and revertant strains after 90 min of atmospheric oxygen exposure, the FeoB2 mutant showed 95% viability loss after 90 min aerobic incubation (Fig. 4A). The viability loss was even more marked when the FeoB2 mutant was incubated aerobically in the presence of hydrogen peroxide. Few surviving bacteria were detected after incubation under these conditions (Fig. 4B). Viability loss was also observed in the wild-type and FeoB2 revertant strains; however, it was delayed compared to the mutant strain (Fig. 4A and B).
FIG. 4.
Survival of P. gingivalis strains in atmosphere and atmosphere supplemented with hydrogen peroxide. P. gingivalis strains were incubated aerobically (A) and aerobically in the presence of hydrogen peroxide (B). Culture samples were withdrawn at 30 min, 90 min, and 180 min and plated on blood agar plates after adequate dilution. The plates were incubated anaerobically at 37°C for 7 days. Data are represented relative to the control at 30 min, which is set as 1. feoB2, feoB2 mutant; rev, same-site revertant.
FeoB2 plays a role in P. gingivalis survival in host cells.
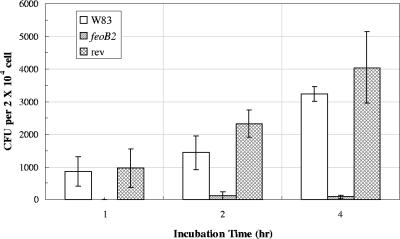
P. gingivalis can invade a variety of host cells (12, 13). Since all eukaryotic cell types (including endothelial cells, vascular smooth muscle cells, and adventitial fibroblasts) (48) can generate intracellular ROS, effective oxidative stress defense mechanisms must be present in P. gingivalis to enable it to survive within the host cells. Our results above indicated that FeoB2 protects the bacteria against atmospheric oxygen and hydrogen peroxide. Therefore, it was plausible that the transporter might have a role in protecting P. gingivalis against oxidative stress encountered in host cells. To determine the role of FeoB2 in this process, we examined the ability of the FeoB2-deficient mutant and the parental strain to invade and survive in host cells. The invasion rates were determined using confocal laser scanning microscopy and are shown in Fig. 5. Bacteria, observed as bright green spots within the host cells, were quantified at two cellular sections (Fig. 5). Similar numbers of intracellular bacteria were detected in HUVECs challenged with either the parental or FeoB2-deficient strains. These data demonstrate that both strains are able to invade HUVECs to a similar degree. The number of P. gingivalis W83 bacteria recovered from HUVECs reflects only viable intracellular bacteria, as extracellular bacteria were killed by treatment of the infected host cells with metronidazole and gentamicin prior to recovery of the intracellular bacteria (Fig. 6). P. gingivalis W83 was found to be capable of invading and surviving inside HUVEC cells. The number of intracellular bacteria increased proportionally with the time of incubation of the infected cells (Fig. 6), indicating that P. gingivalis W83 was multiplying inside the host cells. We recovered only a small portion of the inoculum (equivalent to less than 1 bacterium/cell), indicating that the bacteria can invade (Fig. 5) but do not survive well within the host cells. Although the FeoB2-deficient mutant was also able to invade HUVECs (Fig. 6), approximately 20-fold fewer surviving cells were recovered (Fig. 6). These results indicate that FeoB2 plays a role in the survival of P. gingivalis in HUVECs. We also observed that the rate of killing of the mutant strain was very rapid and that most bacteria were nonviable after 1 h of incubation of the host cells with antibiotics. To further confirm the role of FeoB2 in survival of P. gingivalis in HUVECs, we also tested the FeoB2 revertant strain. As shown in Fig. 6, the survival rate of that strain was restored to that observed in the parental strain. Thus, we conclude that FeoB2 is required for survival of P. gingivalis in host cells.
FIG. 5.
Invasion of HUVECs by P. gingivalis strains. P. gingivalis strains were labeled with BCECF and incubated with HUVECs for 10 min and 30 min as described in Materials and Methods. Infected HUVECs were analyzed for the presence of intracellular bacteria by using confocal laser scanning microscopy. Images shown were taken at the middle section of single HUVECs, and internal bacteria appear bright green (arrows). Images of single HUVECs at two cell sections were used to quantify the bacteria. More than 50 HUVECs were quantified for each bacterial strain for each cross section. WT, wild type; SD, standard deviation.
FIG. 6.
Survival of P. gingivalis in host cells. HUVECs were infected with P. gingivalis W83, V2814 (FeoB2 mutant), and V2831 (revertant) strains as described in Materials and Methods. Intracellular bacteria were recovered from host cells and plated on blood agar plates. The number of colonies recovered after the indicated periods of infection is shown. Results shown are means from one experiment performed in triplicate. The experiment was performed three times with similar results. feoB2, feoB2 mutant; rev, same-site revertant.
DISCUSSION
This is the first study demonstrating a role for manganese in the growth of P. gingivalis. Our study indicates that manganese is required for growth of the bacterium in the presence of oxygen. Manganese is a biologically relevant metal and its role in oxidative stress protection in a variety of organisms is well documented (26). Since atmospheric oxygen can be metabolically converted into a variety of ROS (O2−, H2O2, and HO·) (45), the exact mechanism of protection is unknown. One plausible way may be through the role of manganese as a cofactor for enzymes involved in the oxidative stress defense (26). Manganese is a required cofactor of superoxide dismutase of aerobically grown P. gingivalis (1, 55) and, although the P. gingivalis superoxide dismutase can use both iron and manganese as cofactors, the Mn-superoxide dismutase is more stable in the presence of H2O2 (55). Furthermore, the Mn-superoxide dismutase was demonstrated to be required for survival of the bacteria in the presence of air (34).
The protective role of manganese against reactive oxygen has also been shown to be independent of superoxide dismutase activity (49). Nonenzymatic protection mechanisms mediated by direct dismutation of H2O2 by manganese complexed with bicarbonate have been demonstrated (28). Furthermore, Archibald and Fridovich have shown that manganese can scavenge superoxide nonenzymatically (2). In addition, manganese complexes such as Mn(II)-pyrophosphate and Mn(II)-polyphosphate can block hydroxyl free radical production (6, 9). However, the exact mechanisms of manganese-mediated oxidative stress protection in P. gingivalis remain to be elucidated.
We found that FeoB2, a ferrous iron transport homolog, is a major manganese transporter in P. gingivalis. These data are in agreement with data from Dashper et al., who demonstrated a threefold reduction in manganese accumulation in mutants deficient in the transporter, using inductively coupled plasma-mass spectrometry approaches (11). Since the inhibition profile of FeoB2 has not been determined, the complete metal selectivity spectrum of this transporter is not known. However, as observed for other bacterial divalent metal transporters, it is possible that FeoB2 also transports multiple divalent ions, but with different affinities (22, 25, 36, 54). Our study was carried out using up to 0.9 μM concentrations of 55Fe2+ and did not indicate that FeoB2 mediated iron transport. It is possible that the transporter may transport iron when the extracellular concentrations of the metal are higher; however, such concentrations would probably not be encountered in the natural environment of P. gingivalis (as the free iron concentration in the human host is approximately 10−18 M [4]) and, thus, the role of FeoB2 in uptake of this metal would not be physiologically relevant. Contrary to results predicted for an iron transporter (23), elevated iron uptake was detected in the FeoB2 mutant. This may be due to the increased expression of other transporters primarily involved in iron uptake that also mediate manganese transport. For example, PG1138 (FeoB1), encoding the other FeoB homolog in P. gingivalis, has been shown to transport iron (11), and it is possible that it also transports manganese. In the absence of FeoB2, FeoB1 expression might be increased to compensate for the reduced manganese uptake. This possibility is supported by our findings indicating an increase in the expression level of feoB1 in the feoB2 mutant (unpublished observations). However, detailed analysis of expression of other metal transporter-encoding genes and their products in the FeoB2 mutant will be required to establish the definitive mechanisms of elevated iron uptake in the FeoB2 mutant.
The characteristics of FeoB2-mediated manganese transport indicate that the protein functions as a high-affinity manganese uptake mechanism in P. gingivalis. Since rapid uptake of manganese was observed using concentrations as low as 50 nM of 54Mn2+, we propose that the transporter is physiologically relevant. The concentrations of manganese in mammalian plasma are reported to be as low as 20 nM (29). However, manganese concentrations as high as 36 μM are encountered in saliva (8). Based on the different concentrations of manganese at various sites of the human body, we propose that FeoB2 is required for survival of P. gingivalis when introduced into the circulation and, consequently, for bacterial colonization of heart tissues. Since the role of FeoB2 in manganese uptake at high exogenous concentrations has not been investigated in this study, it is not clear whether it would be essential for survival of P. gingivalis present in saliva.
Manganese uptake in the FeoB2 mutant was only 10% of that of the parental strain, indicating that the protein is a major transporter of the metal in P. gingivalis. Other bacteria have been reported to contain multiple manganese transporters (26). Three classes of manganese transporters have been described in prokaryotes: MntH, SitABCD, and MntA. The first is a homolog of the natural resistance-associated macrophage protein 1 (NRAMP), which is a cation transporter (27), while the second is an ABC-type transporter (20). Although both transporters are present in Salmonella enterica serovar Typhimurium, they transport manganese optimally under different conditions: while MntH is most effective under acidic conditions, the SitABCD system is an alkaline transporter (25). The third transporter type, P-loop ATPase, has to date been reported in only one bacterium, Lactobacillus plantarum (18). Sequence analysis of P. gingivalis W83 revealed no homologs of the NRAMP transporter MntH and the SitABCD system. However, a putative ABC transporter for Zn2+ and Mn2+ is encoded on the genome of P. gingivalis W83 (PG1533), and it is probable that transporter is functional and thus accounts for the growth of the FeoB2 mutant strain in the presence of high manganese concentrations. It is also possible that at higher exogenous concentrations, manganese can be transported by other divalent metal uptake proteins present in P. gingivalis, such as FeoB1, as discussed above.
Our data demonstrate that FeoB2 protects P. gingivalis from hydrogen peroxide and from atmospheric oxygen. These data are in contrast to the report by Dashper et al., who showed that the survival of the FeoB2 mutant is equal to that of the parental strain under aerobic conditions (11). This difference may have resulted from different concentrations of manganese in culture media used to prepare bacteria for the analyses. Since FeoB2 is the major manganese transporter in P. gingivalis, it is probable that the intracellular concentrations of manganese are very low in the FeoB2 mutant. This hypothesis is supported by data reported by Dashper et al., who demonstrated that the concentration of manganese in the FeoB2 mutant is threefold reduced compared to the parental strain (11). Such low concentrations may, in turn, result in reduced activity of Mn-superoxide dismutase due to the absence of a cofactor. In support of this idea, Streptococcus pyogenes deficient in the major manganese transport system MtsABC has been demonstrated to have reduced intracellular manganese concentrations as well as reduced Mn-superoxide dismutase activity (22). Also, the other manganese-dependent oxidative stress protection mechanisms might be impaired in the mutant strain. The elevated expression of feoB2 in the presence of air further suggests that the transporter has a role in oxidative stress protection in P. gingivalis. Contrary to the previously reported requirement of manganese for growth of the feoB2 mutant in anaerobic conditions (11), our data indicate that neither manganese nor this transporter are required for growth of the bacterium in the absence of oxygen. However, our data clearly indicate that manganese and the manganese transporter have a role when the bacterium is grown in the presence of 6% oxygen. In the presence of oxygen, the bacterium is exposed to oxidative stress. Therefore, our data support the role of manganese in oxidative stress protection of P. gingivalis and other bacteria (57).
Elevated expression observed in the presence of atmospheric oxygen was also observed for the S. enterica serovar Typhimurium SitABCD transporter (20, 25, 39). Control of expression of the operon was shown to involve two regulators, the ferric uptake regulator (Fur) and the manganese-dependent regulator (MntR). Since the regulation involved metal-dependent regulators rather than oxygen-dependent regulators, this indicates that metal availability may be related to the presence of oxygen. The insoluble oxidized form of iron (Fe3+) leads to reduced transport and availability of the metal as a cofactor for the Fur protein. Since transcription of the sit operon was also affected by growth phase, it is possible that other as yet uncharacterized mechanisms play a role in regulation of the locus. Furthermore, another manganese transporter, MntH, has also been shown to be regulated by Fur and MntR. However, MntH expression was also induced by hydrogen peroxide in an OxyR-dependent manner (24).
An open reading frame is located upstream of feoB2 that encodes a putative DtxR/MntR-like regulator (PG0931) (35). The absence of promoter sequences in the PG0931-feoB2 intergenic region suggests that the genes are cotranscribed. Open reading frames encoding homologs of Fur and OxyR are present on the genome of P. gingivalis W83 (35) (http://www.oralgen.lanl.gov); however, analysis of the sequences present upstream of the PG0931-feoB2 locus revealed no consensus sequences for Fur and OxyR binding. These findings are consistent with the lack of effect of EDTA and dipyridyl (data not shown) on feoB2 expression but give no insights into the oxygen-dependent regulation of the transporter.
P. gingivalis can invade a variety of eukaryotic cells (12, 13, 44), which may promote escape from host defense mechanisms as well as colonization of sites distant to the mouth, such as atherosclerotic lesions (19). Recently, considerable progress has been made in understanding the invasion process of host cells by P. gingivalis (17, 38, 42, 56, 58). Nevertheless, despite the survival of P. gingivalis in host cells, the mechanisms mediating this process are not well understood. Since eukaryotic cells produce intracellular ROS (51), efficient oxidative stress protection mechanisms would be expected to have a role in mediating the survival of intracellular bacteria. This is indicated by the findings of Ueshima et al., demonstrating that Dps promotes survival of P. gingivalis in HUVECs (52). Dps is a ferritin-like protein shown to attenuate ROS production in bacteria (7). Our data demonstrate that FeoB2 also contributes to survival of P. gingivalis in host cells, as a drastic reduction in survival of the mutant strain was observed at 1 h postinvasion. Due to the required 1 h antibiotic treatment (previously demonstrated to be effective in killing extracellular bacteria), we were not able to examine the survival rates at shorter postinvasion times. Prompted by the role of FeoB2 in oxidative stress protection, we suggest that the FeoB2-mediated attenuation of ROS is likely the major mechanism promoting intracellular survival of P. gingivalis. However, further investigations are required to establish definitely the mechanism by which FeoB2 contributes to survival of the bacterium in eukaryotic cells.
In summary, we report on the major manganese transporter in P. gingivalis. This is also the first functionally established manganese transporter in anaerobic bacteria. So far, three classes of manganese transporters have been reported. Our studies show the presence of a fourth class of Mn2+ transporter in bacteria, that of the GTPase type. Further, our findings that the transporter has a role in oxidative stress protection have been corroborated by data demonstrating that the protein promotes survival of P. gingivalis in host cells. Since oxygen tolerance and oxidative stress protection mechanisms are especially important for pathogenicity of anaerobic bacteria, our study may open new avenues for the development of intervention strategies for bacterial infections.
Acknowledgments
This research was supported by USPHS grant 5K22DE14180 from the National Institute of Dental and Craniofacial Research awarded to Janina P. Lewis.
The determination of the genomic sequence of P. gingivalis was carried out collaboratively by The Institute for Genomic Research (TIGR) and the Forsyth Dental Center database with support from NIDCR. The genomic sequence of P. gingivalis W83 was obtained from TIGR (http://www.tigr.org) and the Los Alamos Oral Pathogen Sequence Database (http://www.oralgen.lanl.gov). We thank Cynthia Nau Cornelissen for help with the radioactive manganese uptake experiments and helpful discussions. We also thank Todd Kitten for help with the microaerophilic system. Lastly, we thank Stuart Dashper for helpful discussions regarding metal uptake in P. gingivalis. The real-time RT-PCR analyses were performed by the Nucleic Acid Core Facility at Virginia Commonwealth University. The confocal laser scanning fluorescent microscopy experiments were performed by the VCU Flow Cytometry and Imaging Shared Resource Facility and supported in part by NIH Grant P30CA16059.
Editor: D. L. Burns
REFERENCES
- 1.Amano, A., S. Shizukuishi, H. Tamagawa, K. Iwakura, S. Tsunasawa, and A. Tsunemitsu. 1990. Characterization of superoxide dismutases purified from either anaerobically maintained or aerated Bacteroides gingivalis. J. Bacteriol. 172:1457-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald, F. S., and I. Fridovich. 1982. The scavenging of superoxide radical by manganous complexes: in vitro. Arch. Biochem. Biophys. 214:452-463. [DOI] [PubMed] [Google Scholar]
- 3.Baughn, A. D., and M. H. Malamy. 2004. The strict anaerobe Bacteroides fragilis grows in and benefits from nanomolar concentrations of oxygen. Nature 427:441-444. [DOI] [PubMed] [Google Scholar]
- 4.Bullen, J. J., H. J. Rogers, P. B. Spalding, and C. G. Ward. 2005. Iron and infection: the heart of the matter. FEMS Immunol. Med. Microbiol. 43:325-330. [DOI] [PubMed] [Google Scholar]
- 5.Chapple, I. L. 1997. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 24:287-296. [DOI] [PubMed] [Google Scholar]
- 6.Cheton, P. L., and F. S. Archibald. 1988. Manganese complexes and the generation and scavenging of hydroxyl free radicals. Free Radic. Biol. Med. 5:325-333. [DOI] [PubMed] [Google Scholar]
- 7.Chiancone, E., P. Ceci, A. Ilari, F. Ribacchi, and S. Stefanini. 2004. Iron and proteins for iron storage and detoxification. Biometals 17:197-202. [DOI] [PubMed] [Google Scholar]
- 8.Chicharro, J. L., V. Serrano, R. Urena, A. M. Gutierrez, A. Carvajal, P. Fernandez-Hernando, and A. Lucia. 1999. Trace elements and electrolytes in human resting mixed saliva after exercise. Br. J. Sports Med. 33:204-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coassin, M., F. Ursini, and A. Bindoli. 1992. Antioxidant effect of manganese. Arch. Biochem. Biophys. 299:330-333. [DOI] [PubMed] [Google Scholar]
- 10.Dahlen, G., F. Manji, V. Baelum, and O. Fejerskov. 1992. Putative periodontopathogens in “diseased” and “non-diseased” persons exhibiting poor oral hygiene. J. Clin. Periodontol. 19:35-42. [DOI] [PubMed] [Google Scholar]
- 11.Dashper, S. G., C. A. Butler, J. P. Lissel, R. A. Paolini, B. Hoffmann, P. D. Veith, N. M. O'Brien-Simpson, S. L. Snelgrove, J. T. Tsiros, and E. C. Reynolds. 2005. A novel Porphyromonas gingivalis FeoB plays a role in manganese accumulation. J. Biol. Chem. 280:28095-28102. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande, R. G., M. B. Khan, and C. A. Genco. 1998. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect. Immun. 66:5337-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn, B. R., W. A. Dunn, Jr., and A. Progulske-Fox. 1999. Invasion of human coronary artery cells by periodontal pathogens. Infect. Immun. 67:5792-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezzo, P. J., and C. W. Cutler. 2003. Microorganisms as risk indicators for periodontal disease. Periodontol. 2000 32:24-35. [DOI] [PubMed] [Google Scholar]
- 15.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher, H. M., H. A. Schenkein, R. M. Morgan, K. A. Bailey, C. R. Berry, and F. L. Macrina. 1995. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect. Immun. 63:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handfield, M., J. J. Mans, G. Zheng, M. C. Lopez, S. Mao, A. Progulske-Fox, G. Narasimhan, H. V. Baker, and R. J. Lamont. 2005. Distinct transcriptional profiles characterize oral epithelium-microbiota interactions. Cell. Microbiol. 7:811-823. [DOI] [PubMed] [Google Scholar]
- 18.Hao, Z., S. Chen, and D. B. Wilson. 1999. Cloning, expression, and characterization of cadmium and manganese uptake genes from Lactobacillus plantarum. Appl. Environ. Microbiol. 65:4746-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haraszthy, V. I., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554-1560. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda, J. S., A. Janakiraman, D. G. Kehres, M. E. Maguire, and J. M. Slauch. 2005. Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. J. Bacteriol. 187:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubovics, N. S., and H. F. Jenkinson. 2001. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147:1709-1718. [DOI] [PubMed] [Google Scholar]
- 22.Janulczyk, R., S. Ricci, and L. Björck. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 71:2656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kammler, M., C. Schön, and K. Hantke. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 175:6212-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+. J. Bacteriol. 184:3151-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehres, D. G., and M. E. Maguire. 2003. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol. Rev. 27:263-290. [DOI] [PubMed] [Google Scholar]
- 27.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 28.Kozlov, Y., A. A. Kazakova, and V. V. Klimov. 1997. Changes in the redox potential and catalase activity of Mn2+ ions during formation of Mn-bicarbonate complexes. Membr. Cell Biol. 11:115-120. [PubMed] [Google Scholar]
- 29.Krachler, M., E. Rossipal, and D. Micetic-Turk. 1999. Concentrations of trace elements in sera of newborns, young infants, and adults. Biol. Trace Elem. Res. 68:121-135. [DOI] [PubMed] [Google Scholar]
- 30.Lamont, R. J., A. Chan, C. M. Belton, K. T. Izutsu, D. Vasel, and A. Weinberg. 1995. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 63:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamont, R. J., and H. F. Jenkinson. 2000. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol. Immunol. 15:341-349. [DOI] [PubMed] [Google Scholar]
- 33.Lewis, J. P., J. A. Dawson, J. C. Hannis, D. Muddiman, and F. L. Macrina. 1999. Hemoglobinase activity of the lysine gingipain protease (Kgp) of Porphyromonas gingivalis W83. J. Bacteriol. 181:4905-4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama, K. 1994. Rapid viability loss on exposure to air in a superoxide dismutase-deficient mutant of Porphyromonas gingivalis. J. Bacteriol. 176:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson, K. E., R. D. Fleischmann, R. T. DeBoy, I. T. Paulsen, D. E. Fouts, J. A. Eisen, S. C. Daugherty, R. J. Dodson, A. S. Durkin, M. Gwinn, D. H. Haft, J. F. Kolonay, W. C. Nelson, T. Mason, L. Tallon, J. Gray, D. Granger, H. Tettelin, H. Dong, J. L. Galvin, M. J. Duncan, F. E. Dewhirst, and C. M. Fraser. 2003. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J. Bacteriol. 185:5591-5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paik, S., A. Brown, C. L. Munro, C. N. Cornelissen, and T. Kitten. 2003. The sloABCR operon of Streptococcus mutans encodes an Mn and Fe transport system required for endocarditis virulence and its Mn-dependent repressor. J. Bacteriol. 185:5967-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papapanou, P. N., J. Sandros, K. Lindberg, M. J. Duncan, R. Niederman, and U. Nannmark. 1994. Porphyromonas gingivalis may multiply and advance within stratified human junctional epithelium in vitro. J. Periodontal Res. 29:374-375. [DOI] [PubMed] [Google Scholar]
- 38.Park, Y., Ö. Yilmaz, I.-Y. Jung, and R. J. Lamont. 2004. Identification of Porphyromonas gingivalis genes specifically expressed in human gingival epithelial cells by using differential display reverse transcription-PCR. Infect. Immun. 72:3752-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patzer, S. I., and K. Hantke. 2001. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J. Bacteriol. 183:4806-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robey, M., and N. P. Cianciotto. 2002. Legionella pneumophila feoAB promotes ferrous iron uptake and intracellular infection. Infect. Immun. 70:5659-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues, P. H., and A. Progulske-Fox. 2005. Gene expression profile analysis of Porphyromonas gingivalis during invasion of human coronary artery endothelial cells. Infect. Immun. 73:6169-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rudney, J. D., R. Chen, and G. J. Sedgewick. 2001. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect. Immun. 69:2700-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudney, J. D., R. Chen, and G. J. Sedgewick. 2005. Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythensis are components of a polymicrobial intracellular flora within human buccal cells. J. Dent. Res. 84:59-63. [DOI] [PubMed] [Google Scholar]
- 45.Storz, G., L. A. Tartaglia, S. B. Farr, and B. N. Ames. 1990. Bacterial defenses against oxidative stress. Trends Genet. 6:363-368. [DOI] [PubMed] [Google Scholar]
- 46.Supek, F., L. Supekova, H. Nelson, and N. Nelson. 1997. Function of metal-ion homeostasis in the cell division cycle, mitochondrial protein processing, sensitivity to mycobacterial infection and brain function. J. Exp. Biol. 200:321-330. [DOI] [PubMed] [Google Scholar]
- 47.Touati, D. 2000. Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373:1-6. [DOI] [PubMed] [Google Scholar]
- 48.Touyz, R. M., and E. L. Schiffrin. 2004. Reactive oxygen species in vascular biology: implications in hypertension. Histochem. Cell Biol. 122:339-352. [DOI] [PubMed] [Google Scholar]
- 49.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 40:1175-1186. [DOI] [PubMed] [Google Scholar]
- 50.Tsolis, R. M., A. J. Bäumler, F. Heffron, and I. Stojiljkovic. 1996. Contribution of TonB- and Feo-mediated iron uptake to growth of Salmonella typhimurium in the mouse. Infect. Immun. 64:4549-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turpaev, K. T. 2002. Reactive oxygen species and regulation of gene expression. Biochemistry (Moscow) 67:281-292. [DOI] [PubMed] [Google Scholar]
- 52.Ueshima, J., M. Shoji, D. B. Ratnayake, K. Abe, S. Yoshida, K. Yamamoto, and K. Nakayama. 2003. Purification, gene cloning, gene expression, and mutants of Dps from the obligate anaerobe Porphyromonas gingivalis. Infect. Immun. 71:1170-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Steenbergen, T. J., M. D. Petit, L. H. Scholte, U. van der Velden, and J. de Graaff. 1993. Transmission of Porphyromonas gingivalis between spouses. J. Clin. Periodontol. 20:340-345. [DOI] [PubMed] [Google Scholar]
- 54.Velayudhan, J., N. J. Hughes, A. A. McColm, J. Bagshaw, C. L. Clayton, S. C. Andrews, and D. J. Kelly. 2000. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol. Microbiol. 37:274-286. [DOI] [PubMed] [Google Scholar]
- 55.Yamakura, F., R. L. Rardin, G. A. Petsko, D. Ringe, B. Y. Hiraoka, K. Nakayama, T. Fujimura, H. Taka, and K. Murayama. 1998. Inactivation and destruction of conserved Trp159 of Fe-superoxide dismutase from Porphy-romonas gingivalis by hydrogen peroxide. Eur. J. Biochem. 253:49-56. [DOI] [PubMed] [Google Scholar]
- 56.Yilmaz, O., P. A. Young, R. J. Lamont, and G. E. Kenny. 2003. Gingival epithelial cell signalling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology 149:2417-2426. [DOI] [PubMed] [Google Scholar]
- 57.Zaharik, M. L., and B. B. Finlay. 2004. Mn2+ and bacterial pathogenesis. Front. Biosci. 9:1035-1042. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, Y., T. Wang, W. Chen, O. Yilmaz, Y. Park, I. Y. Jung, M. Hackett, and R. J. Lamont. 2005. Differential protein expression by Porphyromonas gingivalis in response to secreted epithelial cell components. Proteomics 5:198-211. [DOI] [PMC free article] [PubMed] [Google Scholar]