Abstract
Yersinia enterocolitica invasin (Inv) protein confers internalization into and expression of proinflammatory cytokines by host cells. Both events require binding of Inv to β1 integrins, which initiates signaling cascades including activation of focal adhesion complexes, Rac1, mitogen-activated protein kinase, and NF-κB. Here we tested whether Inv might be suitable as a delivery molecule and adjuvant if used as a component of a vaccine. For this purpose, hybrid proteins composed of Inv and ovalbumin (OVA) were prepared, applied as a coating to microparticles, and used for vaccination. Fusion of OVA to Inv did not significantly disturb the ability of Inv to promote host cell binding, internalization, and interleukin-8 (IL-8) secretion when applied as a coating to microparticles. The microparticles were used for vaccination of mice adoptively transferred with OVA-specific T cells from OT-1 or DO11.10 mice. Administration of OVA-Inv-coated microparticles induced OVA-specific T-cell responses. OVA-specific CD4 T cells produced both gamma interferon (IFN-γ) and IL-4 as determined by enzyme-linked immunosorbent assay. Likewise, pronounced OVA-specific CD8 T-cell responses associated with IFN-γ production were observed. Together, these results suggest that Inv might be an attractive tool in vaccination as it confers both host cell uptake and adjuvant activity by engagement of β1 integrins of host cells, which leads to CD4 as well as CD8 T-cell responses.
Yersinia enterocolitica is a gram-negative, predominantly extracellularly located pathogen that causes food-borne acute or chronic gastrointestinal diseases. The pathogenicity of Y. enterocolitica depends on a virulence plasmid encoding pathogenicity factors such as secreted proteins (Yops) which by means of the type III secretion system are translocated directly into the cytosol of the host cells or the outer membrane protein YadA (9-12). In addition, several chromosome-encoded factors including yersiniabactin, invasin (Inv) (46, 47), SodA (51), and Irp-1 (51) promote the pathogenicity of Y. enterocolitica.
Yersinia Inv has been demonstrated to bind to αβ1 integrin heterodimers of host cells (29, 35). Upon clustering of integrins Inv activates signaling cascades. One signaling pathway causes activation of components of focal adhesion complexes including Src, focal adhesion kinase, and cytoskeletal proteins, leading to the formation of pseudopods that engulf the bacteria into the host cell (2, 3). A second pathway including activation of Rac1, NF-κB, and mitogen-activated protein kinase leads to production of proinflammatory cytokines (20, 56, 63).
Y. enterocolitica is taken up by M cells within the follicle-associated epithelium of Peyer's patches (22, 23). The Yersinia Inv protein has been demonstrated to play an important role in this process as M cells, but not enterocytes, expose β1 integrins at their apical/luminal side, which upon engagement by Inv promotes translocation of Y. enterocolitica (57). Infection of mice with an Inv-deficient Y. enterocolitica mutant strain results in delayed initiation of the infection process although the 50% lethal dose of this mutant is unaltered (47, 48). Subsequently to translocation via M cells, yersiniae colonize the Peyer's patches and may eventually disseminate to lymph nodes, liver, lung, and spleen. Immunohistological analyses suggest that innate host defense mechanisms are involved in control of Yersinia in Peyer's patches (5). In addition, clearance of infection involves the activation of an adaptive immune response including CD8+ and CD4+ TH1 cells (6-8, 17, 42).
Attenuated Yersinia strains, created by targeted disruption of virulence-associated genes, have been demonstrated to be suitable as live carrier vaccines. In fact, YadA-, YopH-, or SodA-deficient Y. enterocolitica mutant strains turned out to be safe even in immunocompromised hosts (15). Moreover, such strains can induce T- and B-cell responses in vaccinated hosts. In previous studies, immunization experiments with various attenuated Y. enterocolitica and Yersinia pseudotuberculosis strains as carriers for heterologous antigens induced protective immune responses against challenge with, e.g., cholera toxin or Listeria monocytogenes in mice (52, 53, 59). In fact, delivery of heterologous antigens via the type III secretion system may induce both CD4 and CD8 T-cell responses in mice. More recent analysis, however, indicated that ovalbumin (OVA)-specific CD4 T-cell responses are not significantly induced upon delivery of OVA along with YopE (64).
In this study we used another Yersinia component as a targeting and delivery system of a vaccine. To induce optimal immune responses, antigens have to be administered along with an adjuvant. The Inv protein might exert all these functions. In fact, the Inv protein promotes host cell targeting via β1 integrins followed by internalization as well as inflammatory host cell reactions including production of chemokines. Therefore, we used the Inv protein fused to OVA as an antigen to create microparticles for vaccination of mice. Microparticles were used according to previous observations that in contrast to soluble Inv (unpublished data), Inv applied as a coating to microparticles elicits a proinflammatory response and is internalized by host cells (14, 56). The data presented demonstrate that by this means both CD4 and CD8 T-cell responses can be enhanced.
MATERIALS AND METHODS
DNA constructs.
A part of the ova gene encoding 108 amino acids (aa 248 to 355) was amplified from pCI-2/OVA by PCR using the forward primer 5′-GGATCCGATGAAGTCTCAGGCCTTGAGC-3′ (BamHI restriction site underlined) and the reverse primer 5′-GAATTCAGGACGCTTGCAGCATCCAC-3′ (EcoRI restriction site underlined). The ova fragment was cloned using the Zero Blunt TOPO PCR cloning kit (Invitrogen, Karlsruhe, Germany), digested with EcoRI and BamHI, and cloned into pGEX-4T-3 expression vector (Amersham Biosciences, Freiburg, Germany) and pInv397 (56), resulting in pOVA108 and pOVA-Inv397, respectively.
Expression and purification of fusion proteins.
Escherichia coli BL21 harboring pGEX-4T-3, pInv397, pOVA108, or pOVA-Inv397 was grown at 27°C in yeast extract tryptone medium to an optical density at 600 nm of 0.5 to 0.8. Expression of glutathione S-transferase (GST) and GST fusion proteins was induced with IPTG (isopropyl-β-d-thiogalactopyranoside) at a final concentration of 0.2 mM. Cells were grown for 4 additional hours before being harvested by centrifugation and stored at 4°C. Cells were resuspended in STE buffer (25 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA) containing lysozyme (2 mg/ml) and a complete protease inhibitor mixture (Roche, Mannheim, Germany). Prior to disruption of bacterial cells by ultrasonication, Sarkosyl was added to a final concentration of 1.5%. Lysates were then cleared from cellular debris by centrifugation. GST, GST-Inv397, GST-OVA108, and GST-OVA-Inv397 were purified from supernatants using affinity chromatography on a GSTPrep FF 16/10 column (Amersham Biosciences) and gel filtration on a HiLoad 26/60 Superdex 75 Prepgrade column (Amersham Biosciences). Purity and identity of GST and GST fusion proteins were analyzed by sodium-dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Protein concentration was determined by the Bio-Rad protein assay (Bio-Rad, Munich, Germany). Contamination with lipopolysaccharide (LPS) was measured by the QCL-1000 Limulus amebocyte lysate assay (BioWhittaker, Maryland) according to the instructions of the manufacturers.
Application of proteins as a coating to microparticles.
For noncovalent coating of microparticles, purified protein was dialyzed against 0.3× phosphate-buffered saline (PBS), pH 7.0. Microparticles (polystyrene, 1-μm diameter; Molecular Probes, Leiden, The Netherlands) were washed with PBS and resuspended in 1 ml of fusion protein solution (0.5 to 1 mg/ml) adjusted to pH 6. Adsorption to the microparticles was allowed for 3 h at room temperature with permanent agitation. After the protein solution was removed, 1 ml of 20-mg/ml bovine serum albumin in PBS was added and incubation was continued at room temperature for another hour. Then microparticles were washed in PBS containing 1 mg/ml bovine serum albumin and stored at 4°C. Coupling efficiency and integrity of coated proteins were checked by SDS-PAGE. Before use, the microparticles were resuspended in PBS, pH 7.0.
Cell culture and stimulation protocols.
Human HeLa cervical epithelial cells (ATCC CCL-2.1) and mouse spleen cells were grown in RPMI 1640 (Biochrom, Berlin, Germany) and 10% heat-inactivated fetal calf serum (Sigma, Taufkirchen, Germany) supplemented with 2 mM l-glutamine (Invitrogen, Karlsruhe, Germany) in a humidified 5% CO2 atmosphere at 37°C. For primary cells the medium was additionally supplemented with penicillin (100 U/ml) (Biochrom), streptomycin (100 μg/ml) (Biochrom), 0.05 mM 2-mercaptoethanol (Sigma), 1 mM sodium pyruvate (Biochrom), and 1% (vol/vol) nonessential amino acids (Biochrom). HeLa cells (1 × 105) were seeded in a 24-well tissue culture plate (Nunc, Wiesbaden, Germany) and grown overnight. Confluent monolayers of cells were incubated with protein-coated microparticles with a ratio of 4,000 microparticles/cell for 2 h. After removal of the medium, cultures were washed three times with PBS, fresh medium was added, and incubation was continued for an additional 6 h. The amount of interleukin-8 (IL-8) secreted by the HeLa cells into the supernatant was determined by enzyme-linked immunosorbent assay (ELISA). Spleens from mice 5 days after immunization were homogenized and adjusted to a concentration of 1.5 × 106 or 3 × 106 cells/ml, distributed into a 24-well plate, and restimulated with 1 or 10 μg/ml of the respective ovalbumin-peptide OVA323-339 or OVA257-264 (SIINFEKL) for 3 days. The amount of cytokines secreted by the restimulated spleen cells into the supernatant was determined by ELISA.
Cytokine ELISA.
ELISAs were performed in Maxisorb-ELISA plates (Nunc) to determine the amount of secreted cytokines in the supernatant. IL-8 secreted by HeLa cells was detected as previously described (55) with optimal concentrations of a mouse anti-IL-8 monoclonal antibody (BD Pharmingen, Heidelberg, Germany) and a biotinylated mouse anti-IL-8 (BD Pharmingen) as detection antibody. Gamma interferon (IFN-γ) secretion was determined by using a capture ELISA including anti-IFN-γ monoclonal antibody (AN 18.17.24) and biotin-labeled anti-IFN-γ monoclonal antibody (R4-6A2) described previously (4). Cytokine concentrations were calculated from the straight-line portion of a standard curve using recombinant human IL-8 (BD Pharmingen) or recombinant mouse IFN-γ (Bender, Vienna, Austria), respectively. The amount of secreted IL-4 was determined using the BD OptEIA Set mouse IL-4 kit (BD Biosciences, Heidelberg, Germany) according to the manufacturer's instructions.
Immunofluorescence microscopy.
HeLa cells (5 × 104) were seeded on coverslips in a 24-well tissue culture plate (Nunc) and grown overnight. Semiconfluent monolayers of cells were incubated with protein-coated microparticles with a ratio of 100 microparticles/cell for 1 h to allow adherence and internalization. After removal of the medium, cultures were washed three times with PBS, fixed with 3.75% paraformaldehyde (Sigma), and blocked with 1% fetal calf serum in PBS. Extracellular microparticles were stained with polyclonal rabbit anti-Inv antibodies (diluted 1:100) (fusion proteins containing Inv397) or polyclonal goat anti-GST antibodies (Amersham Biosciences) (diluted 1:100) (proteins without Inv397), respectively, followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit or donkey anti-goat antibodies (Dianova, Hamburg, Germany). After permeabilization with 2% Triton X-100 in PBS and blocking, intracellularly located microparticles were stained with the same primary antibodies as were the extracellular microparticles, followed by Cy-5-conjugated goat anti-rabbit or rabbit anti-goat antibodies, respectively. F-actin was stained by tetramethyl rhodamine isothiocyanate-conjugated phalloidin (Sigma). The fluorescence images were obtained with a Leica DM RE microscope (Leica, Bensheim, Germany).
Immunization of mice.
Eight- to 10-week-old female C57BL/6 and BALB/c (Harlan Winkelmann, Borchen, Germany), transgenic DO11.10 (40), and OT-1 (25) mice were kept in individually ventilated cages (Tecniplast, Italy) under specific-pathogen-free conditions. Transgenic DO11.10 mice (BALB/c background) express the mouse α- and β-chain T-cell receptor (TCR) that pairs with the CD4 coreceptor and which is specific for chicken OVA323-339 (OVA323-339 peptide) in the context of I-Ad (class II restricted). Transgenic OT-1 mice (C57BL/6 background) express a TCR which pairs with the CD8 coreceptor and which is specific for chicken OVA257-264 in the context of Kβ (class I restricted). Sterile food and water were provided ad libitum. Three mice per group were immunized by intraperitoneal administration of 2 × 1010 microparticles coated with 90 to 150 μg protein.
Adoptive transfer of T cells.
Spleen cells from DO11.10 mice were stained with anti-DO11.10 clonotypic TCR-FITC (clone KJ1-26) (24) (BD Pharmingen) and anti-CD4-phycoerythrin (PE) antibodies (BD Pharmingen), and spleen cells from OT-1 mice were stained with anti-Vα2 TCR-FITC (49) (BD Pharmingen) and anti-CD8-PE (BD Pharmingen) antibodies and analyzed on a FACSCalibur flow cytometer (BD Biosciences) to determine the number of transgenic T cells. Spleen cells derived from DO11.10 mice or OT-1 mice containing 3 × 106 T cells were injected in a volume of 200 μl PBS into the tail vein of BALB/c or C57BL/6 mice, respectively.
Analysis of in vivo frequency of transgenic OVA-specific T cells.
Three days after the adoptive transfer of T cells, 2 × 1010 GST fusion protein-coated microparticles were injected intraperitoneally, and 5 days later the mice were sacrificed. The spleens were aseptically removed and homogenized. An aliquot of approximately 1 × 106 cells was stained with anti-DO11.10 clonotypic TCR-FITC and anti-CD4-PE antibodies for DO11.10 cells and with anti-Vα2 TCR-FITC and anti-CD8-PE antibodies for OT-1 cells and analyzed by flow cytometry.
Analysis of cytokine production in T cells by flow cytometry.
For staining of intracellular IFN-γ, 1.5 × 106 spleen cells from immunized mice were seeded into a 24-well tissue culture plate. All samples were restimulated with 1 μg/ml of the respective OVA peptide. Two hours after addition of stimuli, monensin (BD Pharmingen) was added for 4 h to stop cytokine secretion. After blocking of Fc receptors and permeabilization, intracellular staining was performed using allophycocyanin-conjugated anti-IFN-γ antibodies (BD Pharmingen) and the reagents provided with the Cytofix/Cytoperm Plus kit (BD Pharmingen) following the manufacturer's instructions. Staining of cell surface markers was done before permeabilization as described above.
Statistics.
Data shown in the figures are from representative experiments. Comparable results were obtained in additional experiments. Differences between mean values were analyzed using the Student t test. A value for P of <0.05 was considered statistically significant.
RESULTS
Construction of fusion proteins and coating of microparticles.
In order to investigate the ability of Y. enterocolitica Inv protein to function as an adjuvant and targeting molecule for vaccination, we constructed a fusion protein of the C-terminal 397 amino acids of Inv with a part of OVA consisting of 108 amino acids comprising dominant T- and B-cell epitopes (data not shown). To accomplish this, an ova gene fragment encoding aa 248 to 355 was amplified from the plasmid pCI-2/OVA and ligated into the plasmid pInv397 carrying a part of the inv gene corresponding to the C-terminal 397 aa, resulting in the plasmid pOVA-Inv397. The plasmids described in Materials and Methods were introduced into E. coli BL21, and the proteins (GST, GST-Inv397, GST-OVA, and GST-OVA-Inv397) were expressed and purified (data not shown). Subsequently, these proteins were applied as a coating to microparticles and the amount of protein bound to the microparticles was estimated by SDS-PAGE by comparison with a bovine serum albumin standard (data not shown). About 300 to 500 μg of protein was detectable per ml of a suspension containing 6.7 × 1010 microparticles. In the following vaccination experiments, each mouse was immunized with 300 μl containing 2 × 1010 microparticles with 90 to 150 μg of fusion protein. To estimate the LPS contamination of the protein preparations, the LPS concentration was measured using a Limulus amebocyte lysate assay. LPS concentrations in the different protein preparations ranged from 50 to 100 ng LPS per 2 × 1010 microparticles.
In vitro characterization of protein-coated microparticles.
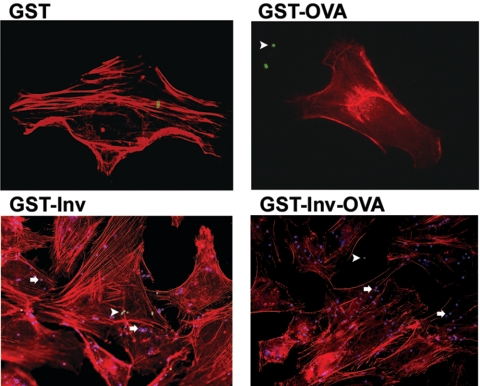
To elucidate whether the OVA protein impairs the Inv protein in its functions which are relevant for vaccination, namely, (i) stimulation of internalization and (ii) triggering proinflammatory host cell reactions via binding to β1 integrins, we performed in vitro experiments as previously published (56). HeLa cells were incubated for 1 h with microparticles coated with GST, GST-Inv397, GST-OVA, or GST-OVA-Inv397, and adhesion as well as internalization of microparticles was analyzed. Immunofluorescence microscopy showed that microparticles coated with GST or GST-OVA did not significantly adhere to HeLa cells and thus were not significantly internalized (Fig. 1). In contrast, microparticles coated with GST-Inv397 or GST-OVA-Inv397 efficiently adhered to and were internalized by HeLa cells (Fig. 1).
FIG. 1.
Internalization of protein-coated microparticles into HeLa cells. HeLa cells were incubated with microparticles at a ratio of 100 microparticles/cell for 1 h at 37°C. Internalization was visualized by immunofluorescence staining. The cytoskeleton was stained with phalloidin (red). Extracellularly localized microparticles are green (arrowheads); intracellularly localized microparticles are blue (arrows). The results are representative of two independent experiments.
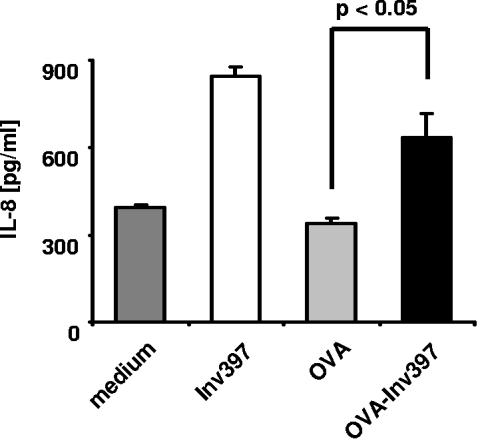
Next, IL-8 was determined by ELISA in culture supernatants of HeLa cells exposed to microparticles. The data indicate that Inv-coated microparticles induce IL-8 production of HeLa cells as previously reported (56). GST-OVA-Inv397-coated microparticles induced about 75% of IL-8 levels compared with GST-Inv397 microparticles but twofold-higher IL-8 levels than stimulation with GST-OVA108-coated microparticles or medium (Fig. 2). These experiments show that the OVA fragment fused to Inv does not significantly impair the ability of Inv to confer internalization and IL-8 production by host cells and thus maintains the putative targeting and adjuvant activity of Inv.
FIG. 2.
IL-8 production by HeLa cells induced by GST fusion protein-coated microparticles. HeLa cells were incubated with microparticles coated with fusions of the indicated proteins to GST at a ratio of 4,000 microparticles/cell or with medium alone. After 8 h, culture supernatants were collected and the levels of IL-8 were determined by ELISA. Values represent the means ± standard deviations of triplicate samples. The results are representative of three independent experiments.
OVA-specific CD4 T-cell responses after immunization with OVA-Inv-coated microparticles.
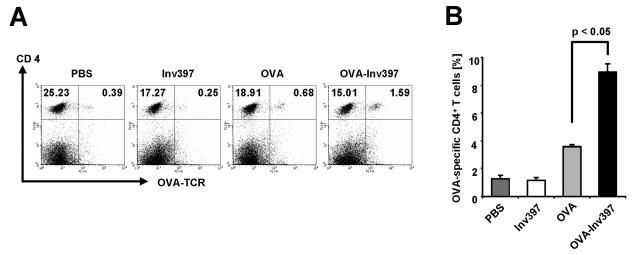
To determine the ability of Inv to induce T-helper cell responses against OVA, we analyzed the number of OVA-specific CD4 T cells upon adoptive transfer of OVA-specific T cells into mice prior to immunization with microparticles. Naive BALB/c mice received OVA-specific splenic T cells from transgenic DO11.10 mice (40) and were immunized 3 days later with 2 × 1010 microparticles coated with fusion proteins. Five days later, the frequency of transgenic CD4 T cells out of splenic CD4 T cells was analyzed by flow cytometry (Fig. 3). CD4 T-cell responses were not enhanced over background levels by immunization with Inv397 microparticles (1.2% specific T cells versus 1.3% in PBS controls). Administration of OVA-coated microparticles increased the proportion of OVA-specific CD4 T cells almost threefold (3.6% specific T cells), but significantly higher numbers (8.9%) could be detected when OVA-Inv397-coated microparticles were administered. From these data we can conclude that OVA-Inv-coated microparticles induce significant OVA-specific CD4 T-cell responses in mice.
FIG. 3.
Frequency of antigen-specific CD4+ T cells in vivo. Three mice received transgenic T cells 3 days prior to immunization with protein-coated microparticles or PBS. The percentage of T cells positive for specific TCR and CD4 out of all CD4+ T cells was determined by fluorescence-activated cell sorting analysis. (A) Double staining with anti-CD4-PE and anti-DO11.10 clonotypic TCR-FITC antibody. Numbers in quadrants indicate the percentages of gated viable cells. (B) CD4+ OVA-specific TCR+ T cells per number of total splenic CD4+ T cells after intraperitoneal immunization. Means ± standard deviations of three animals per group are shown. The results are representative of three independent experiments.
Cytokine production by OVA-specific CD4 T cells.
To address the type of CD4 T-cell response obtained by immunization with OVA-Inv397 microparticles, spleen cells were isolated from mice at day 5 after immunization and cultured for another 3 days in the presence or absence of OVA323-339 peptide. The supernatants were analyzed by ELISA for IFN-γ and IL-4. We observed significantly higher IFN-γ production after immunization with OVA-Inv397 microparticles than after that with the OVA controls (24 ng/ml versus 10 ng/ml) or the other controls (Fig. 4). Even in the absence of additional OVA antigen in the in vitro cultures, significant IFN-γ levels were found in the cultures of spleen cells from OVA-Inv397-immunized mice. Moreover, we observed a profound IL-4 response by spleen cells from mice immunized with OVA-Inv397 microparticles (80 pg/ml) (Fig. 4). This response was significantly higher than that of controls (10 to 20 pg/ml) including spleen cells from mice immunized with OVA-coated microparticles.
FIG. 4.
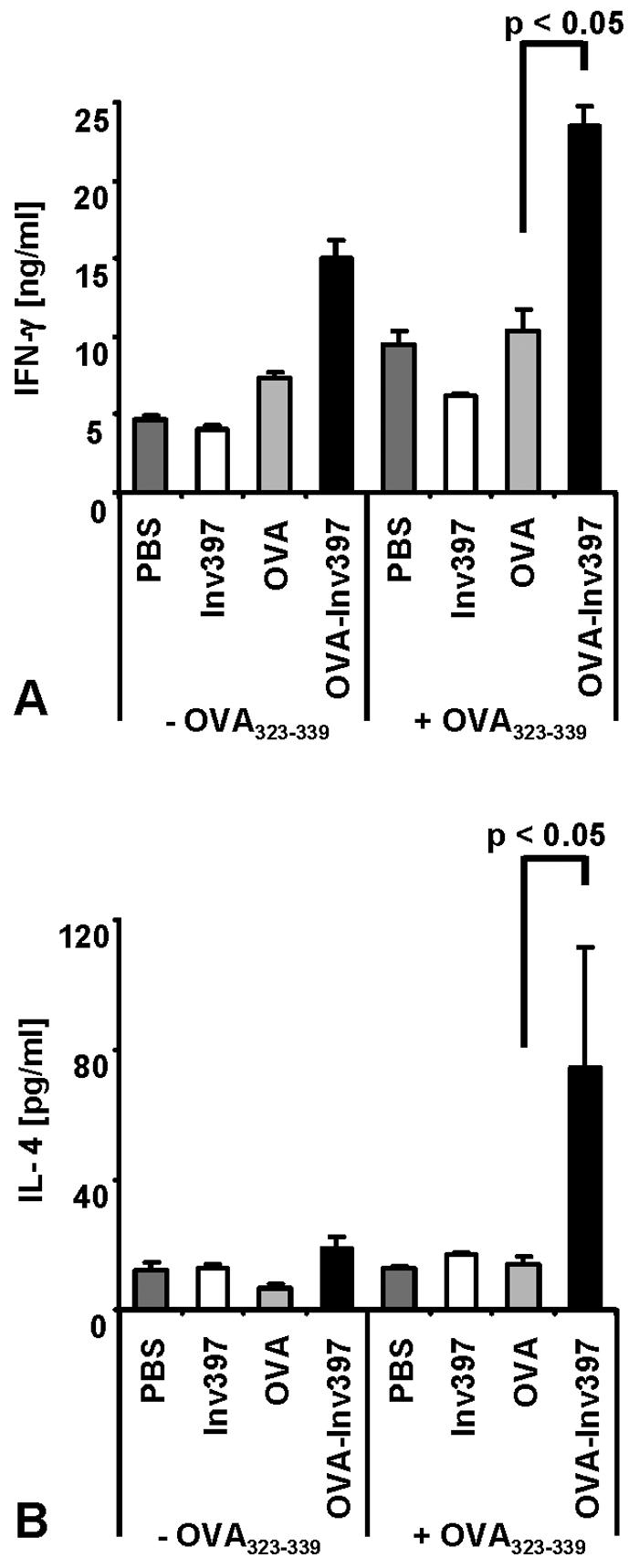
Cytokine production by OVA-specific CD4+ T cells. Five days after immunization, three spleens per group were pooled. Spleen cells were prepared and restimulated with OVA323-339. (A) Supernatants of cultures 3 days after restimulation of 3 × 106 T cells with 10 μg/ml of OVA peptide were tested for IFN-γ by ELISA. (B) Supernatants of cultures 3 days after stimulation of 1.5 × 106 T cells with 1 μg/ml of OVA peptide were tested for IL-4 by ELISA using triplicates. Means ± standard deviations of triplicate samples per group are shown. The results are representative of three independent experiments.
OVA-specific CD8 T-cell responses after immunization with OVA-Inv-coated microparticles.
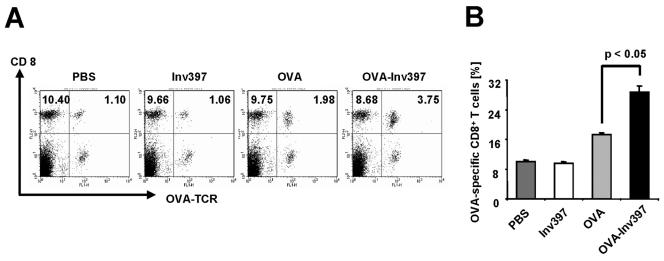
To investigate whether the OVA-Inv-coated microparticles also induce CD8 T-cell responses, OVA-specific splenic T cells from transgenic OT-1 mice were adoptively transferred into C57BL/6 mice. Three days after T-cell transfer, 2 × 1010 microparticles coated with fusion proteins were injected into mice. Five days later, the frequency of transgenic CD8 T cells in splenic CD8 T cells was analyzed by flow cytometry. As depicted in Fig. 5, an increase in OVA-specific CD8 T cells could be observed upon vaccination with OVA-coated microparticles (17% OVA-specific T cells versus 10% in controls). However, a significantly higher frequency of OVA-specific T cells (30%) was found upon administration of OVA-Inv397-coated microparticles. These results show that OVA-Inv-coated microparticles induce significant OVA-specific CD8 T-cell responses in mice.
FIG. 5.
Frequency of antigen-specific CD8+ T cells in vivo. Three mice received transgenic T cells 3 days prior to immunization with protein-coated microparticles or PBS. Percentages of T cells positive for specific TCR and CD8 out of all CD8+ T cells were determined by fluorescence-activated cell sorting analysis. (A) Double staining with anti-CD8-PE and anti-Vα2-FITC antibody. Numbers in quadrants indicate the percentages of gated viable cells. (B) Ratio of CD8+ OVA-specific TCR+ T cells/number of splenic CD8+ T cells after intraperitoneal immunization. Means ± standard deviations of three animals per group are shown. The results are representative of three independent experiments.
Cytokine production by OVA-specific CD8 T cells.
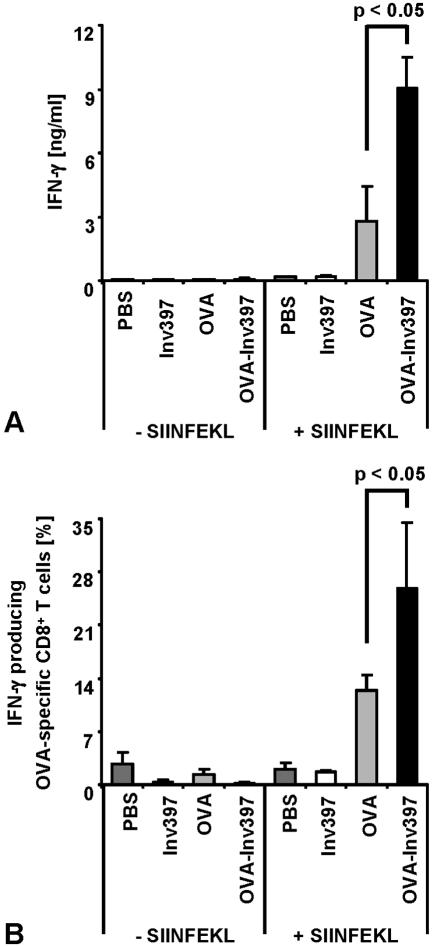
To address the cytokine production of the expanding OVA-specific CD8 T cells, spleen cells were recovered on day 5 after immunization and cultivated for another 3 days in the presence of SIINFEKL peptide, and the supernatants were analyzed for IFN-γ by ELISA. We observed significantly increased IFN-γ production (9 ng/ml) in cultures from mice after immunization with OVA-Inv397 microparticles compared to T cells from mice after immunization with OVA microparticles (3 ng/ml) or controls (Fig. 6A). In addition, we performed intracellular staining for IFN-γ to determine the proportion of IFN-γ-producing T cells among the spleen cells. Flow cytometry analysis revealed that about 26% of OVA-specific CD8 T cells produced IFN-γ after immunization with the OVA-Inv397 microparticles whereas only 12% of these cells produced IFN-γ after immunization with OVA microparticles (Fig. 6B).
FIG. 6.
IFN-γ production by OVA-specific CD8+ T cells. Five days after immunization, three spleens per group were pooled. Spleen cells were prepared and stimulated with OVA257-264 (SIINFEKL) peptide (1 μg/ml). (A) Supernatants of cultures 3 days after restimulation were tested for the presence of IFN-γ by ELISA. (B) Flow cytometry analysis for intracellular IFN-γ was performed using anti-CD8-PE, anti-Vα2-FITC, and anti-IFN-γ-allophycocyanin antibody. Percentages of T cells positive for IFN-γ, OVA-specific TCR, and CD8 out of all CD8+ T cells are shown. Means ± standard deviations of triplicates per group are shown. The results are representative of three independent experiments.
DISCUSSION
Vaccination is an important measure in the prevention of infectious diseases. Due to the low immunogenicity of many antigens, immunogenic carriers or adjuvants are required and included in a vaccine. Several microbial products have been described elsewhere as adjuvants for the induction of specific immune responses (30). Induction of antigen-specific responses to protein antigens requires the activation of T cells. In this study we investigated the potential of an outer membrane protein of Y. enterocolitica, the Inv protein, to induce OVA-specific T-cell responses in mice as this protein targets β1 integrins of host cells, resulting in activation of several signaling pathways. These signaling events confer (i) rearrangement of the actin cytoskeleton leading to, e.g., host cell internalization of Y. enterocolitica and (ii) activation of NF-κB and mitogen-activated protein kinase leading to, e.g., IL-8 production.
We constructed a hybrid protein consisting of Inv and the model antigen OVA and applied this protein as a coating to microparticles as carriers. As the C-terminal two domains of Inv confer binding to β1 integrins (35), the OVA protein was fused to the N terminus of the Inv protein fragment. Herein we show in vitro that fusion of OVA to Inv did not significantly disturb the ability of Inv to promote host cell binding, internalization, and IL-8 responses. Upon exposure to HeLa cells, the microparticles coated with Inv or OVA-Inv were efficiently taken up and induced the secretion of IL-8. From these data we conclude that the OVA-Inv microparticles might exert adjuvant activity in vivo while maintaining their targeting properties for β1 integrins. This was a prerequisite for the following in vivo studies by which we analyzed the influence of Inv on OVA-specific T-cell responses in an adoptive transfer model. IL-8 is a potent chemokine that binds to the chemokine receptors CXCR1 and CXCR2 (34). Recent work demonstrates that, in addition to, e.g., neutrophils, these receptors are also expressed on CD4 and CD8 T cells (38, 60, 61). Our data show that Inv-OVA-, but not OVA-coated, microparticles promote both significant CD4 and CD8 T-cell responses, suggesting that Inv acts as an adjuvant.
The classical model of antigen presentation suggests that exogenous antigens collected from the environment by phagocytosis enter the endocytic pathway which results in degradation of the antigens in phagolysosomes and their presentation via major histocompatibility complex (MHC) class II molecules to CD4 T cells (50). In contrast, endogenous cytosolic proteins may be degraded by the proteasome and translocated via transporter associated with antigen processing (TAP) transporters to the endoplasmic reticulum and are loaded onto and presented by MHC class I molecules to CD8 T cells (21). In accordance with this model we observed pronounced in vivo CD4 T-cell responses against OVA when it was fused to Inv. In fact, Inv promotes the uptake of particles via a zipper-like mechanism into host cells (28, 29). We were surprised to observe that the OVA-specific CD4 T cells produced IFN-γ as well as IL-4. This is in contrast to our previous study demonstrating that various live attenuated Y. enterocolitica mutant strains promote IFN-γ but not IL-4 production in OVA-specific T cells (15, 64). As IFN-γ is a marker for Th1 cells and IL-4 a marker for Th2 cells, it seems that Inv is able to promote the differentiation of Th0 CD4 T cells into both of these subtypes. Further studies are needed to find out whether this depends on the type of antigen or mouse strain or whether this is a feature of Inv as an adjuvant.
We could also detect pronounced OVA-specific CD8 T-cell responses upon vaccination with OVA-Inv-coated microparticles. According to the aforementioned classical model of antigen presentation, this was unexpected as Inv-triggered endocytosis should promote MHC class II-restricted antigen presentation. However, one mechanism by which this could be explained is that the microparticles may promote cross-presentation (for a review see reference 1). In fact, OVA linked to microparticles, but not soluble OVA, may be presented with MHC class I molecules upon conventional phagocytosis by macrophages (32). Moreover, phagosomes from mouse J774.1 macrophages are fully competent compartments to generate simultaneously both MHC class I- and MHC class II-restricted antigenic peptides after internalization of OVA-coated latex microparticles, and the MHC class I-restricted peptide (SIINFEKL) rapidly exits from the phagosome to the cytosol (39). In addition, recent work demonstrated that Rac1 plays an important role in the capacity of CD8+ dendritic cells to endocytose apoptotic cells and prime T cells via cross-presentation (31). The fact that Inv (via its high affinity for β1 integrins) efficiently activates Rac1 (20) may thus explain the ability of Inv-coated microparticles to induce CD8 T-cell responses by cross-presentation of OVA.
Although several new types of vaccines such as synthetic peptides or plasmid DNA with high efficacy have been developed, more-potent adjuvants are still needed. Adjuvants such as microbial proteins or carbohydrates that act as danger signals may activate antigen-presenting cells (45). Proliferative responses of CD4 as well as CD8 T cells are enhanced when an antigen is fused to bacterial flagellin or Bordetella pertussis adenylate cyclase CyaA, respectively, as flagellin is a ligand for TLR 5 and CyaA for the β2 integrin CD11b/CD18 (13, 54). In addition it was shown that immunization of mice with Yersinia pestis F1 antigen combined with flagellin as a adjuvant leads to increased anti-F1 immunoglobulin G levels and protective immunity after challenge of mice with Y. pestis (26). In the present study we fused an antigen to a bacterial surface component that unifies both immunostimulating and targeting properties and applied this hybrid protein as a coating to polystyrene microparticles also to take advantage of a particulate delivery system. Particulate antigen delivery systems such as microparticles increase the accessibility of antigens to the immune system. Microparticles have been demonstrated to promote the induction of cytotoxic T-lymphocyte, CD4, and antibody responses in mice (16, 36, 41, 44, 62). The immunogenicity of microparticles with adsorbed antigens has been improved by coadministration with adsorbed immunostimulatory adjuvants (58). Our results support these data and demonstrate that microparticles upon appropriate coating can in fact trigger strong CD4 and CD8 T-cell responses.
We also addressed the influence of contaminating bacterial products in the protein preparations used for coating of microparticles as these compounds (LPS and CpG DNA) may also show adjuvant activity. We determined the concentration of LPS in the protein solutions used for coating of the microparticles. The LPS concentration ranged between 50 and 100 ng per 2 × 1010 microparticles. Further attempts to reduce LPS contamination by polymyxin agarose beads were not feasible as the GST-Inv protein was removed as efficiently as LPS. Although the amounts of LPS in the recombinant protein preparations used were comparable in GST, GST-Inv, GST-Ova, and GST-OVA-Inv preparations, only the last induced significant T-cell responses, suggesting that LPS contamination did not significantly account for the profound T-cell responses of OVA-Inv-coated microparticles. Moreover, in preliminary experiments with, e.g., TLR-4-deficient mice (O. T. Bühler et al., unpublished), we were not able to differentiate between “nonspecific” (via LPS) and “specific” (via Inv) adjuvant effects exerted by microparticles.
The ability of microparticles to induce immune responses upon mucosal administration depends on their uptake into the specialized mucosa-associated lymphoid tissue (43). In this process M cells play an important role although dendritic cells may also be directly involved in antigen sampling from the gut (19, 33). For mucosal delivery, lectins have been used to target microparticles to the mucosa-associated lymphoid tissue (18, 37) and to enhance the extent of uptake of microparticles following oral delivery (27). Yersinia Inv has been proven to exploit β1 integrins of M cells for translocation (57). Future studies will have to demonstrate whether the microparticles coated with Yersinia Inv may also be used for oral vaccination. For this purpose, however, we first need to develop an appropriate formulation for oral vaccination that protects the coated microparticles from gastric acid and digestive enzymes.
Together, our results further support the idea that recombinant proteins fused to microbial ligands and bound to microparticles induce strong immune responses. The Inv protein of Y. enterocolitica might be particularly suitable due to its multifunctional properties including cell targeting and induction of inflammatory host responses.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft.
Editor: J. B. Bliska
REFERENCES
- 1.Ackerman, A. L., and P. Cresswell. 2004. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 5:678-684. [DOI] [PubMed] [Google Scholar]
- 2.Alrutz, M. A., and R. R. Isberg. 1998. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc. Natl. Acad. Sci. USA 95:13658-13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alrutz, M. A., A. Srivastava, K. W. Wong, C. D'Souza-Schorey, M. Tang, L. E. Ch'Ng, S. B. Snapper, and R. R. Isberg. 2001. Efficient uptake of Yersinia pseudotuberculosis via integrin receptors involves a Rac1-Arp 2/3 pathway that bypasses N-WASP function. Mol. Microbiol. 42:689-703. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth, I. B., M. Beer, E. Bohn, S. H. E. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autenrieth, I. B., V. Kempf, T. Sprinz, S. Preger, and A. Schnell. 1996. Defence mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autenrieth, I. B., A. Tingle, A. Reske Kunz, and J. Heesemann. 1992. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect. Immun. 60:1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autenrieth, I. B., U. Vogel, S. Preger, B. Heymer, and J. Heesemann. 1993. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 61:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohn, E., and I. B. Autenrieth. 1996. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J. Immunol. 156:1458-1468. [PubMed] [Google Scholar]
- 9.Cornelis, G. R. 1994. Yersinia pathogenicity factors. Curr. Top. Microbiol. Immunol. 192:243-263. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 97:8778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelis, G. R., A. Boland, A. P. Boyd, C. Geuijen, M. Iriarte, C. Neyt, M. P. Sory, and I. Stainier. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuadros, C., F. J. Lopez-Hernandez, A. L. Dominguez, M. McClelland, and J. Lustgarten. 2004. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect. Immun. 72:2810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dersch, P., and R. R. Isberg. 1999. A region of the Yersinia pseudotuberculosis invasin protein enhances integrin-mediated uptake into mammalian cells and promotes self-association. EMBO J. 18:1199-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Genaro, M. S., M. Waidmann, U. Kramer, N. Hitziger, E. Bohn, and I. B. Autenrieth. 2003. Attenuated Yersinia enterocolitica mutant strains exhibit differential virulence in cytokine-deficient mice: implications for the development of novel live carrier vaccines. Infect. Immun. 71:1804-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eldridge, J. H., J. K. Staas, J. A. Meulbroek, T. R. Tice, and R. M. Gilley. 1991. Biodegradable and biocompatible poly(dl-lactide-co-glycolide) microspheres as an adjuvant for staphylococcal enterotoxin B toxoid which enhances the level of toxin-neutralizing antibodies. Infect. Immun. 59:2978-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falgarone, G., H. S. Blanchard, B. Riot, M. Simonet, and M. Breban. 1999. Cytotoxic T-cell-mediated response against Yersinia pseudotuberculosis in HLA-B27 transgenic rat. Infect. Immun. 67:3773-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster, N., M. A. Clark, M. A. Jepson, and B. H. Hirst. 1998. Ulex europaeus 1 lectin targets microspheres to mouse Peyer's patch M-cells in vivo. Vaccine 16:536-541. [DOI] [PubMed] [Google Scholar]
- 19.Foti, M., and P. Ricciardi-Castagnoli. 2005. Antigen sampling by mucosal dendritic cells. Trends Mol. Med. 11:394-396. [DOI] [PubMed] [Google Scholar]
- 20.Grassl, G. A., M. Kracht, A. Wiedemann, E. Hoffmann, M. Aepfelbacher, C. Eichel-Streiber, E. Bohn, and I. B. Autenrieth. 2003. Activation of NF-κB and IL-8 by Yersinia enterocolitica invasin protein is conferred by engagement of Rac1 and MAP kinase cascades. Cell. Microbiol. 5:957-971. [DOI] [PubMed] [Google Scholar]
- 21.Gromme, M., and J. Neefjes. 2002. Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways. Mol. Immunol. 39:181-202. [DOI] [PubMed] [Google Scholar]
- 22.Grutzkau, A., C. Hanski, H. Hahn, and E. O. Riecken. 1990. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut 31:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanski, C., U. Kutschka, H. P. Schmoranzer, M. Naumann, A. Stallmach, H. Hahn, H. Menge, and E. O. Riecken. 1989. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect. Immun. 57:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haskins, K., R. Kubo, J. White, M. Pigeon, J. Kappler, and P. Marrack. 1983. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J. Exp. Med. 157:1149-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogquist, K. A., S. C. Jameson, W. R. Heath, J. L. Howard, M. J. Bevan, and F. R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Immunity 76:17-27. [DOI] [PubMed] [Google Scholar]
- 26.Honko, A. N., N. Sriranganathan, C. J. Lees, and S. B. Mizel. 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 74:1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain, N., P. U. Jani, and A. T. Florence. 1997. Enhanced oral uptake of tomato lectin-conjugated nanoparticles in the rat. Pharm. Res. 14:613-618. [DOI] [PubMed] [Google Scholar]
- 28.Isberg, R. R. 1991. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science 252:934-938. [DOI] [PubMed] [Google Scholar]
- 29.Isberg, R. R., and J. M. Leong. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Immunity 60:861-871. [DOI] [PubMed] [Google Scholar]
- 30.Kenney, R. T., and R. Edelman. 2003. Survey of human-use adjuvants. Expert Rev. Vaccines 2:167-188. [DOI] [PubMed] [Google Scholar]
- 31.Kerksiek, K. M., F. Niedergang, P. Chavrier, D. H. Busch, and T. Brocker. 2005. Selective Rac1 inhibition in dendritic cells diminishes apoptotic cell uptake and cross-presentation in vivo. Blood 105:742-749. [DOI] [PubMed] [Google Scholar]
- 32.Kovacsovics-Bankowski, M., K. Clark, B. Benacerraf, and K. L. Rock. 1993. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc. Natl. Acad. Sci. USA 90:4942-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucharzik, T., N. Lugering, K. Rautenberg, A. Lugering, M. A. Schmidt, R. Stoll, and W. Domschke. 2000. Role of M cells in intestinal barrier function. Ann. N. Y. Acad. Sci. 915:171-183. [DOI] [PubMed] [Google Scholar]
- 34.Larsen, C. G., A. O. Anderson, E. Appella, J. J. Oppenheim, and K. Matsushima. 1989. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science 243:1464-1466. [DOI] [PubMed] [Google Scholar]
- 35.Leong, J. M., R. S. Fournier, and R. R. Isberg. 1990. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 9:1979-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maloy, K. J., A. M. Donachie, D. T. O'Hagan, and A. M. Mowat. 1994. Induction of mucosal and systemic immune responses by immunization with ovalbumin entrapped in poly(lactide-co-glycolide) microparticles. Immunology 81:661-667. [PMC free article] [PubMed] [Google Scholar]
- 37.Manocha, M., P. C. Pal, K. T. Chitralekha, B. E. Thomas, V. Tripathi, S. D. Gupta, R. Paranjape, S. Kulkarni, and D. N. Rao. 2005. Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex Europaeus-I lectin as M cell target. Vaccine 23:5599-5617. [DOI] [PubMed] [Google Scholar]
- 38.Moepps, B., E. Nuesseler, M. Braun, and P. Gierschik. 2006. A homolog of the human chemokine receptor CXCR1 is expressed in the mouse. Mol. Immunol. 43:897-914. [DOI] [PubMed] [Google Scholar]
- 39.Muno, D., E. Kominami, and T. Mizuochi. 2000. Generation of both MHC class I- and class II-restricted antigenic peptides from exogenously added ovalbumin in murine phagosomes. FEBS Lett. 478:178-182. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, K. M., A. B. Heimberger, and D. Y. Loh. 1990. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250:1720-1723. [DOI] [PubMed] [Google Scholar]
- 41.Nixon, D. F., C. Hioe, P. D. Chen, Z. Bian, P. Kuebler, M. L. Li, H. Qiu, X. M. Li, M. Singh, J. Richardson, P. McGee, T. Zamb, W. Koff, C. Y. Wang, and D. O'Hagan. 1996. Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine 14:1523-1530. [DOI] [PubMed] [Google Scholar]
- 42.Noll, A., and I. B. Autenrieth. 1996. Yersinia-HSP60-reactive T cells are efficiently stimulated by peptides of 12 and 13 amino acid residues in a MHC class II (I-Ab)-restricted manner. Clin. Exp. Immunol. 105:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Hagan, D. T. 1996. The intestinal uptake of particles and the implications for drug and antigen delivery. J. Anat. 189:477-482. [PMC free article] [PubMed] [Google Scholar]
- 44.O'Hagan, D. T., H. Jeffery, M. J. Roberts, J. P. McGee, and S. S. Davis. 1991. Controlled release microparticles for vaccine development. Vaccine 9:768-771. [DOI] [PubMed] [Google Scholar]
- 45.Pashine, A., N. M. Valiante, and J. B. Ulmer. 2005. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 11:S63-S68. [DOI] [PubMed] [Google Scholar]
- 46.Pepe, J. C., M. R. Wachtel, E. Wagar, and V. L. Miller. 1995. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect. Immun. 63:4837-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pepe, J. C., and V. L. Miller. 1993. The biological role of invasin during a Yersinia enterocolitica infection. Infect. Agents Dis. 2:236-241. [PubMed] [Google Scholar]
- 48.Pepe, J. C., and V. L. Miller. 1993. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc. Natl. Acad. Sci. USA 90:6473-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pircher, H., N. Rebai, M. Groettrup, C. Gregoire, D. E. Speiser, M. P. Happ, E. Palmer, R. M. Zinkernagel, H. Hengartner, and B. Malissen. 1992. Preferential positive selection of V alpha 2+ CD8+ T cells in mouse strains expressing both H-2k and T cell receptor V alpha a haplotypes: determination with a V alpha 2-specific monoclonal antibody. Eur. J. Immunol. 22:399-404. [DOI] [PubMed] [Google Scholar]
- 50.Ramachandra, L., E. Noss, W. H. Boom, and C. V. Harding. 1999. Phagocytic processing of antigens for presentation by class II major histocompatibility complex molecules. Cell. Microbiol. 1:205-214. [DOI] [PubMed] [Google Scholar]
- 51.Roggenkamp, A., T. Bittner, L. Leitritz, A. Sing, and J. Heesemann. 1997. Contribution of the Mn-cofactored superoxide dismutase (SodA) to the virulence of Yersinia enterocolitica serotype O8. Infect. Immun. 65:4705-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russmann, H., U. Gerdemann, E. I. Igwe, K. Panthel, J. Heesemann, S. Garbom, H. Wolf-Watz, and G. Geginat. 2003. Attenuated Yersinia pseudotuberculosis carrier vaccine for simultaneous antigen-specific CD4 and CD8 T-cell induction. Infect. Immun. 71:3463-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russmann, H., A. Weissmuller, G. Geginat, E. I. Igwe, A. Roggenkamp, A. Bubert, W. Goebel, H. Hof, and J. Heesemann. 2000. Yersinia enterocolitica-mediated translocation of defined fusion proteins to the cytosol of mammalian cells results in peptide-specific MHC class I-restricted antigen presentation. Eur. J. Immunol. 30:1375-1384. [DOI] [PubMed] [Google Scholar]
- 54.Schlecht, G., J. Loucka, H. Najar, P. Sebo, and C. Leclerc. 2004. Antigen targeting to CD11b allows efficient presentation of CD4+ and CD8+ T cell epitopes and in vivo Th1-polarized T cell priming. J. Immunol. 173:6089-6097. [DOI] [PubMed] [Google Scholar]
- 55.Schulte, R., and I. B. Autenrieth. 1998. Yersinia enterocolitica-induced interleukin-8 secretion by human intestinal epithelial cells depends on cell differentiation. Infect. Immun. 66:1216-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulte, R., G. A. Grassl, S. Preger, S. Fessele, C. A. Jacobi, M. Schaller, P. J. Nelson, and I. B. Autenrieth. 2000. Yersinia enterocolitica invasin protein triggers IL-8 production in epithelial cells via activation of Rel p65-p65 homodimers. FASEB J. 14:1471-1484. [DOI] [PubMed] [Google Scholar]
- 57.Schulte, R., S. Kerneis, S. Klinke, H. Bartels, S. Preger, J. P. Kraehenbuhl, E. Pringault, and I. B. Autenrieth. 2000. Translocation of Yersinia entrocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to beta1 integrins apically expressed on M-like cells. Cell. Microbiol. 2:173-185. [DOI] [PubMed] [Google Scholar]
- 58.Singh, M., G. Ott, J. Kazzaz, M. Ugozzoli, M. Briones, J. Donnelly, and D. T. O'Hagan. 2001. Cationic microparticles are an effective delivery system for immune stimulatory cpG DNA. Pharm. Res. 18:1476-1479. [DOI] [PubMed] [Google Scholar]
- 59.Sory, M. P., and G. R. Cornelis. 1990. Delivery of the cholera toxin B subunit by using a recombinant Yersinia enterocolitica strain as a live oral carrier. Res. Microbiol. 141:921-929. [DOI] [PubMed] [Google Scholar]
- 60.Takata, H., H. Tomiyama, M. Fujiwara, N. Kobayashi, and M. Takiguchi. 2004. Cutting edge: expression of chemokine receptor CXCR1 on human effector CD8+ T cells. J. Immunol. 173:2231-2235. [DOI] [PubMed] [Google Scholar]
- 61.Tani, K., S. B. Su, I. Utsunomiya, J. J. Oppenheim, and J. M. Wang. 1998. Interferon-gamma maintains the binding and functional capacity of receptors for IL-8 on cultured human T cells. Eur. J. Immunol. 28:502-507. [DOI] [PubMed] [Google Scholar]
- 62.Westwood, A., S. J. Elvin, G. D. Healey, E. D. Williamson, and J. E. Eyles. 2006. Immunological responses after immunisation of mice with microparticles containing antigen and single stranded RNA (polyuridylic acid). Vaccine 24:1736-1743. [DOI] [PubMed] [Google Scholar]
- 63.Wiedemann, A., S. Linder, G. Grassl, M. Albert, I. Autenrieth, and M. Aepfelbacher. 2001. Yersinia enterocolitica invasin triggers phagocytosis via beta1 integrins, CDC42Hs and WASp in macrophages. Cell. Microbiol. 3:693-702. [DOI] [PubMed] [Google Scholar]
- 64.Wiedig, C. A., U. Kramer, S. Garbom, H. Wolf-Watz, and I. B. Autenrieth. 2005. Induction of CD8+ T cell responses by Yersinia vaccine carrier strains. Vaccine 23:4984-4998. [DOI] [PubMed] [Google Scholar]