Abstract
In this work, we report the cloning and characterization of the first cell surface casein kinase II (CKII) substrate (Tc-1) of Trypanosoma cruzi, the causative agent of Chagas' disease. Analysis of the gene sequence revealed a 1,653-bp open reading frame coding for 550 amino acid residues. Northern blot analysis showed a 4.5-kb transcript that is expressed in invasive trypomastigotes but not in noninvasive epimastigote forms of T. cruzi. Southern blot analysis indicates that Tc-1 is a single-copy gene. At the amino acid level, Tc-1 displayed 95% and 99% identity to two hypothetical proteins recently reported by the T. cruzi genome project. Analysis of the translated amino acid sequence indicates that the Tc-1 gene has a putative transmembrane domain with multiple cytoplasmic and extracellular CKII phosphosites. Exogenous human CKII was able to phosphorylate serine residues on both recombinant Tc-1 and Tc-1 of intact trypomastigotes. This phosphorylation was inhibited by the CKII inhibitors heparin and 4,5,6,7,-tetrabromo-2-azabenzimidazole. Immunoblots of solubilized trypomastigotes, epimastigotes, and amastigotes probed with anti-recombinant Tc-1 immunoglobulin G revealed a 62-kDa protein that is expressed only in infective trypomastigotes. Immunoprecipitation of labeled surface proteins of trypomastigotes indicated that the 62-kDa protein is a surface protein, and we found that the protein is uniformly distributed on the surface of trypomastigotes by direct immunofluorescence. Antibodies to Tc-1 effectively blocked trypomastigote invasion of host cells and consequently reduced parasite load. Preincubation of either trypomastigotes or myoblasts with CKII inhibitors blocked T. cruzi infection. Thus, for the first time, we describe a cell surface CKII substrate of a protozoan parasite that is phosphorylated by human CKII and that is involved in cellular infection.
Trypanosoma cruzi, the protozoan parasite that causes Chagas' disease, is an obligate intracellular parasite that must attach to several cell types in the body in order to gain entry and establish infection. The disease causes significant morbidity and mortality in the Americas, yet it remains incurable. Chagas' disease is now viewed as an emerging illness associated with human immunodeficiency virus type 1 infection, where it induces dramatic brain pathology and early death (20). A better understanding of the molecular mechanisms of host cell invasion by T. cruzi is critically important not only for the understanding of the pathogenesis of infection but also for the development of novel means for molecular intervention.
A number of T. cruzi surface glycosylphosphatidylinositol membrane-anchored molecules, including mucins, trans-sialidases, and trans-sialidase-like molecules (8, 17, 29-31), as well as host factors (9, 12, 14, 16, 19, 22, 28) have been implicated in the initial process of infection. This suggests that it is a complex process that requires intense investigation. It has been shown that during the early process of cellular infection by T. cruzi, the parasite activates some host signal transduction molecules and pathways (3) including Ca2+ (4), transforming growth factor β (10), cyclic AMP (cAMP) (4), phosphatidylinositol 3-kinase (24, 32), mitogen-activated protein kinase (29), and protein kinase C (PKC) (30) to gain entry.
Here, we explored the parasite's ability to utilize host cell casein kinase II (CKII) during the initial stages of infection. CKII is a ubiquitous, pleiotropic, and constitutively active protein kinase, and CKII substrates play critical roles in essential cellular functions such as protein synthesis, metabolic activity, signaling, and viral infection (15). In the latter case, the constitutive activity of CKII may be the reason viruses have exploited CKII as a phosphorylating agent of proteins essential to their life cycle (12, 18).
In this study, we have cloned and characterized a novel cell surface protein of T. cruzi that is expressed only in invasive trypomastigotes, is phosphorylated by host cell CKII, and is involved in the early process of cellular infection.
MATERIALS AND METHODS
Parasite cultures.
The highly infective trypomastigote clone MMC 20A, derived from the Tulahuen strain of T. cruzi (13), was used. Pure-culture trypomastigotes were obtained from the supernatant of heart myoblast monolayers as described previously (13). Epimastigotes were produced as previously described (25). Amastigotes were produced as described previously (26).
Infection assays.
To investigate the ability of purified anti-Tc-1 immunoglobulin G (IgG) to block the infection process, trypomastigotes were preincubated with increasing concentrations (0.03 to 1.0 μg/ml) of purified anti-Tc-1 IgG or with purified preimmune IgG for 1 h at 4°C. The parasites were then incubated with monolayers of rat heart myoblasts for 2 h as described previously (13). To investigate the effect of CKII inhibitors on infection, trypomastigotes (4 × 107 parasites/ml) were preincubated with different concentrations of CKII inhibitors (2.5 to 80 μM) or mock treated for 30 min and then exposed to untreated myoblasts. Myoblasts were also preincubated with the same concentrations of CKII inhibitors or mock treated as described above for 30 min and then incubated with untreated trypomastigotes. The CKII inhibitors used were 4,5,6,7,-tetrabromo-2-azabenzimidazole (TBB), 2-dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole (DMAT), and 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) from EMD Biosciences (La Jolla, CA). Stocks of CKII inhibitors were dissolved in dimethyl sulfoxide and then diluted in Dulbecco's modified Eagle's medium (DMEM) to the appropriate concentrations for the assays. The concentrations of CKII inhibitors used were not toxic for either myoblasts or parasites and did not affect their motility. Unbound parasites were removed, and trypomastigote binding to rat heart myoblasts was evaluated at 2 h by fluorescence microscopy using fluorescein isothiocyanate (FITC)-labeled antibodies specific for a trypomastigote surface protein and DAPI (4′,6′-diamidino-2-phenylindole) (11). The number of bound FITC-fluorescent parasites per 200 cells was determined. Parasite entry was evaluated at the same 2-h time point. The number of bound FITC-fluorescent parasites was obtained by subtracting the number of internalized parasites from the total number of DAPI-stained kinetoplast DNA parasites per 200 host cells. Parasite multiplication within cells was evaluated at 72 h using standardized procedures (13). Infection assays were done in triplicate, and experiments were repeated three times. The percent inhibition of trypanosome binding and entry was also determined.
Genomic Tc-1 clone.
A T. cruzi λZAPII genomic expression library was screened with monoclonal antibody 4A4 against the surface of T. cruzi trypomastigotes that blocked trypomastigote binding to mammalian cells (27). One of the three clones selected, designated the Tc-1 clone, was investigated in the present study.
RACE.
Trypomastigotes were solubilized in TRIzol (Invitrogen, Grand Island, NY), and total RNA was purified according to the manufacturer's instructions. The integrity of RNA was determined with a Bioanalyzer (Agilent Technologies, Palo Alto, CA). We used a 3′ and 5′ rapid amplification of cDNA ends (RACE) kit (Invitrogen) to amplify the cDNA ends of the Tc-1 gene. For the 3′ RACE, the gene-specific primers GSP1 (5′-TCCATTGACTCCATTGCG-3′) and GSP2 (5′-GTTTTGACTCAGAAGTGACCTC-3′) were used. For 5′ RACE, the primers used were GSP1 (5′-CTGATTTGGCAATAAGGGC-3′) and GSP2 (5′-CTCCTCTTGTCGTGGTAATG-3′). The specific amplicons obtained were cloned into the TOPO-TA cloning vector (Invitrogen), sequenced, and aligned with the original sequences.
Cloning and expression of Tc-1.
To clone Tc-1 in an expression vector, a forward primer, 5′-ATGGCGCGAAAACGCCGAACCGT-3′, and a reverse primer, 5′-TACACGTTACCGGGCCCCCCCTCGT-3′, were used to PCR amplify the entire open reading frame of Tc-1 using the following conditions: an initial denaturation step at 95°C for 4 min and 35 cycles at 94°C for 30 s, 56°C for 1 min, and 72°C for 2 min followed by 72°C for 10 min. The amplicons were cloned into the pCRT7/NT-TOPO vector (Invitrogen), and the sequence was verified. The plasmid was used to transform BL21-AI cells (Invitrogen) according to the manufacturer's instructions. The transformed Escherichia coli was induced with l-arabinose at a final concentration of 0.2%. The expressed recombinant protein was purified over a Ni-nitrilotriacetic acid affinity matrix (QIAGEN, Valencia, CA) according to the manufacturer's instructions. The purified protein was analyzed by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Southern and Northern blots.
For Southern blots, T. cruzi genomic DNA (5 μg) was digested with EcoRI, BamHI, HindIII, and XhoI; separated on a 1.2% agarose gel; transferred onto nylon membranes; and probed with 32P-labeled Tc-1 using high-stringency conditions. Labeled Tc-1 probe was made by random primer extension (21). For Northern blots of trypomastigotes and epimastigotes, total RNA (20 μg) was separated on formaldehyde agarose gels, blotted onto nylon membranes, and probed with 32P-labeled Tc-1 according to the NorthernMax Ultrahyb kit protocol (Ambion, Austin, TX).
Antibodies.
IgG monospecific to Tc-1 was produced by immunizing goats with highly purified recombinant Tc-1 as a histidine-tagged protein mixed with Freund's adjuvant. Tc-1 IgG or preimmune IgG was chromatographically purified. IgG monospecific to Tc-1 was purified by adsorption and desorption on immobilized purified recombinant Tc-1.
Immunoblotting.
Trypomastigotes, epimastigotes, and amastigotes (2 × 107) were solubilized in 0.1% CHAPS {3-[(3-cholamidylpropyl)-dimethylammonio]-1-propanesulfonate} containing protease inhibitors (GE Healthcare, Piscataway, NJ), and the same protein concentrations were loaded onto a 12% SDS-PAGE gel, transferred onto nitrocellulose membranes, probed with goat anti-Tc-1 IgG, and developed with peroxidase-conjugated mouse anti-goat IgG by ECL (GE Healthcare, Piscataway, NJ) as described previously (2). Blots were stripped and reprobed with monoclonal IgG specific for β-actin.
Immunoprecipitation.
Surface proteins of trypomastigotes, epimastigotes, and amastigotes were labeled with biotin as described previously (13). The same concentration of parasites was solubilized in Tris-buffered saline (pH 8.0) containing 1% Triton X-100, 1% bovine serum albumin, 1 mM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride, and 0.02% NaN3 and preabsorbed with protein G (Pierce, Rockford, IL). The same protein concentration of each preabsorbed solubilized T. cruzi stage was incubated with the same concentration of IgG specific for Tc-1 or preimmune IgG and protein G as described previously (11). The same volume of immunoprecipitates was separated by SDS-PAGE, blotted onto nitrocellulose membranes, and probed with IgG specific for Tc-1. Blots were incubated with avidin-horseradish peroxidase and developed by ECL (GE Healthcare) as described previously (2).
Immunofluorescence.
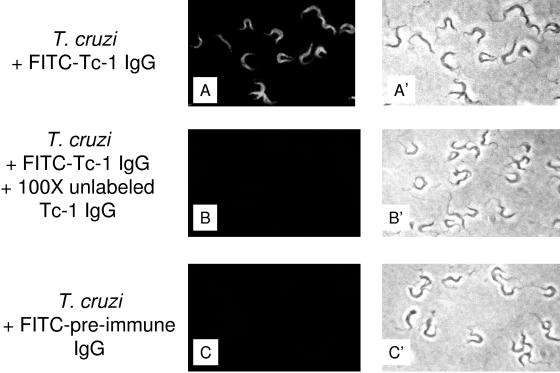
Monospecific IgG against Tc-1 or preimmune IgG was labeled with FITC (11) for studies of binding of trypomastigotes to host cells as described previously (11). Briefly, trypomastigotes (1 × 106) fixed with 1% paraformaldehyde were incubated with FITC-labeled IgG specific for Tc-1 (1 μg/ml in phosphate-buffered saline) in the presence or absence of a 100× excess of unlabeled IgG specific for Tc-1 in phosphate-buffered saline supplemented with 0.1% bovine serum albumin for 1 h at 37°C. Paraformaldehyde-fixed trypomastigotes were also incubated with FITC-labeled preimmune IgG under the same conditions, and the fluorescence was visualized with a Nikon fluorescence microscope.
Phosphorylation of Tc-1 by CKII.
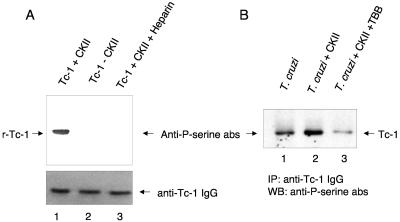
Purified recombinant Tc-1 was dialyzed against CKII phosphorylation buffer (20 mM MOPS [morpholinepropanesulfonic acid], pH 7.2, 25 mM β-glycerol phosphate, 5 mM EGTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol) supplemented with magnesium-ATP cocktail and PKA inhibitor cocktail (Upstate Cell Signaling Solutions, Charlottesville, VA). Recombinant Tc-1 (40 μg) or intact trypomastigotes (7 × 107) previously starved in DMEM for 2 h were incubated with 20 ng of human CKII (Upstate Cell Signaling Solutions) without CKII or with 20 ng CKII in the presence of the CKII inhibitor heparin or TBB in supplemented phosphorylation buffer for 10 min at 30°C. For immunoblots, the same protein concentration of samples containing Tc-1 was separated by 12% SDS-PAGE, blotted onto nitrocellulose membranes, blocked with 2% gelatin, and probed with rabbit anti-phosphoserine antibody (Chemicon, Temecula, CA). The blots were incubated with mouse anti-rabbit IgG-biotin followed with Streptavidin-horseradish peroxidase and developed by ECL (GE Healthcare). The membranes were stripped and reprobed with anti-Tc-1 IgG and developed by ECL. For immunoprecipitations, the same protein concentration of solubilized CKII-treated trypanosomes, under conditions indicated above, was immunoprecipitated with anti-Tc-1 IgG using protein G. The same aliquots of immunoprecipitates were separated by SDS-PAGE, blotted onto nitrocellulose, probed with anti-phosphoserine antibodies, and developed by ECL.
Presentation of results and statistical analysis.
The results shown in this work were from triplicate determinations and represent three independent experiments performed by identical methods. The results are expressed as the mean ± 1 standard deviation. Differences were considered to be statistically significant if the P value was <0.05 by the Student's t test.
Nucleotide sequence accession number.
The complete coding sequence of the Tc-1 gene described in this paper was deposited in the GenBank database under accession no. AY167028 on 21 October 2002.
RESULTS
Molecular characterization of Tc-1.
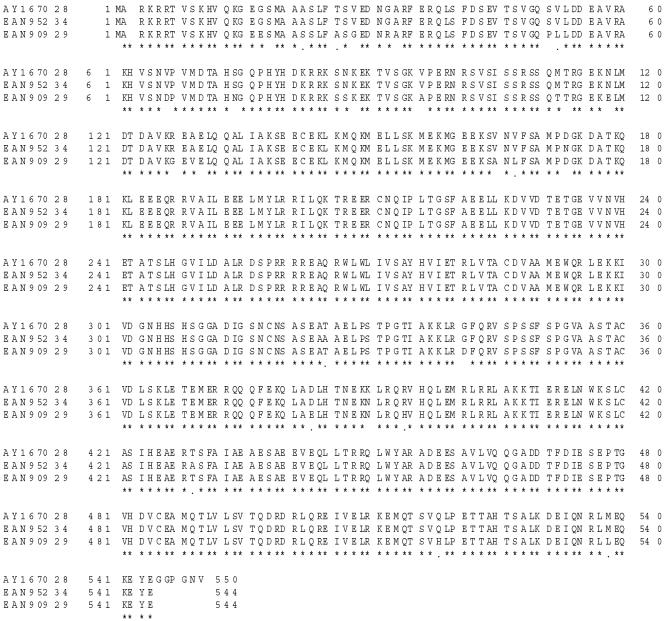
Sequencing analysis of the Tc-1 gene revealed a 1,653-bp coding sequence and its corresponding 550-amino-acid (aa) sequence. The protein has a predicted molecular mass of about 62 kDa and a pI of 6.1. Figure 1 shows that Tc-1 has 99% identity (540/544) with a T. cruzi hypothetical protein (GenBank accession no. EAN95234) (5) and 95% identity (521/544) with another T. cruzi hypothetical protein (GenBank accession no. EAN90929) (6) from the recently completed T. cruzi genome project (5, 6). It is important to mention that our laboratory had initially identified the Tc-1 gene and deposited its coding sequence in GenBank prior to the reporting of the results of the T. cruzi genome project.
FIG. 1.
Alignment of the deduced amino acid sequences of the Tc-1 gene (GenBank accession no. AY167028) with those of two genes coding for two hypothetical proteins (GenBank accession no. EAN95234 and EAN90929), which were reported as a result of the completion of the T. cruzi genome project (4, 5). BLASTp analysis revealed that Tc-1 had 99% and 95% identity to two hypothetical proteins of T. cruzi. Identical amino acid residues are indicated with an asterisk (*).
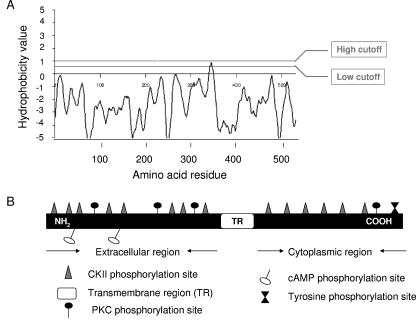
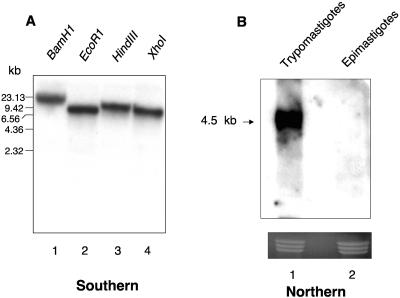
A genomic BLASTn search indicated that Tc-1 is a novel gene. A comprehensive search of the completed T. cruzi genome verified that the gene is indeed present in the parasite genome. Toppred protein analysis predicted that Tc-1 is a single-pass transmembrane protein with aa 1 to 343 lying in the extracellular region, aa 344 to 364 being a predicted transmembrane region with a hydrophobicity value of 0.889 (1 being the upper cutoff), and aa 365 to 550 lying in the cytoplasm (Fig. 2A). The Toppred protein analysis used the Goldman, Engelman, and Steitz hydrophobicity scale, a window size of 21, a lower cutoff value of 0.6, and an upper cutoff value of 1.0 (7). Prosite proteomics software analysis using the CKII phosphorylation site motif S-X-X-E/D (X indicates any amino acid) predicted the presence of 14 phosphorylation sites in the protein, 8 extracellular and 6 intracellular, along with other phosphorylation sites as shown in the model (Fig. 2B). Southern blot analysis indicates that Tc-1 is present in the parasite genome as a single-copy gene (Fig. 3A), whereas analysis by Northern blot showed a 4.5-kb transcript expressed in invasive trypomastigotes but not in noninvasive epimastigotes (Fig. 3B).
FIG. 2.
Hydrobobicity profile of Tc-1 showing a single transmembrane region and the predicted model of the Tc-1 protein structure showing its phosphorylation sites. (A) Hydrophobicity plot of the Tc-1 protein predicts a single transmembrane region between amino acids 344 and 364 using Toppred protein analysis (7). (B) Envisage model of the Tc-1 protein structure showing its phosphorylation sites. Prosite and Scansite software analysis of Tc-1 showed 16 CKII phosphorylation sites, 4 PKC phosphorylation sites, a tyrosine phosphorylation site, and 2 cAMP sites.
FIG. 3.
Tc-1 is a single-copy gene that is expressed in invasive trypomastigotes but not in noninvasive epimastigotes. (A). Southern blot analysis of T. cruzi indicates that Tc-1 is a single-copy gene. T. cruzi DNA (5 μg) was digested with several restriction enzymes, separated on an agarose gel, blotted onto nylon membranes, and probed with 32P-labeled Tc-1 as indicated in Materials and Methods. (B) Northern blot analysis indicates that the Tc-1 gene shows a 4.5-kb transcript that is expressed only in trypomastigotes. Total RNA (20 μg) of trypomastigotes and epimastigotes was separated on an agarose gel, blotted onto nylon membranes, and probed with 32P-labeled Tc-1. The bottom of panel B represents ethidium bromide staining of the gel as RNA loading controls. The results presented in panels A and B are representative of one experiment of three performed with similar results.
Cloning of Tc-1 into the pCRT7/NT-TOPO vector allowed the proper expression of the recombinant Tc-1 gene product in E. coli. The expressed recombinant His6-tagged protein was purified on a Ni-nitrilotriacetic acid affinity matrix and analyzed on a 12.5% SDS-PAGE gel stained with Coomassie blue. This showed a purified recombinant 62-kDa protein.
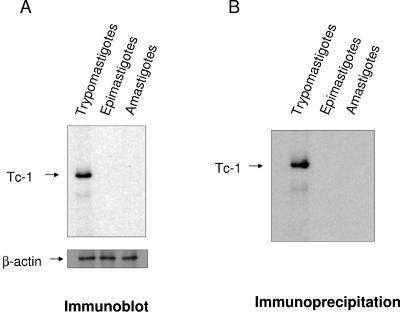
Tc-1 is expressed as a surface protein in invasive trypomastigotes but not in noninvasive epimastigotes or amastigotes.
Immunoblots of trypomastigotes, epimastigotes, and amastigotes probed with IgG specific for Tc-1 indicated that Tc-1 is a 62-kDa protein present only in invasive trypomastigotes (Fig. 4A). Thus, Tc-1 is developmentally regulated in the cell cycle of T. cruzi. Immunoprecipitation of biotin-labeled surface proteins of trypomastigotes, epimastigotes, and amastigotes with IgG specific for Tc-1 confirms that Tc-1 is expressed only in trypomastigotes and that it is a surface protein (Fig. 4B). Immunofluorescence studies were performed to pinpoint the surface distribution of Tc-1 on trypomastigotes. FITC-labeled IgG specific for Tc-1 binds to the surface of trypomastigotes in a uniform manner (Fig. 5A). The binding is specific, since a 100× excess of unlabeled IgG specific for Tc-1 completely inhibited the binding of FITC-labeled IgG specific for Tc-1 (Fig. 5B). FITC-labeled preimmune IgG did not bind to trypomastigotes (Fig. 5C). The immunofluorescence studies also confirmed the immunoprecipitation studies that showed that Tc-1 is a surface protein.
FIG. 4.
T. cruzi trypomastigotes but not epimastigotes or amastigotes express Tc-1 on their surface. (A) Immunoblotting analysis indicates that invasive trypomastigotes but not noninvasive epimastigotes or amastigotes express Tc-1. The same concentration of solubilized trypomastigotes, epimastigotes, and amastigotes was separated by SDS-PAGE, transferred onto nitrocellulose membranes, probed with Tc-1 IgG, and developed by ECL as indicated in Materials and Methods. Blots were stripped and probed with β-actin antibody as protein loading controls. (B) Immunoprecipitation of biotin-labeled surface proteins of T. cruzi indicates that Tc-1 is expressed as a surface protein in trypomastigotes. The same numbers of trypomastigotes, epimastigotes, and amastigotes were biotinylated and solubilized, and the same concentration of protein was immunoprecipitated with Tc-1 IgG supplemented with protein G. The immunoprecipitated material was separated by SDS-PAGE, blotted onto nitrocellulose, probed with Tc-1 IgG, and developed by ECL as described in Materials and Methods. Blots presented in panels A and B are representative of one experiment of three performed with similar results.
FIG. 5.
FITC-labeled Tc-1 IgG specifically binds to the surface of trypomastigotes. Trypomastigotes were washed with Hanks' balanced salt solution, fixed with 1% paraformaldehyde, and incubated with FITC-labeled Tc-1 IgG, FITC-labeled Tc-1 IgG in the presence of a 100× excess of unlabeled Tc-1 IgG, or FITC-labeled preimmune IgG. (A) FITC-labeled IgG to Tc-1 binds uniformly to the surface of trypomastigotes. (B) Binding of FITC-labeled IgG to Tc-1 is abolished by a 100× excess of unlabeled Tc-1 IgG. (C) FITC-labeled preimmune IgG does not bind to trypomastigotes. A′, B′, and C′ show that trypanosomes were present in the preparations shown in A, B, and C, as seen in their respective light fields. This is a representative experiment of three independent experiments performed with similar results.
Human CKII phosphorylates Tc-1.
Incubation of purified recombinant Tc-1 with highly purified human CKII, analysis of the products by SDS-PAGE followed by blotting onto nitrocellulose membranes, and probing with anti-phosphoserine antibodies showed that the recombinant Tc-1 was phosphorylated on serine residues as shown in Fig. 6A. This reaction was blocked in the presence of heparin, a CKII inhibitor, and was not seen when the kinase was replaced with reaction buffer. These results indicate that that exogenous human CKII phosphorylates Tc-1 on serine residues. Exogenous CKII phosphorylates Tc-1 on serine residues of intact trypomastigotes, and TBB, a casein kinase inhibitor, inhibited this phosphorylation (Fig. 6B).
FIG. 6.
Human exogenous CKII phosphorylates recombinant Tc-1 or Tc-1 of intact T. cruzi trypomastigotes on serine residues, and the phosphorylation is inhibited by the CKII inhibitor heparin or TBB. (A) Phosphorylation of recombinant Tc-1 (r-Tc-1) by exogenous CKII. Recombinant Tc-1 (40 μg) was incubated with 20 ng of human CKII, with phosphorylation buffer in place of CKII, or with 20 ng CKII in the presence of heparin, a CKII inhibitor, for 10 min at 30°C. The same amount of each sample was separated by SDS-PAGE, blotted onto nitrocellulose membranes, probed with anti-phosphoserine antibodies (Anti-P-serine abs), and developed by ECL as described in Materials and Methods. Lane 1 shows the phosphorylation of serine residues on Tc-1 by CKII. Lane 2 (control) shows that Tc-1 is not phosphorylated in the presence of reaction buffer alone. Lane 3 shows that heparin inhibits the phosphorylation of Tc-1 by CKII. This is a representative experiment of three experiments performed with similar results. (B) Trypomastigotes (7 × 107) were starved in DMEM and incubated with 20 ng of human CKII, with phosphorylation buffer in place of CKII, or with 20 ng CKII in the presence of TBB, a CKII inhibitor, for 10 min at 30°C. The same protein concentration of solubilized samples was immunoprecipitated (IP) with anti-Tc-1 IgG, separated by SDS-PAGE, blotted onto nitrocellulose membranes, probed with anti-phosphoserine antibodies, and developed by ECL. Lane 1 (control) shows endogenous phosphorylation of serine residues on Tc-1 of trypomastigotes in the absence of exogenous CKII. Lane 2 shows the phosphorylation of serine residues on Tc-1 of intact T. cruzi by exogenous CKII. Lane 3 shows that TBB inhibits the phosphorylation of Tc-1 on intact T. cruzi by exogenous CKII. This is a representative experiment of three experiments performed with similar results. WB, Western blot.
Anti-Tc-1 IgG and CKII inhibitors block parasite infection of host cells.
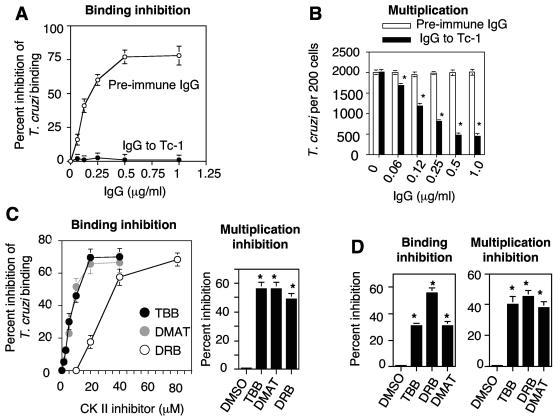
Preincubation of invasive trypomastigotes with increasing concentrations of IgG specific for Tc-1 significantly inhibited the binding of trypomastigotes to rat heart myoblasts in a concentration-dependent and saturable manner (Fig. 7A). Preincubation of trypomastigotes with preimmune IgG did not affect parasite binding to cells at all concentrations tested. The minimal concentration of Tc-1 IgG that caused inhibition was 0.06 μg/ml, whereas the maximal concentration of Tc-1 IgG that caused maximal inhibition (75%) was 0.5 μg/ml. The fact that fewer trypanosomes were bound to cells at 2 h when they were preincubated with Tc-1 IgG (Fig. 7A) resulted in a smaller number of parasites entering and multiplying within these cells at 72 h with respect to trypanosomes that were preincubated with preimmune IgG. Analysis of parasite multiplication at 72 h indicated that heart myoblasts that were exposed to parasites preincubated with preimmune IgG showed a higher number of parasites per cell, whereas heart myoblasts exposed to trypanosomes that were preincubated with IgG to Tc-1 had a significantly lower number of parasites per cell (Fig. 7B). The inhibitory effect of Tc-1 IgG on T. cruzi infection of heart myoblasts requires very low concentrations of Ig and is concentration dependent and saturable. Pretreatment of trypomastigotes with the CKII inhibitors TBB, DMAT, and DRB significantly inhibited trypomastigote binding to myoblasts at 2 h in a concentration-dependent and saturable manner (Fig. 7C, left panel) and consequently caused an inhibition of parasite multiplication at 72 h (Fig. 7C, right panel). Pretreatment of myoblasts with TBB, DMAT, and DRB also inhibited trypomastigote binding to myoblasts at 72 h (Fig. 7D, left panel) and parasite multiplication at 72 h with respect to mock-treated myoblasts (Fig. 7D, right panel).
FIG. 7.
Tc-1 IgG and CKII inhibitors prevent the binding of trypomastigotes to heart myoblasts at 2 h, causing a significant reduction in the parasite load at 72 h. (A) Tc-1 IgG inhibits trypomastigote binding to heart myoblasts in a concentration-dependent and saturable manner, whereas preimmune IgG has no effect. Trypomastigotes were preincubated with increasing concentrations of either Tc-1 IgG or preimmune IgG and then exposed to heart myoblast monolayers at a ratio of 5 parasites per cell for 2 h, and trypanosome binding was evaluated as described in Materials and Methods. (B) Reduction of trypanosome binding caused by Tc-1 IgG reduced parasite multiplication in heart myoblasts. Trypomastigotes were preincubated with either Tc-1 IgG or preimmune IgG and then exposed to myoblast monolayers for 2 h as described in panel A. After unbound trypanosomes were removed, the cell monolayers received fresh complete medium and were postincubated for 72 h, where T. cruzi multiplication was evaluated. (C) Pretreatment of trypomastigotes with CKII inhibitors inhibits trypomastigote binding to myoblasts at 2 h (left panel), and the reduction of parasite binding caused by CKII inhibitors decreased parasite load at 72 h (right panel). DMSO, dimethyl sulfoxide. (D) Pretreatment of myoblasts with CKII inhibitors at the concentration of 40 μM inhibited parasite binding at 2 h (left panel) and multiplication at 72 h (right panel). Parasite binding and multiplication assays were performed as described above and as described in Materials and Methods. Bars represent the means ± 1 standard deviation of results from triplicate samples in one representative experiment (±1 standard deviation) selected from three experiments with similar results. *, significant difference compared to control values (P < 0.05).
DISCUSSION
In this study, we describe, for the first time, a cell surface CKII substrate of invasive trypomastigote forms of T. cruzi that is capable of being phosphorylated by human CKII and is involved in cellular infection.
Viruses have exploited the uniqueness of the host's CKII constitutive activity by using CKII to phosphorylate proteins involved in viral infection (18). Here, we show that a CKII substrate on the surface of the intracellular protozoan T. cruzi plays an important role in the infection process. This is supported by the fact that human CKII strongly phosphorylates the cell surface Tc-1 protein of trypomastigotes and by the fact that antibodies raised against Tc-1 strongly inhibit T. cruzi infection of host cells in a concentration-dependent and saturable manner by up to 75%. The residual T. cruzi infection seen indicates that the parasite can infect cells through other mechanisms that are Tc-1 independent. Furthermore, the fact that pretreatment of trypomastigotes with CKII inhibitors reduces the infection process suggests that T. cruzi may depend on its CKII to infect cells. In addition, our findings showing that pretreatment of myoblasts with CKII inhibitors inhibited infection also suggest that the host enzyme is playing a role in the infection process.
CKII's enzymatic activity is always turned on, and its substrates are involved in very essential cell functions including viral infection and cell survival. Basically, cells cannot survive without CKII activity, and substrates for the enzyme fall into many critical functional categories including cell cycle regulation, protein synthesis, metabolic activity, signaling, DNA/RNA synthesis, and viral infection (15). It has also been reported that the slender form of Trypanosoma brucei has a higher CKII activity than the stumpy form, but its biological role remains unclear (1). The fact that Tc-1 presents two cAMP and three PKC phosphorylation sites, which may involve Ca2+ mobilization, would correlate with the fact that T. cruzi entry into cells triggers the elevation of the intracellular concentration of free Ca2+ in host cells (23).
Multiple acidic residues located downstream from the phosphorylatable amino acids, with serine being preferred over threonine, specify CKII phosphorylation. The acidic residue at the +3 position has the most crucial function (15). The CKII sites found on Tc-1 fulfill the minimum consensus sequence S-x-x-E/D. Putative CKII targets have multiple phosphorylation sites, and this is also the case with Tc-1, which has multiple sites for PKC, cAMP, and tyrosine phosphorylation in close proximity to the CKII phosphosites.
Tc-1 appears to be the first described transmembrane surface protein of T. cruzi involved in T. cruzi-host cell interactions that leads to infection, since other molecules that are apparently implicated in the infection process are glycosylphosphatidylinositol membrane-anchored molecules, which are released from the trypanosome by action of the trypanosome phospholipase C. The fact that Tc-1 is expressed only in invasive trypomastigotes but not in noninvasive epimastigotes or amastigotes indicates that the Tc-1 gene is developmentally regulated in the cell cycle of parasites. Furthermore, the fact that T. cruzi infection is inhibited when Tc-1 is blocked by specific antibodies suggests that the Tc-1 gene may be an invasive gene in the parasite that regulates the pathogenesis of T. cruzi infection.
In view of the predicted structure of Tc-1, we speculate that T. cruzi exploits the host CKII to phosphorylate the extracellular domain of Tc-1, which may be important for cellular invasion. The CKII sites in the cytoplasmic region of Tc-1 would be phosphorylated by the parasite's own CKII, which in turn might activate a series of signal cascades that may be important for parasite invasion or parasite biology.
Since there are no homologs of Tc-1 in humans, this gene and its encoded protein could be excellent targets for molecular intervention in Chagas' disease and might lead to the development of novel drugs for chemotherapy. Furthermore, the fact that Tc-1 is highly immunogenic, as evidenced by the high antibody titer (1:50,000) produced in experimental immunizations of goats for the characterization of Tc-1 in this study, and the fact that antibodies raised against Tc-1 neutralize T. cruzi infection of mammalian cells suggest that Tc-1 could also be a candidate for vaccine development.
Acknowledgments
This work was supported in part by NIH grants GM 08037, GM 05994, AI 07281, AI 056667, HL 007737, MD 000104, and RR 003032.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aboagye-Kwarteng, T., K. ole-MoiYoi, and J. D. Lonsdale-Eccles. 1991. Phosphorylation differences among proteins of bloodstream developmental stages of Trypanosoma brucei brucei. Biochem. J. 275:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, D. A., F. Villalta, and M. F. Lima. 2003. Transforming growth factor α binds to Trypanosoma cruzi amastigotes to induce signaling and cellular proliferation. Infect. Immun. 71:4201-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burleigh, B. B., and A. M. Woolsey. 2002. Cell signaling and Trypanosoma cruzi invasion. Cell. Microbiol. 4:701-711. [DOI] [PubMed] [Google Scholar]
- 4.Caler, E. V., R. E. Morty, B. A. Burleigh, and N. W. Andrews. 2000. Dual role of signaling pathways leading to Ca2+ and cyclic AMP elevation in host cell invasion by Trypanosoma cruzi. Infect. Immun. 68:6602-6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Sayed, N. M., P. J. Myler, G. Blandin, M. Berriman, J. Crabtree, G. Aggarwal, E. Caler, H. Renauld, E. A. Worthey, C. Hertz-Fowler, E. Ghedin, C. Peacock, D. C. Bartholomeu, B. J. Haas, A. N. Tran, J. R. Wortman, U. C. Alsmark, S. Angiuoli, A. Anupama, J. Badger, F. Bringaud, E. Cadag, J. M. Carlton, G. C. Cerqueira, T. Creasy, A. L. Delcher, A. Djikeng, T. M. Embley, C. Hauser, A. C. Ivens, S. K. Kummerfeld, J. B. Pereira-Leal, D. Nilsson, J. Peterson, S. L. Salzberg, J. Shallom, J. C. Silva, J. Sundaram, S. Westenberger, O. White, S. E. Melville, J. E. Donelson, B. Andersson, K. D. Stuart, and N. Hall. 2005. Comparative genomics of trypanosomatid parasitic protozoa. Science 309:404-409. [DOI] [PubMed] [Google Scholar]
- 6.El-Sayed, N. M., P. J. Myler, D. C. Bartholomeu, D. Nilsson, G. Aggarwal, A. N. Tran, E. Ghedin, E. A. Worthey, A. L. Delcher, G. Blandin, S. J. Westenberger, E. Caler, G. C. Cerqueira, C. Branche, B. Haas, A. Anupama, E. Arner, L. Aslund, P. Attipoe, E. Bontempi, F. Bringaud, P. Burton, E. Cadag, D. A. Campbell, M. Carrington, J. Crabtree, H. Darban, J. F. da Silveira, P. de Jong, K. Edwards, P. T. Englund, G. Fazelina, T. Feldblyum, M. Ferella, A. C. Frasch, K. Gull, D. Horn, L. Hou, Y. Huang, E. Kindlund, M. Klingbeil, S. Kluge, H. Koo, D. Lacerda, M. J. Levin, H. Lorenzi, T. Louie, C. R. Machado, R. McCulloch, A. McKenna, Y. Mizuno, J. C. Mottram, S. Nelson, S. Ochaya, K. Osoegawa, G. Pai, M. Parsons, M. Pentony, U. Pettersson, M. Pop, J. L. Ramirez, J. Rinta, L. Robertson, S. L. Salzberg, D. O. Sanchez, A. Seyler, R. Sharma, J. Shetty, A. J. Simpson, E. Sisk, M. T. Tammi, R. Tarleton, S. Teixeira, S. Van Aken, C. Vogt, P. N. Ward, B. Wickstead, J. Wortman, O. White, C. M. Fraser, K. D. Stuart, and B. Andersson. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas' disease. Science 309:409-415. [DOI] [PubMed] [Google Scholar]
- 7.Engelman, D. M., T. A. Steitz, and A. Goldman. 1986. Identifying nonpolar transmembrane helices in amino acid sequences of membrane proteins. Annu. Rev. Biophys. Biophys. Chem. 15:321-353. [DOI] [PubMed] [Google Scholar]
- 8.Frasch, A. C. 2000. Functional diversity in the trans-sialidase and mucin families in Trypanosoma cruzi. Parasitol. Today 16:282-286. [DOI] [PubMed] [Google Scholar]
- 9.Giordano, R., D. L. Fouts, D. Tewari, W. Colli, J. E. Manning, and M. J. M. Alves. 1999. Cloning of a surface membrane glycoprotein specific for the infective form of Trypanosoma cruzi having adhesive properties to laminin. J. Biol. Chem. 274:3461-3468. [DOI] [PubMed] [Google Scholar]
- 10.Hall, B. S., and M. A. Pereira. 2000. Dual role for transforming growth factor β-dependent signaling in Trypanosoma cruzi infection of mammalian cells. Infect. Immun. 68:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleshchenko, Y. Y., T. N. Moody, V. A. Furtak, J. Ochieng, M. F. Lima, and F. Villalta. 2004. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect. Immun. 72:6717-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazaro, J. B., J. Boretto, B. Selmi, J. P. Capony, and B. Canard. 2000. Phosphorylation of AZT-resistant human immunodeficiency virus type 1 reverse transcriptase by casein kinase II in vitro: effects on inhibitor sensitivity. Biochem. Biophys. Res. Commun. 275:26-32. [DOI] [PubMed] [Google Scholar]
- 13.Lima, M. F., and F. Villalta. 1989. Trypanosoma cruzi trypomastigote clones differentially express a parasite cell adhesion molecule. Mol. Biochem. Parasitol. 33:159-170. [DOI] [PubMed] [Google Scholar]
- 14.Magdesian, M. H., R. Giordano, H. Ulrich, M. A. Juliano, L. Juliano, R. I. Schumacher, W. Colli, and M. J. Alves. 2001. Infection by Trypanosoma cruzi. Identification of a parasite ligand and its host cell receptor. J. Biol. Chem. 276:19382-19389. [DOI] [PubMed] [Google Scholar]
- 15.Meggio, F., and L. A. Pinna. 2003. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17:349-368. [DOI] [PubMed] [Google Scholar]
- 16.Moody, T. N., J. Ochieng, and F. Villalta. 2000. Novel mechanism that Trypanosoma cruzi uses to adhere to the extracellular matrix mediated by human galectin-3. FEBS Lett. 470:305-308. [DOI] [PubMed] [Google Scholar]
- 17.Nde, P. N., K. J. Simmons, Y. Y. Kleshchenko, S. Pratap, M. F. Lima, and F. Villalta. 2006. Silencing of laminin γ-1 gene blocks Trypanosoma cruzi infection. Infect. Immun. 73:1643-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtsuki, K., T. Maekawa, S. Harada, A. Karino, Y. Morikawa, and M. Ito. 1998. Biochemical characterization of HIV-1 Rev as a potent activator of casein kinase II in vitro. FEBS Lett. 428:235-240. [DOI] [PubMed] [Google Scholar]
- 19.Ouaissi, M. A. D., D. Afchain, A. Capron, and J. A. Grimaud. 1984. Fibronectin receptors on Trypanosoma cruzi trypomastigotes and their biological function. Nature 308:380-382. [DOI] [PubMed] [Google Scholar]
- 20.Rosemberg, S., C. J. Chaves, M. L. Higuchi, M. B. Lopes, L. H. Castro, and L. R. Machado. 1992. Fatal meningoenchephalitis caused by reactivation of Trypanosoma cruzi infection in a patient with AIDS. Neurology 42:640-642. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Simmons, K. J., P. N. Nde, Y. Y. Kleshchenko, M. F. Lima, and F. Villalta. 2006. Stable RNA interference of host thrombospondin-1 blocks Trypanosoma cruzi infection. FEBS Lett. 580:2365-2370. [DOI] [PubMed] [Google Scholar]
- 23.Tardieux, I., M. H. Nathanson, and N. W. Andrews. 1994. Role of host cell invasion of Trypanosoma cruzi-induced cytosolic free Ca2+ transients. J. Exp. Med. 179:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todorov, A. G., M. Einicker-Lamas, S. L. de Castro, M. M. Oliveira, and A. Guilherme. 2000. Activation of host cell phosphatidylinositol 3-kinases by Trypanosoma cruzi infection. J. Biol. Chem. 275:32182-32186. [DOI] [PubMed] [Google Scholar]
- 25.Turner, C. W., M. F. Lima, and F. Villalta. 2002. Trypanosoma cruzi uses a 45-kDa mucin for adhesion to mammalian cells. Biochem. Biophys. Res. Commun. 11:29-34. [DOI] [PubMed] [Google Scholar]
- 26.Villalta, F., and F. Kiersenbaum. 1982. Growth of isolated amastigotes of Trypanosoma cruzi in cell-free medium. J. Protozool. 29:570-576. [DOI] [PubMed] [Google Scholar]
- 27.Villalta, F., M. F. Lima, A. Ruiz-Ruano, and L. Zhou. 1992. Attachment of Trypanosoma cruzi to host cells: a monoclonal antibody recognizes a trypomastigote stage-specific epitope on the gp83 required for parasite attachment. Biochem. Biophys. Res. Commun. 182:6-13. [DOI] [PubMed] [Google Scholar]
- 28.Villalta, F., A. Ruiz-Ruano, A. A. Valentine, and M. F. Lima. 1993. Purification of a 74-kilodalton surface protein from heart myoblasts that inhibits binding and entry of Trypanosoma cruzi into heart cells. Mol. Biochem. Parasitol. 61:217-230. [DOI] [PubMed] [Google Scholar]
- 29.Villalta, F., Y. Zhang, K. Bibb, J. M. Burns, Jr., and M. F. Lima. 1998. Signal transduction in human macrophages by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosome entry through the MAP kinase pathway. Biochem. Biophys. Res. Commun. 249:247-252. [DOI] [PubMed] [Google Scholar]
- 30.Villalta, F., Y. Zhang, K. Bibb, S. Pratap, J. M. Burns, Jr., and M. F. Lima. 1999. Signal transduction in human macrophages by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosome entry through protein kinase C activation. Mol. Cell Biol. Res. Commum. 2:67-70. [DOI] [PubMed] [Google Scholar]
- 31.Villalta, F., C. Smith, A. Ruiz-Ruano, and M. F. Lima. 2001. A ligand that Trypanosoma cruzi uses to bind to mammalian cells to initiate infection. FEBS Lett. 505:383-388. [DOI] [PubMed] [Google Scholar]
- 32.Woolsey, A. M., L. Sunwoo, C. A. Petersen, S. M. Brachmann, L. C. Cantley, and B. A. Burleigh. 2003. Novel PI 3-kinase-dependent mechanisms of trypanosome invasion and vacuole maturation. J. Cell Sci. 116:3611-3622. [DOI] [PubMed] [Google Scholar]