Abstract
Germination of Arabidopsis seeds is light dependent and under phytochrome control. Previously, phytochromes A and B and at least one additional, unspecified phytochrome were shown to be involved in this process. Here, we used a set of photoreceptor mutants to test whether phytochrome D and/or phytochrome E can control germination of Arabidopsis. The results show that only phytochromes B and E, but not phytochrome D, participate directly in red/far-red light (FR)-reversible germination. Unlike phytochromes B and D, phytochrome E did not inhibit phytochrome A-mediated germination. Surprisingly, phytochrome E was required for germination of Arabidopsis seeds in continuous FR. However, inhibition of hypocotyl elongation by FR, induction of cotyledon unfolding, and induction of agravitropic growth were not affected by loss of phytochrome E. Therefore, phytochrome E is not required per se for phytochrome A-mediated very low fluence responses and the high irradiance response. Immunoblotting revealed that the need of phytochrome E for germination in FR was not caused by altered phytochrome A levels. These results uncover a novel role of phytochrome E in plant development and demonstrate the considerable functional diversification of the closely related phytochromes B, D, and E.
In many plants, seed germination is light dependent (Casal and Sánchez, 1998). Among plant photoreceptors, only phytochromes have been shown to directly mediate induction of germination. Recently, several new insights into the molecular mechanisms of phytochrome action and its physiological role throughout the whole life cycle of plants were achieved (for review, see Whitelam and Devlin, 1997; Casal, 2000; Smith, 2000). In Arabidopsis, phytochrome is a small gene family, consisting of the five members, PHYA to PHYE (Sharrock and Quail, 1989; Clack et al., 1994). PHYB, PHYD, and PHYE are evolutionary related and clearly separated from PHYA and PHYC (Mathews and Sharrock., 1997). Studies using mutants and overexpressor lines demonstrated that individual phytochrome family members have overlapping and distinct functions (Reed et al., 1994; Smith et al., 1997). phyA and phyB are the best characterized phytochromes in Arabidopsis. phyA mediates the high irradiance response in far red light (FR; FR-HIR) and the very low fluence response (VLFR), which can also be induced by FR. In contrast, phyB mediates the red light (R)/FR-reversible low fluence response (LFR; Casal et al., 1998). Less is known about the other phytochromes. An LFR-type action of phyD could be observed in phyB seedlings (Aukerman et al., 1997; Hennig et al., 1999a). The high sequence identity of phyB and phyD (>80%) suggests very similar roles of these two phytochromes, albeit certain physiological differences have been reported (Hennig et al., 1999a). Finally, for phyE, mainly functions in the shade avoidance syndrome of adult plants have been described (Devlin et al., 1996, 1998). Furthermore, phyE has been shown to be capable of signaling to the circadian clock in seedlings (Devlin and Kay, 2000).
Light-induced germination has been studied extensively in Arabidopsis (for review, see Casal and Sánchez, 1998). This work revealed that induction of germination by R is mediated by phyB and phyA, whereas induction by FR is mediated only by phyA (Shinomura et al., 1996, 1998). Casal and coworkers demonstrated that phyA induces germination when acting in the VLFR mode. In contrast, continuous FR effectively opposes germination in many plant species, although not in Arabidopsis (Botto et al., 1996; Casal and Sánchez, 1998). Moreover, both phyB and phyD have been shown to interfere with phyA-mediated induction of germination by FR (Hennig et al., 2001). However, phyA and phyB are clearly not the only phytochromes participating in light-induced germination. R/FR-reversible induction of germination was observed in phyA phyB double mutants (Poppe and Schäfer, 1997). Nonetheless, it has remained completely unknown which other phytochromes are involved in this reaction. Here, we used additional phyD and phyE photoreceptor mutant combinations to investigate phytochrome-mediated germination in Arabidopsis in more detail.
RESULTS
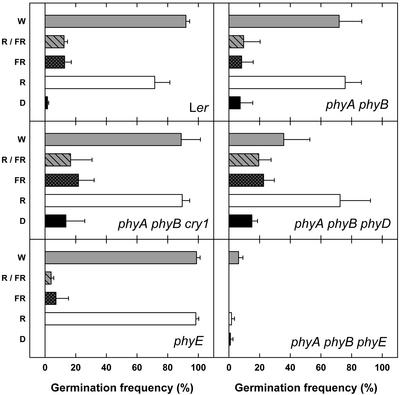
Poppe and Schäfer (1997) observed R/FR-reversible induction of germination in phyA phyB double mutants. Using the original light regime of hourly pulses for 3 d, we analyzed additional photoreceptor mutants (Fig. 1). Wild-type (WT) seeds germinated efficiently in continuous white light (W) and after R pulses (30 s, 39 μmol m−2 s−1) but germinated poorly in darkness and after long wavelength FR (3 min, 35 μmol m−2 s−1) or R/FR pulses. As demonstrated before (Poppe and Schäfer, 1997), germination of phyA phyB double mutants did not differ from WT under these conditions. Likewise, phyA phyB cry1 and phyA phyB phyD triple mutants behaved in a very similar way to WT, except for less efficient germination of phyA phyB phyD in W. Moreover, phyE mutants showed an unaltered germination. In contrast, basically no light-inducible germination could be observed for seeds of phyA phyB phyE triple mutants.
Figure 1.
Induction of germination by light pulses. After sowing, seeds were incubated for 24 h at 4°C in darkness, followed by continuous W or hourly pulses of the indicated light quality at 25°C for 3 d. Germination frequencies were determined after further incubation for 3 d in darkness (D).
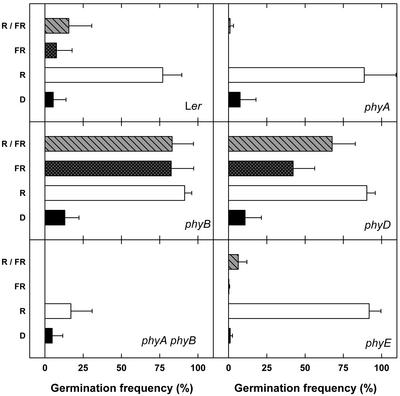
Induction of germination by FR depends on phyA and is inhibited by phyB and phyD (Hennig et al., 2001). Because phyB, phyD, and phyE are evolutionary closely related, we tested the influence of phyE on phyA-mediated germination in FR. Seeds were incubated for 24 h at 4°C in darkness; after treatment with indicated light qualities for 3 h, the seeds were stored at 25°C in darkness for 3 d. In agreement with previous reports, only R lead to high germination rates in WT and phyA under these conditions (Fig. 2). In contrast, FR and R followed by FR were as efficient as R in inducing germination of phyB. For phyD, FR and R/FR were slightly less effective than R. Seeds of the phyA phyB double mutant germinated poorly under all light conditions. Importantly, phyE seeds did not germinate appreciably after FR or R/FR and thus behaved like WT rather than phyB or phyD.
Figure 2.
Induction of germination by light periods of 3 h. After sowing, seeds were incubated for 24 h at 4°C in darkness, followed by a treatment at 25°C with R or FR for 3 h or with R for 3 h, followed by FR for 3 h (R/FR). Germination frequencies were determined after further incubation for 6 d in darkness (D).
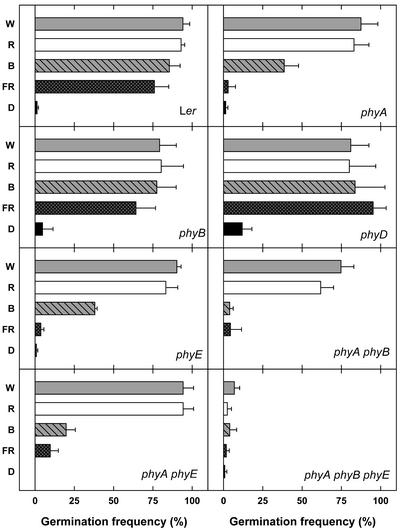
To further characterize the role of phyE in germination, we analyzed induction of germination by continuous irradiation in more detail. Seeds were incubated for 24 h at 4°C in darkness. After treatment with indicated light qualities for 3 d, seeds were stored at 25°C in darkness for another 3 d. Continuous irradiation with W, blue (B), R, or FR for 3 d induced high germination frequencies in WT (Fig. 3). In contrast, phyA seeds germinated poorly after B and not at all after FR treatment. Germination of phyB and phyD was indistinguishable from WT. Seeds of the phyA phyB double mutant germinated after neither B nor FR treatment. Surprisingly, phyE seeds behaved in a very similar way to phyA; germination after B was impaired and after FR it was completely abolished. These results indicate a requirement of phyE for phyA-mediated induction of germination under continuous FR. Similarly, the phyA phyE double mutant germinated to a low extent after B and barely after FR. Finally, the phyA phyB phyE triple mutant completely failed to show light-inducible germination under these conditions.
Figure 3.
Induction of germination by continuous light. After sowing, seeds were incubated for 24 h at 4°C and for 24 h at 25°C in darkness, followed by a treatment with the indicated light quality at 25°C for 3 d. Germination frequencies were determined after further incubation for 3 d in darkness (D).
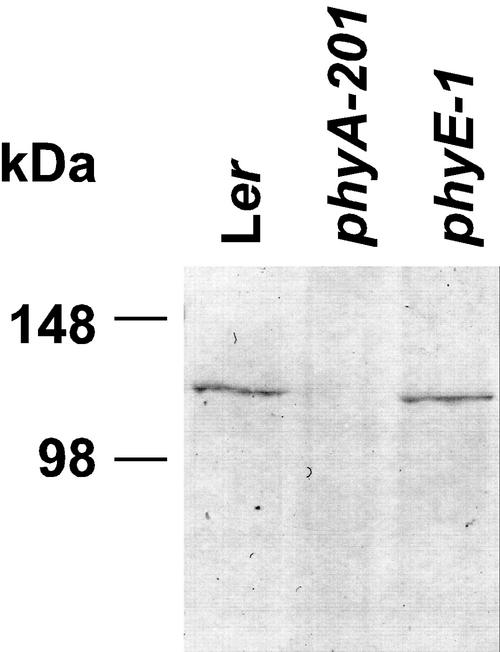
The requirement of phyE on germination in FR might be caused by altered phyA levels in phyE seeds. To test this hypothesis, we analyzed total phyA amounts by immunoblotting. Total protein (25 μg) extracted from seeds, which had been allowed to imbibe in darkness for 24 h at 4°C and for 24 h at 25°C was used. No signal was detected in phyA mutant seeds. Importantly, WT and phyE contained similar phyA levels (Fig. 4). Thus, the inability of phyE seeds to germinate under FR is not caused by strongly decreased phyA amounts.
Figure 4.
Contents of phyA in Arabidopsis seeds after storage for 24 h at 4°C and for 24 h at 25°C in darkness. Samples were analyzed by immunoblotting of 25 μg of protein and probing with an antiserum against phyA.
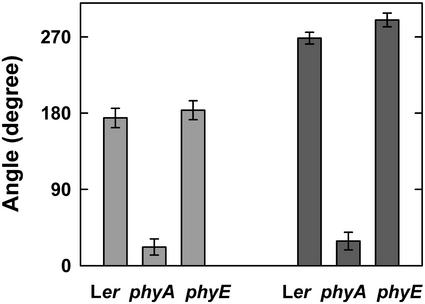
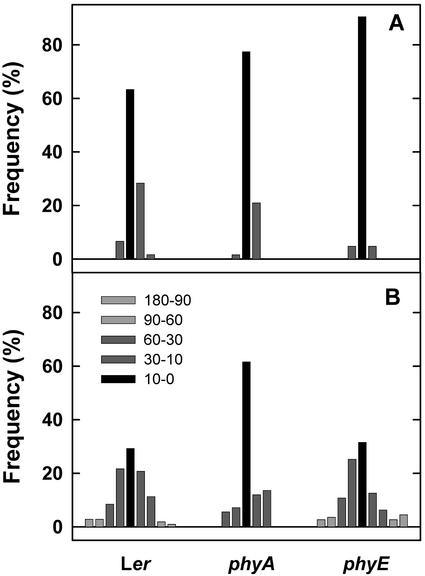
Subsequently, we tested whether phyE is also required for other phyA-mediated processes. To this end, seedlings were exposed to hourly FR pulses (36 μmol m−2 s−1) or continuous FR (3 μmol m−2 s−1). After 5 d cotyledon opening angles were measured. The results are shown in Figure 5. Both FR pulses and continuous FR induced a strong opening of cotyledons in WT and phyE but failed to do so in phyA. Therefore, only phyA but not phyE is required for induction of cotyledon opening in Arabidopsis. Another well-documented response of phyA is its interference with the negative gravitropism of seedling growth. Here, we measured the randomization of growth orientation after 3 d in darkness (Fig. 6A) or after exposure to hourly FR pulses (10 μmol m−2 s−1) for 3 d (Fig. 6B). In darkness, >60% of all seedlings (WT, phyA, and phyE) differed <10 degrees from the vertical. Seedlings of WT and phyE, which were treated with FR pulses, displayed a wide range of hypocotyl angles, reflecting agravitropism. In contrast, phyA seedlings treated with FR pulses remained predominantly vertical.
Figure 5.
Induction of cotyledon opening by FR. After induction of germination, seeds were exposed either to hourly FR pulses of 5 min (36 μmol m−2 s−1; light-gray bars) or to continuous FR (3 μmol m−2 s−1; dark-gray bars). After 5 d, seedlings were photographed and measurements were taken.
Figure 6.
Suppression of gravitropic growth by FR. After induction of germination, seeds were kept in darkness (A) or exposed to hourly FR pulses of 5 min (10 μmol m−2 s−1; B). Growth orientation of at least 60 seedlings was determined after 3 d. Displayed are frequencies of seedlings with angles falling into the indicated ranges (light-gray bars, <−60 or >+60 degrees; dark-gray bars, <−10 or > +10 degrees; black bars, 0 ± 10 degrees).
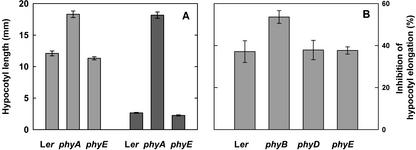
Next, we tested whether phyE might effect growth under FR, either by positive or by negative interference with phyA action. Seedlings of WT, phyA, and phyE were exposed to hourly FR pulses (36 μmol m−2 s−1) or continuous FR (3 μmol m−2 s−1). After 5 d hypocotyl lengths were measured. The results (Fig. 7A) show that WT and phyE seedlings did not differ either under FR pulses or under continuous FR. In contrast, phyA seedlings remained elongated under both conditions. Interaction of photoreceptors can be observed more easily under nonsaturating conditions (Hennig et al., 2001). Consequently, seedlings of WT, phyA, phyB, phyD, and phyE were grown under nonsaturating continuous FR (0.6 μmol m−2 s−1). WT seedlings showed an inhibition of elongation of about 40% under these conditions (Fig. 7B). In contrast, growth of phyB mutants was inhibited by >50%. Nonetheless, neither phyD nor phyE differed from the WT.
Figure 7.
Inhibition of hypocotyl elongation in FR. A, After induction of germination, seeds were exposed either to hourly FR pulses of 5 min (36 μmol m−2 s−1; light-gray bars) or to continuous FR (3 μmol m−2 s−1; dark-gray bars). After 5 d, seedlings were photographed and measurements were taken. B, After induction of germination, seeds were exposed to 0.6 μmol m−2 s−1 FR. Hypocotyl lengths of at least 25 seedlings were determined after 3 d.
DISCUSSION
Induction of germination by light is controlled by phyA and phyB (Shinomura et al., 1996). However, in addition to these two photoreceptors other pigments have been shown to be involved in this process. R/FR-reversible germination of phyA phyB double mutants led to the conclusion that at least one other member of the phytochrome family controls germination in Arabidopsis (Poppe and Schäfer, 1997). Moreover, no reports about a possible involvement of other photoreceptors, e.g. cryptochromes, exist. To determine the role of additional phytochromes in germination, we used several photoreceptor single, double, and triple mutant combinations.
phyE Controls R/FR-Reversible Germination in phyA phyB
A program of hourly light pulses applied for 3 d induced R/FR-reversible germination in phyA phyB double mutants. Both, phyA phyB phyD and phyA phyB cry1 triple mutants displayed the same pattern of germination. Therefore, neither phyD nor cry1 are required for germination under these conditions. Given the high sequence conservation of phyB and phyD, it was surprising that absence of phyD did not further impair R-induced germination. Under none of the diverse conditions tested was any direct demonstration of germination control by phyD obtained. In contrast, phyA phyB phyE triple mutants were severely impaired in light-induced germination and lacked any R/FR-reversible germination. We conclude, therefore, that phyE plays an active role in germination of Arabidopsis. If any additional photoreceptors were involved in this process, they clearly depend on the presence of at least one of phyA, phyB, or phyE. Moreover, the R/FR-reversible nature of induction of germination by phyE implies that this phytochrome can function in the LFR mode of phytochrome action.
The similar actions of phyB and phyE in germination prompted us to investigate negative effects of these phytochromes on phyA action. Previously, we described inhibition of phyA-mediated germination by phyB and phyD (Hennig et al., 2001). However, we were not able to detect any negative interference of phyE with phyA function. This difference could be due to different expression levels of phyB, phyD, and phyE. Alternatively, it could reflect inherent mechanistic differences in the biological actions of these phytochromes.
phyE Is Required for Germination in Continuous FR but Not for Other phyA Responses
In addition to its involvement in germination after pulsed irradiations, phyE proved to affect germination in continuous light. Surprisingly, phyE was required for induction of germination by continuous FR. This observation was not expected, because phyA is generally regarded as being both necessary and sufficient for effects of FR. Responses of phyA to FR are usually attributed to either the VLFR or the HIR mode of phytochrome action (Casal et al., 1998). We analyzed the consequences of phyE deficiency on additional responses of either the VLFR or HIR type. None of three other VLFR reactions (namely, induction of cotyledon opening, interference with gravitropism, and growth inhibition by FR pulses) was altered in phyE mutants. Further experiments showed that phyE was also not required for control of hypocotyl growth under continuous FR involving an HIR. However, phyE also had no negative influence on inhibition of hypocotyl elongation by intermediate fluence rates of FR. In this respect, phyE behaves like phyD and not like phyB, which counteracts the FR-HIR (Hennig et al., 1999b). Therefore, only germination under FR, but not all phyA-mediated responses to FR, depend on the presence of phyE.
Immunoblots showed that the amount of phyA in seeds is not influenced by the presence or absence of phyE. Consequently, the failure of phyE seeds to germinate under FR is not caused by reduced phyA levels. Several other mechanisms could account for the behavior of the phyE mutant. phyE might control the level of signaling intermediates required by phyA for inducing germination. Alternatively, phyE might act as an additional photoreceptor for FR. In this regard, it is noteworthy that a recent report described profound differences between the photochemical properties of phyB and phyE (Eichenberg et al., 2000). Further investigations will be required to resolve this issue.
The Physiological Roles of phyB, phyD, and phyE Differ Considerably
The three closely related photoreceptors phyB, phyD, and phyE have been shown to be involved in responses to R/FR (Devlin et al., 1998, 1999). All three phyB-like phytochromes can function in the LFR mode of phytochrome action. Moreover, they are all capable of signaling to the circadian clock (Devlin and Kay, 2000). For phyB and phyD, but not for phyE, an involvement in control of hypocotyl elongation has been shown (Reed et al., 1993; Aukerman et al., 1997; Hennig et al., 1999a). Here, we report that phyB and phyE, but not phyD, participate in R/FR-reversible germination. Poppe and Schäfer (1997) demonstrated that the photobiology of induction of germination by phyE, termed phyX by these authors, differed greatly from that of phyB: Requirement of prolonged irradiation and fluence rate response curves were more similar to an HIR than to the classical LFR. Likewise, the loss-of-reversibility kinetics were much faster for the phyE than for the phyB response (Poppe and Schäfer, 1997). Our results show that phyB and phyE also have opposing effects. Whereas phyB exerts an inhibitory action on a diverse set of phyA functions under FR, including germination (Hennig et al., 2001), phyE is required for germination under FR. Taken together, phyB is more similar to phyD than to phyE regarding interference with phyA-mediated germination. In contrast, phyB is more similar to phyE than to phyD regarding positive control of germination after R pulses. Hence, the physiological functions of the closely related phyB, phyD, and phyE are only partly redundant but differ in several aspects.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Light Sources
The Landsberg erecta (Ler) ecotype of Arabidopsis (L.) Heynh. was used (obtained from Lehle Seeds, Tuscon, AZ). The mutants were phyA-201 (Nagatani et al., 1993; Reed et al., 1994), phyB-5 (Koornneef et al., 1980; Reed et al., 1993), phyD-1 (Aukerman et al., 1997), phyE-1 (Devlin et al., 1998), and hy4-2.23n (containing a defect CRY1 gene, Koornneef et al., 1980; Ahmad and Cashmore, 1993). Double and triple mutants were generated by crossing (Devlin et al., 1998, 1999; Hennig et al., 1999a).
Seeds were plated on four layers of water-soaked filter paper, which were placed into clear plastic boxes. A 24-h dark treatment at 4°C was followed by induction of germination by the light quality and duration indicated in Figures 1 to 4 and 7B. Subsequently, samples were further incubated in the dark at 25°C. Standard B (436 nm, 35 μmol m−2 s−1), R (656 nm, 30 μmol m−2 s−1), or FR (730 nm, 20 μmol m−2 s−1) fields were used. Repetitive pulse irradiation with monochromatic light was achieved using computer-controlled Leitz Prado light projectors (Leitz, Wetzlar, Germany) with 660 nm of DAL interference filters or RG9 color glasses (Schott, Mainz, Germany). Alternatively, seeds were sown onto plates (0.8% agar Lehle medium, Lehle Seeds) and chilled at 4°C for 3 d. Germination was induced by 30 min of white fluorescent light (80 μmol m−2 s−1), and plates were incubated in the dark at 22°C for 24 h, after which irradiation was started (Figs. 5, 6, and 7A).
Protein Extraction and Immunoblotting
Seeds were ground in liquid nitrogen and extracted with preheated SDS sample buffer (65 mm Tris-HCl, pH 6.8, 4 m urea, 3% [w/v] SDS, 10% [v/v] glycerol, 10 mm dithioerythritol, 0.05% [w/v] bromphenol blue). After heating to 95°C for 5 min, the crude extracts were clarified by centrifugation for 15 min at 20,000g (25°C).
SDS-PAGE, protein blotting, and immunodetection were performed as described by Harter et al. (1993) using CSPD-STAR (New England Biolabs, Beverly, MA) as the substrate for alkaline phosphatase-conjugated secondary antibodies. Monoclonal antibodies against phyA of Arabidopsis were a generous gift of P. Quail (Albany, CA; Hirschfeld et al., 1998).
Determination of Hypocotyl Length and Germination Frequencies
Hypocotyl lengths were measured manually for at least 20 seedlings. The mean value and se from at least three independent experiments (Fig. 7B) or the results of one representative experiment are shown (Fig. 7A). Significant differences of hypocotyl lengths of dark-grown seedlings were not observed (data not shown). Germination percentages of 80 to 120 seeds were determined by taking the protrusion of the radicle as the criterion of germination. The mean value and se of at least four independent experiments are shown. At least two independent seed batches per mutant were used for germination experiments.
Determination of Hypocotyl Growth Orientation
Seeds were sown onto plates (1.2% agar Lehle medium, Lehle Seeds) and chilled at 4°C for 3 d. Germination was induced by 30 min of white fluorescent light (80 μmol m−2 s−1), and plates were orientated vertically and incubated in the dark at 22°C for 24 h, after which irradiation was started. Plates were maintained at a vertical orientation throughout. After 3 d of treatments, plates were photographed, and measurements were taken of at least 60 (dark control) or 100 seedlings. The angles of hypocotyls relative to vertical were recorded, resulting in values from 0 (vertical) to ±180 degrees.
ACKNOWLEDGMENTS
We thank Peter H. Quail for the antiserum against phyA. We thank Dr. Trish Ingles for help with the experiments on growth orientation. Furthermore, we thank Rena Wiehe for excellent technical assistance and Claudia Büche for critical reading of the manuscript.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft (grant no. SFB592 to E.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010559.
LITERATURE CITED
- Ahmad M, Cashmore AR. HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto JF, Sanchez RA, Whitelam GC, Casal JJ. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ. Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol. 2000;71:1–11. doi: 10.1562/0031-8655(2000)071<0001:pcppii>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Sánchez RA. Phytochromes and seed germination. Seed Sci Res. 1998;8:317–329. [Google Scholar]
- Casal JJ, Sánchez RA, Botto JF. Modes of action of phytochromes. J Exp Bot. 1998;49:127–138. [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Halliday KJ, Harberd NP, Whitelam GC. The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: Novel phytochromes control internode elongation and flowering time. Plant J. 1996;10:1127–1134. doi: 10.1046/j.1365-313x.1996.10061127.x. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Kay SA. Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell. 2000;12:2499–2510. doi: 10.1105/tpc.12.12.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Patel SR, Whitelam GC. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1487. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Robson PRH, Patel SR, Goosey L, Sharrock R, Whitelam GC. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberg K, Bäurle I, Paulo N, Sharrock RA, Rüdiger W, Schäfer E. Arabidopsis phytochromes C and E have different spectral characteristics from those of phytochromes A and B. FEBS Lett. 2000;470:107–112. doi: 10.1016/s0014-5793(00)01301-6. [DOI] [PubMed] [Google Scholar]
- Harter K, Talke-Messerer C, Barz W, Schäfer E. Light-dependent and sucrose-dependent gene expression in photomixotrophic cell suspension cultures and protoplasts of rape (Brassica napus L.) Plant J. 1993;4:507–516. [Google Scholar]
- Hennig L, Funk M, Whitelam GC, Schäfer E. Functional interaction of cryptochrome 1 and phytochrome D. Plant J. 1999a;20:289–294. doi: 10.1046/j.1365-313x.1999.t01-1-00599.x. [DOI] [PubMed] [Google Scholar]
- Hennig L, Poppe C, Sweere U, Martin A, Schäfer E. Negative interference of endogenous phytochrome B with phytochrome A function in Arabidopsis. Plant Physiol. 2001;125:1036–1044. doi: 10.1104/pp.125.2.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig L, Poppe C, Unger S, Schäfer E. Control of hypocotyl elongation in Arabidopsis thaliana by photoreceptor interaction. Planta. 1999b;208:257–263. doi: 10.1007/s004250050557. [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Tepperman JM, Clack T, Quail PH, Sharrock RA. Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics. 1998;149:523–535. doi: 10.1093/genetics/149.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Mathews S, Sharrock RA. Phytochrome gene diversity. Plant Cell Environ. 1997;20:666–671. [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Schäfer E. Seed germination of Arabidopsis thaliana phyA/phyB double mutants is under phytochrome control. Plant Physiol. 1997;114:1487–1492. doi: 10.1104/pp.114.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red-far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Hanzawa H, Schäfer E, Furuya M. Mode of phytochrome B action in the photoregulation of seed germination in Arabidopsis thaliana. Plant J. 1998;13:583–590. doi: 10.1046/j.1365-313x.1998.00049.x. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. Action spectra for phyto-chrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Phytochromes and light signal perception by plants: an emerging synthesis. Nature. 2000;407:585–591. doi: 10.1038/35036500. [DOI] [PubMed] [Google Scholar]
- Smith H, Xu Y, Quail PH. Antagonistic but complementary actions of phytochromes A and B allow optimum seedling de-etiolation. Plant Physiol. 1997;114:637–641. doi: 10.1104/pp.114.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Devlin PF. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 1997;20:752–758. [Google Scholar]