Abstract
Targeted gene disruption has proved to be a powerful approach for studying the function of important ligands involved in erythrocyte invasion by the extracellular merozoite form of the human malaria parasite, Plasmodium falciparum. Merozoite invasion proceeds via a number of seemingly independent alternate pathways, such that entry can proceed with parasites lacking particular ligand-receptor interactions. To date, most focus in this regard has been on single-pass (type 1) membrane proteins that reside in the secretory organelles. Another class of merozoite proteins likely to include ligands for erythrocyte receptors are the glycosylphosphatidyl inositol (GPI)-anchored membrane proteins that coat the parasite surface and/or reside in the apical organelles. Several of these are prominent vaccine candidates, although their functions remain unknown. Here, we systematically attempted to disrupt the genes encoding seven of the known GPI-anchored merozoite proteins of P. falciparum by using a double-crossover gene-targeting approach. Surprisingly, and in apparent contrast to other merozoite antigen classes, most of the genes (six of seven) encoding GPI-anchored merozoite proteins are refractory to genetic deletion, with the exception being the gene encoding merozoite surface protein 5 (MSP-5). No distinguishable growth rate or invasion pathway phenotype was detected for the msp-5 knockout line, although its presence as a surface-localized protein was confirmed.
Invasion of erythrocytes by the extracellular merozoite form of the malaria parasite is an essential step in the life cycle of this important pathogen. Proteins residing on the merozoite surface and within its secretory apical organelles form specific interactions with host receptors to initiate erythrocyte attachment and invasion. These proteins are also key targets of protective antibody responses, and as a consequence, several have emerged as promising candidates for inclusion in a vaccine to control Plasmodium falciparum, the most important cause of human malaria.
From electron microscopy studies, it appears that the thick fibrillar coat surrounding the merozoite surface is involved in the initial, long-range (20 to 40 nm) and apparently reversible contact between the merozoite and the erythrocyte (4, 24, 34). This surface coat is almost entirely comprised of glycosylphosphatidyl inositol (GPI)-anchored membrane proteins together with several associating peripheral proteins. Nine GPI-anchored merozoite proteins have been identified in P. falciparum to date: merozoite surface protein 1 (MSP-1) (30), MSP-2 (53), MSP-4 (36), MSP-5 (37), MSP-10 (6), rhoptry-associated membrane antigen (RAMA) (55), Pf92, Pf38, and Pf12, the last three of which were only recently identified (52). With the exception of RAMA, all GPI-anchored merozoite proteins appear to reside on the surface at least to some degree (note that MSP-8, now renamed RMP-1, was originally designated as a surface protein, but it is expressed in the ring stage and it does not appear that the full-length protein is present on the surface of the mature parasite, although the C-terminal-processed fragment containing two epidermal growth factor [EGF]-like domains can be found in isolated merozoites [8, 19]). A number of cysteine-rich globular domains are found in the GPI-anchored surface proteins, including EGF-like domains, “six-cysteine” domains which are predicted to be structurally homologous to surface proteins of Toxoplasma gondii (26) and potentially novel folds such as that present in Pf92 (52). These regions are obvious candidates for mediating protein-protein interactions, including receptor-binding function.
While an involvement in primary recognition and attachment to erythrocytes is likely for at least some of the GPI-anchored surface proteins, to date only minimal experimental evidence in support of this has been published. For example, a number of investigations have reported binding of MSP-1 or MSP-1-processed fragments to erythrocyte receptors (43, 47), and more-recent data suggest that the C-terminal MSP-119 fragment binds to erythrocyte receptor band 3 (28). However, as is the case with all other GPI-anchored surface proteins, the biological significance of MSP-1-receptor interactions has not been confirmed using reverse genetics.
In contrast, molecular genetic approaches, especially the generation of gene knockout lines, have proved very useful in defining the relevance of interactions between apical proteins and erythrocyte receptors. These apical proteins include two distinct families, Duffy-binding-like proteins, referred to in P. falciparum as erythrocyte binding antigens (1, 25), and the reticulocyte binding protein homologue family (48, 49). Targeted disruption of the genes encoding all members of these families has been achieved, in many cases on more than one genetic background (14, 20, 22, 27, 31, 35, 50, 54). While these gene knockout lines continue to effectively invade erythrocytes, as is necessary to allow generation of the mutant line, they may do so by switching the invasion pathway used to attach to cells. That is, in the absence of particular apical proteins, alternate receptor-ligand interactions can be employed. Such phenotypic switching can be revealed using a combination of erythrocytes with altered surface receptors and antibodies against the relevant parasite ligands.
A particularly important technological advance that facilitated the generation of most of these knockout lines is that of double-crossover homologous recombination, a system that utilizes a negative selectable marker to select for integrants (21). P. falciparum transfection can be performed only with circular plasmid DNA, and parasites initially maintain episomally replicating forms of the plasmid (44). While single-crossover integration can be achieved by various positive selection strategies and has proved useful for both gene disruption and allelic replacement, it is a rare event that relies on parasites maintaining the integrated form of the plasmid outgrowing those maintaining episomally replicating forms (13, 16). Moreover, unlike with double-crossover technology, sequence is not deleted using single-crossover targeting. Attempts to disrupt genes encoding several GPI-anchored merozoite surface proteins using single-crossover gene-targeting transfection technology have not proved successful (12, 45). Here, we attempt to knock out seven GPI-anchored merozoite proteins in P. falciparum, utilizing the double-crossover integration approach. We show that this particular set of merozoite proteins is generally refractory to genetic deletion, suggesting that most play important roles in blood-stage development. The exception to this is the msp-5 gene, which was successfully disrupted in P. falciparum 3D7 parasites without a notable change in growth rate or invasion pathway phenotype.
MATERIALS AND METHODS
Plasmids.
5′ (F1) and 3′ (F2) flanking regions of the target gene(s) were cloned on either side of the human dihydrofolate reductase (hDHFR) cassette of the pHTK or pCC-1 double recombination construct incorporating the thymidine kinase (TK) or cytosine deaminase (CD) negative selectable marker, respectively (21; A. G. Maier and A. F. Cowman, unpublished data). MSP-2, MSP-4, MSP-5, and RAMA sequences were amplified from P. falciparum 3D7 genomic DNA, and MSP-1 and MSP-10 sequences were amplified from P. falciparum D10 genomic DNA, using the following oligonucleotides: MSP1 5′ Fwd, CCGCGGCTAATGTAAAATGCAAAAATAAATGTATAC (SacII); MSP1 5′ Rev, CAGATCTCGCTTATTAAATTATGTGCTTCTTG (BglII); MSP1 3′ Fwd, GAATTCACTAACGACTTCGAAGCAAATAAAAAATTG (EcoRI); MSP1 3′ Rev, CCATGGAATGTTTATAAAAAAAAAAATGCTTTTTATGTATGCG (NcoI); MSP2 5′ Fwd, TCCCCGCGGCAATTACGATATAAAACCTAGTATCTTTTC; MSP2 5′ Rev, GAAGATCTCTTTTGGATTTGTTTCGGCATTTTTATG; MSP2 3′ Fwd, CCATCGATGAGAAGTTCAAGAACCAAATCAAGC; MSP2 3′ Rev, CATGCCATGGGACCTTATAACATGTACGCCTTTTTAAC; MSP4 5′ Fwd, TCCCCGCGGGTATATAAAATAACACGAATGTGTTACCC; MSP4 5′ Rev, GAAGATCTGGAGACTTTTCTAGAACCTTTTCTTG; MSP4 3′ Fwd, CCATCGATGTTCAACCAAGTTCATCAAATTCAGG; MSP4 3′ Rev, CATGCCATGGGTACCTAT GTATGTATATATGTATACATCC; MSP5 5′ Fwd, TCCCCGCGGCACCATGTTA TTAATTTATGACAATATATATTTG; MSP5 5′ Rev, GAAGATCTCTGATATATC AACAGAACGTGTTGC; MSP5 3′ Fwd, CCATCGATTCTTGAAATTAATGAGAA TGCAGAAATAGG; MSP5 3′ Rev, CATGCCATGGGAAATTAAAAAAAAAAAA AAGAGTCTTACTGCAC; MSP10 5′ Fwd, TCCCCGCGGGTTTCTTTTAATTCGATACGACTGCTTTCATG (SacII); MSP10 3′ Rev, CTAGACTAGTCTAGGGAAAAATGCTTCAATTAAATCTTCTTC (SpeI); MSP10 3′ Fwd, CCCATCGATCTCTGAAAACATTCAAGAAATTCTCAGTACGG (ClaI); MSP10 3′ Rev, CATGCCATGGACCCAAAATAGGAAAACGTTATTTTAATTTTTGAATGAG (NcoI); RAMA 5′ Fwd, GGACTAGTAAAAAATGGTATAAGCGGTC (SpeI); RAMA 5′ Rev, GAAGATCTCTTCATATTTCATTTGTTCG (BglII); RAMA 3′ Fwd, CCGGAATTCTTATGAAGAATATGAAGACG (EcoRI); and RAMA 3′ Rev, CATGCCATGGCCTTATTTTCCATGTCTTCC (NcoI). 5′ and 3′ MSP-1, MSP-2, MSP-4, MSP-5, MSP-10, and RAMA PCR products were cloned into the pHTK vector (21). Pf92 sequences were amplified from the P. falciparum CS2 line, using the following nucleotides: Pf92 5′ Fwd, ATCCCGCGGTACACACATGCGTATGTTCG (SacII); Pf92 5′ Rev, GATACTAGTCCAGAGTAAACAGCTGGGGTAG (SpeI); Pf92 3′ Fwd, ATCGAATTCGATTCAGGAAGGATATTTCAGTAG (EcoRI); and Pf92 3′ Rev, GATCCTAGGTTACAGTATCTGTATCAAACGG (AvrII). (Restriction endonuclease sites are underlined.) The 5′ PCR product was digested with SacII-SpeI and cloned into the SacII-SpeI-digested pCC-1, followed by the insertion of the EcoRI-AvrII-digested 3′ PCR product in the corresponding cloning site of this vector.
Parasite culture and transfection.
P. falciparum parasites were cultured and synchronized as per standard procedures (33, 56). Ring-stage parasites (∼1% parasitemia) were transfected with 100 μg of purified plasmid DNA (plasmid maxi kit; QIAGEN) as described previously (15), except using modified electroporation conditions (23).
Pulsed-field gel electrophoresis.
Chromosomes were separated by pulsed-field gel electrophoresis as described previously (11). Analysis of genomic DNA by Southern blot hybridization was performed using standard procedures.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Parasites were synchronized with 5% sorbitol (Sigma) 4 h apart to ensure a tightly synchronous population of late-blood-stage parasites. Infected erythrocytes were lysed with 0.15% saponin prior to solubilization in nonreducing sample buffer to obtain total parasite protein. Proteins were separated on 4 to 20% polyacrylamide gradient Tris-HEPES-sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels under nonreducing conditions and transferred to Immobilon-P (polyvinylidene difluoride) transfer membranes (Millipore) for Western blotting. Rabbit polyclonal antibodies to MSP-4, MSP-5, and SERA5 were diluted to 1/500 in 5% skim milk-phosphate-buffered saline (PBS), and the rabbit polyclonal antibody to MSP-119 was diluted 1/2,000 in 5% skim milk prior to use.
Indirect immunofluorescence assays (IFA).
Schizont-infected erythrocytes were washed once in PBS and then fixed in PBS containing 4.0% formaldehyde (diluted from 16% electron microscopy-grade paraformaldehyde; Electron Microscopy Services) and 0.0075% glutaraldehyde for 30 min at room temperature. Fixed cells were washed once in PBS, permeabilized for 10 min with 0.1% Triton X-100 (Sigma) in PBS, washed again in PBS, treated for 5 min with 0.1 mg/ml NaBH4 in PBS, washed three times in PBS, and then blocked overnight at 4°C in 0.5% bovine serum albumin (Sigma) in PBS. Fixed P. falciparum-infected erythrocytes were resuspended in PBS containing 1 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI), 1/100 rabbit anti-PfMSP-5w (37), and 1/20,000 mouse monoclonal anti-PfMSP-119 4H9/19 (10). Primary antibodies were incubated with fixed cells for 1 h at room temperature. P. falciparum-infected erythrocytes were then resuspended in PBS containing 10 mg/ml Alexa Fluor 568 goat anti-mouse immunoglobulin G (heavy plus light chains) and 10 mg/ml Alexa Fluor 488 goat anti-rabbit immunoglobulin G (heavy plus light chains) (Molecular Probes). Fixed cells were incubated with secondary antibodies for 1 h at room temperature and then washed four times in PBS prior to being mounted on slides for microscopy. Dual-color fluorescence images were captured using a Carl Zeiss Axioskop microscope with a PCO SensiCam and Axiovision 2 software.
Parasite growth rate assay.
Ring-stage parasites underwent double sorbitol synchronization with a 4-h interval and were plated in triplicate at 0.5% parasitemia and 4% hematocrit in complete culture medium in the absence of drug. Thin blood smears were made, methanol fixed, and Giemsa stained every 8 h for a total of 124 h or every 24 h for the extended 14-day growth rate assay. An addition of fresh media was made every 24 h, and cultures underwent a 1:5 dilution every 48 h with complete culture medium containing 4% hematocrit. Invasion rates were determined by counting the number of infected erythrocytes per 1,000 erythrocytes at each time point as the mean of time points smeared in triplicate.
Enzyme-treated erythrocyte invasion assay.
Schizont-infected erythrocytes in a synchronous culture were adjusted to 1% parasitemia and 4% hematocrit. Aliquots (1 ml) were washed and resuspended in 0.2% NaHCO3-buffered media (RPMI-HEPES) as a control or in buffered media containing either Vibrio cholerae neuraminidase (0.066 units/ml; Calbiochem), l-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin (0.9 mg/ml; Sigma), or α-chymotrypsin (0.9 mg/ml; Sigma). Following incubation for 1 h at 37°C, parasites were washed once with buffered media. Parasite-infected erythrocytes were then incubated with buffered media containing 0.5 mg/ml soybean trypsin inhibitor (Sigma) at 37°C for 20 min and then washed three times. Control and enzyme-treated parasitized erythrocytes were resuspended in culture media to 4% hematocrit and were dispensed into a 96-well tray in triplicate lots of 100 μl. To allow schizont rupture and merozoite reinvasion into enzyme-treated red blood cells, parasites were incubated for an additional 36 to 48 h. Following reinvasion, parasitemia was determined by flow cytometry. This involved exchanging 70 μl of the 100-μl volume of each well with fresh culture media, resuspending the erythrocytes, and transferring 10 μl of resuspended culture into a fluorescence-activated cell-sorting (FACS) tube. Cells were fixed for 24 h at 4°C in 250 μl fax fix (10% formaldehyde, 4% glucose in STE [150 mM NaCl, 10 mM Tris, 1 mM EDTA, pH 7.3]), after which 220 μl of fix was removed from the tubes and fixed erythrocytes were resuspended in 1 ml of Retic-COUNT (Thiazole Orange) reagent (Becton Dickinson) and incubated for 30 min in the dark. Erythrocytes were resuspended in the solution and analyzed by FACS on a FACSort (Becton Dickinson). Each well of a triplicate set was analyzed individually by counting 100,000 red blood cells. Reinvaded erythrocytes were identified by virtue of their fluorescence, using CellQuest V3.3 software to analyze FACS data. Prior to fixing of reinvaded enzyme-treated erythrocytes, 5 μl from each well was used to make thin smears of every sample, which were methanol fixed and Giemsa stained for manual counting of 1,000 erythrocytes to support FACS data.
RESULTS
GPI-anchored merozoite proteins are generally refractory to genetic deletion.
Attempts were made to disrupt each of the genes encoding seven different GPI-anchored merozoite proteins, MSP-1, MSP-2, MSP-4, MSP-5, MSP-10, RAMA, and Pf92, in P. falciparum blood stages. With the exception of Pf92, a single-crossover gene-targeting approach was previously used in an attempt to disrupt each of these genes in the D10 and/or 3D7 parasite line using the pHCl-based (17) or pHH1-based (51) vector. In all instances, stable transformants were derived; however, plasmids remained episomal after two to four drug cycles, and no correct gene targeting was observed (12, 45; our unpublished data). The lack of success with this approach led to the use of the more rigorous negative selection, double-crossover homologous recombination strategy (Fig. 1). This approach has proved far more effective at generating gene knockouts in P. falciparum than the original single-crossover strategy, because of the ability to select for integrants by using a combination of both positive and negative selection. As msp-4 and msp-5 are adjacent on chromosome 2, a plasmid designed to simultaneously disrupt both genes was also constructed. Seven constructs were initially made utilizing the TK negative selectable marker (21): pTKΔMSP1, pTKΔMSP2, pTKΔMSP4, pTKΔMSP5, pTKΔMSP4/5, pTKΔMSP10, and pTKΔRAMA. An additional construct, pCDΔPf92, was generated using the CD negative selectable marker vector (Maier and Cowman, unpublished).
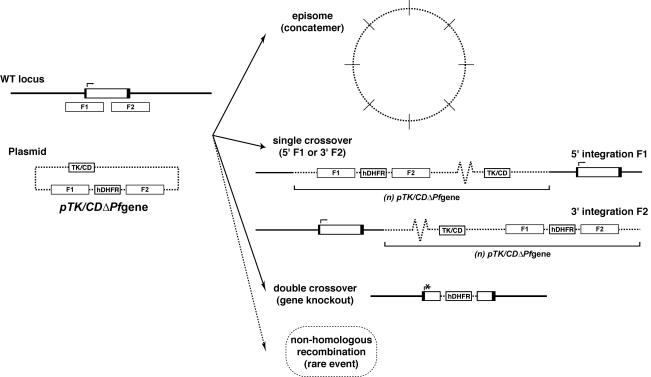
FIG. 1.
Possible genetic outcomes of following negative selection of parasites transfected with gene knockout constructs. Four distinct genetic outcomes are possible during drug cycling with double recombination vectors, including retention of the episomal form as a concatemer, 5′ or 3′ single integration of the vector, double integration representing a targeted genetic deletion, and the more rare event of nonhomologous recombination. WT, wild type.
Constructs were transfected into the 3D7, D10, and/or CS2 line of P. falciparum. Parasite lines were drug cycled on and off the positive selection agent (WR99210), and cultures were treated with a combination of WR99210 and the negative selection agent (ganciclovir or flucytosine for the TK or CD vector, respectively) at the completion of each drug cycle to select for double integrants. While the addition of the negative selection agent causes death of TK (or CD)-expressing parasites and selects for double-crossover events, if the targeted gene is essential and hence cannot be disrupted, other genetic outcomes are observed after recovery of the population in the presence of this drug. Each of these outcomes can be distinguished by Southern blotting to a combination of probes recognizing different sequences in the plasmid (Fig. 1). These include retention of the episome in its concatemeric replicating form, observed as a large smearing band toward the top of the pulsed-field gel electrophoresis Southern blot (44); single-crossover homologous integration into either the 5′ flanking region or the 3′ flanking region cloned into the transfected vector, characterized by hybridization of both the positive selectable marker and the plasmid backbone to the point of migration of the target chromosome; and nonhomologous integration into another chromosome. On the other hand, successful disruption of the targeted gene by a double recombination event is seen by the hybridization of the positive selectable marker to the position of the target chromosome, accompanied by the failure of the plasmid backbone to hybridize to this position on the blot (16).
All plasmids were successfully transfected, as evidenced by the establishment of drug-resistant populations possessing episomally replicating forms of the plasmid several weeks after transfection (data not shown). However, with two exceptions (discussed below), no sign of genetic deletion was observed in transfected lines after drug cycling and the addition of the negative selection agent. Attempts at targeting msp-4, rama, and msp-10 resulted in the retention of the episomally replicating form of the transfected construct, whereas attempts at targeting msp-1, msp-2, and msp-4 plus msp-5 resulted in 5′ or 3′ single integrants that had not compromised the expression of the targeted gene (Table 1). In the case of Pf92, parasites were not recovered following the addition of the negative selection agent, demonstrating that double-crossover integrants were not present in the population and highlighting the power of this negative selection strategy. Taken together, these data suggest that most, if not all, of these genes play an important, perhaps essential, role in blood-stage growth.
TABLE 1.
Summary of double-crossover gene-targeting experiments
| Gene | Construct | Attempt no. | Line | Outcomea |
|---|---|---|---|---|
| msp-1 | pTKΔMSP1 | 1 | D10 | Single crossover (3′) |
| pTKΔMSP1 | 2 | D10 | Single crossover (3′) | |
| pTKΔMSP1 | 3 | D10 | Single crossover (3′) | |
| msp-2 | pTKΔMSP2 | 1 | 3D7 | Single crossover (3′) |
| pTKΔMSP2 | 2 | 3D7 | Single crossover (3′) | |
| msp-4 | pTKΔMSP4 | 1 | 3D7 | Episomal |
| pTKΔMSP4 | 2 | 3D7 | Single crossover | |
| msp-5 | pTKΔMSP5 | 1 | 3D7 | Double crossover (KO) |
| msp-4 plus msp-5 | pTKΔMSP4/5 | 1 | 3D7 | Single crossover (5′) |
| msp-10 | pTKΔMSP10 | 1 | D10 | Episomal |
| rama | pTKΔRAMA | 1 | 3D7 | Episomal |
| pTKΔRAMA | 2 | 3D7 | Episomal | |
| Pf92 | pCDΔPf92 | 1 | CS2 | Lethal |
Single crossover refers to homologous recombination into either the 5′ or the 3′ targeting sequence, as indicated in the brackets. KO refers to gene knockout via the expected double-crossover homologous recombination event, and instances in which populations were not reestablished after the addition of the negative selection agent are referred to as lethal.
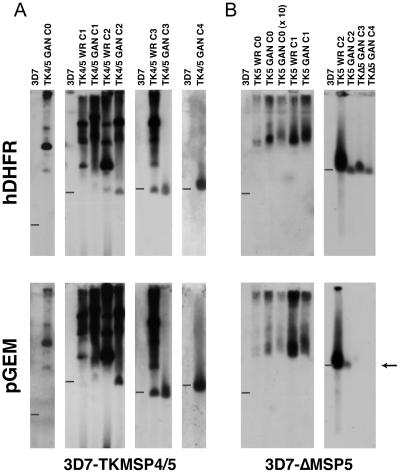
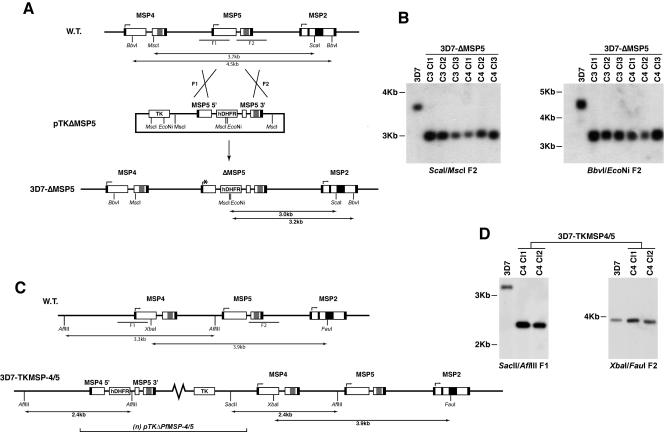
The exceptions were the lines transfected with pTKΔMSP4/5, which resulted in a population of single-crossover integrants potentially representing a truncation of the Pfmsp-4 5′ untranslated region (UTR), and pTKΔMSP5, which resulted in a line that appeared to have integrated into the msp-5 gene by double-crossover homologous recombination (Fig. 2). Deletion of msp-5 by this event was confirmed by Southern blot analysis of ScaI-MscI- and BbvI-EcoNi-restricted genomic DNA from various 3D7-ΔMSP5 clones from late drug cycles (Fig. 3A and B). The loss of a 3.7-kb and 4.5-kb endogenous fragment and the appearance of the expected 3.0-kb and 3.2-kb bands for the ScaI-MscI and BbvI-EcoNi digests, respectively, confirmed the disruption of msp-5 by double-crossover homologous recombination. Southern blot analysis of the 3D7-TKMSP4/5 line confirmed integration of this construct by single-crossover recombination at the 5′ end of msp-4, effectively restricting the 5′ UTR of this gene to 500 bp (Fig. 3C and D).
FIG. 2.
Southern blot hybridizations of pulsed-field gels across drug-cycled transfected lines. (A) 3D7-TKMSP4/5 chromosomes probed with hDHFR and pGem probes across four drug cycles. (B) 3D7-ΔMSP5 chromosomes across four drug cycles also probed in a Southern blot with hDHFR and pGem, indicating the loss of msp-5 in late drug cycles. A 3D7 genomic control was loaded in the leftmost lane of every blot, and the position of migration of chromosome 2 is denoted by the bars. Identical blots were hybridized with the hDHFR probe (positive selectable marker) and the pGEM probe (plasmid backbone). Loss of chromosome 2 signal (arrow) indicates double crossover integration. C, cycle.
FIG. 3.
Mapping of the integration events in MSP-5 knockout and MSP-4 truncated 5′ UTR lines. (A) Schematic diagram detailing the strategy employed in targeting of the endogenous msp-5 gene. The black boxes represent sequences encoding the signal peptides and the GPI attachment moieties. The central repeat regions that define the MSP-2 alleles are also indicated by the black boxes toward the center of the MSP-2 coding sequence. Gray boxes represent the EGF-like domains. (B) Southern blot of restricted genomic DNA isolated from P. falciparum 3D7 parental parasites and a number of 3D7-ΔMSP5 clones (cycles 3 and 4 [C3 and C4]). Both blots (ScaI-MscI and BbvI-EcoNi) were probed with the MSP-5 3′ targeting sequence (F2) delineated in panel A. (C) Schematic diagram detailing the outcome of an attempt to target PfMSP-4 and PfMSP-5 in the one transfected line, resulting in a 5′ single recombination event integrating one or more copies of the episome. (D) Southern blot of restricted genomic DNA isolated from P. falciparum 3D7 parental parasites and two clones of 3D7-TKMSP4/5 parasites from late drug cycles (C4). Blot SacII-AflIII was probed with the MSP-4 5′ targeting sequence (F1), and blot XbaI-FauI was probed with the MSP-5 3′ targeting sequence (F2) delineated in panel C. W.T., wild type; Cl, clone.
Phenotypic analysis of MSP-5 knockout and MSP-4 promoter mutant lines.
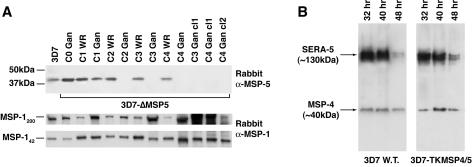
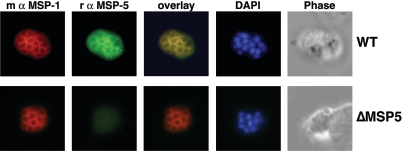
To confirm the loss of expression of MSP-5 in 3D7-ΔMSP5 parasites, total protein extracts were prepared from synchronized late-stage parasites from each drug cycle and analyzed by Western blotting (Fig. 4A). This material was also probed with a polyclonal rabbit anti-MSP-119 antibody serving as a loading control and to ensure that parasite material was from late in the blood-stage cycle. It was clear that parasites at later drug cycles no longer expressed MSP-5 after (but not prior to) the addition of the negative selection agent ganciclovir. Importantly, MSP-5 was absent from several cloned lines. It has previously been reported that localization of MSP-5 by indirect immunofluorescence confocal microscopy is consistent with merozoite surface labeling (60). Here, merozoite surface localization of MSP-5 was confirmed by colocalization with MSP-1 in IFA utilizing a rabbit polyclonal MSP-5 antibody (Fig. 5). Using this same antibody, MSP-5 was not detected above background labeling in MSP-5 knockout parasites, confirming the specificity of the rabbit polyclonal antibody employed.
FIG. 4.
Western blot analysis of MSP-5 knockout and MSP-4 truncated 5′ UTR lines. (A) Western blots of total material from synchronized late-blood-stage parasites comparing the 3D7 wild type with the 3D7-ΔMSP5 line across progressive WR99210 drug cycles, including lines treated with both WR99210 (WR) and ganciclovir (Gan) and three late-drug-cycle 3D7-ΔMSP5 clones probed with rabbit polyclonal anti-MSP-5 C-terminal antibodies (37). The panels below represent a loading control probed with rabbit polyclonal anti-MSP-119 antibodies (18). (B) Western blots of total material from parental 3D7 (wild type [W.T.]) and 3D7-TKMSP4/5-synchronized parasites at 32, 40, and 48 h postinvasion. Blots were probed with a rabbit polyclonal anti-MSP-4 antibody to confirm MSP-4 expression in 3D7-TKMSP4/5 and with rabbit polyclonal anti-SERA5 antibody (29) as a loading control. C, cycle; cl, clone.
FIG. 5.
Confirmation that P. falciparum MSP-5 localizes to the merozoite surface. Fixed 3D7 (wild type [WT]) or 3D7-ΔMSP5 (ΔMSP5) parasites were analyzed by double-label immunofluorescence with a polyclonal rabbit (r) anti-MSP5 antibody (green) and a mouse (m) monoclonal anti-MSP-119 antibody (4H9/19 [10]) (red). Colocalization of MSP-1 and MSP-5 is evident upon merging of images. DAPI was used for nuclear staining (blue).
Western blot analysis of a single clone of 3D7-TKMSP4/5 in which integration had occurred upstream of the MSP-5 start codon revealed no notable loss of expression of MSP-4 in comparison to that of the 3D7 wild-type parasite line (Fig. 4B). A SERA5 internal control was included as a loading control. Total parasite material was sampled from synchronized 3D7 wild-type and 3D7-TKMSP4/5 parasites at 32 h, 40 h, and 48 h postinvasion. These data, in addition to an independent 8-h time course assay over the entire 48-h life cycle (data not shown), confirmed that the timing and level of expression of MSP-4 in 3D7-TKMSP4/5 parasites resembled those of the parental line. This indicates that truncation of the putative msp-4 promoter to approximately 500 bp by 5′ integration does not disrupt regular expression of MSP-4. This suggests either the possible presence of a fully active MSP-4 promoter sequence within approximately 500 bp upstream of the MSP-4 start codon or that MSP-4 expression in the 3D7-TKMSP4/5 line is augmented by the calmodulin promoter, which drives expression of the TK cassette that has integrated adjacent to the msp-4 in a head-to-head orientation. The calmodulin promoter has previously been reported to have the capacity for bidirectional transcription (15, 17).
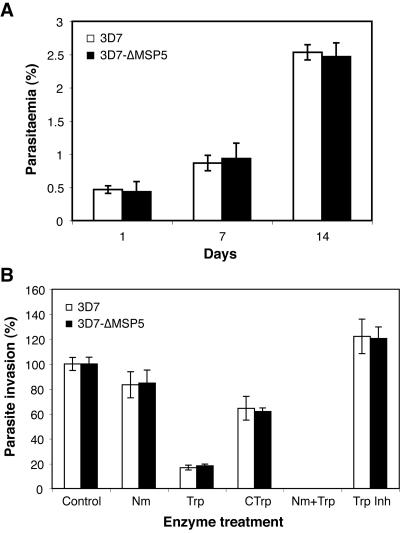
Having confirmed the deletion of msp-5 in 3D7-ΔMSP5 parasites, we investigated growth rates over a 104-h period, comparing 3D7-ΔMSP5 with 3D7 wild-type parasites. Starting levels of parasitemia were identical, parasite cultures were diluted equally every 48 h, and parasitemia levels were recorded in triplicate every 8 h. While no significant differences in growth rates were found between the two parasite lines, at 32-h and 64-h time points, it appeared that the parasitemia level of 3D7-ΔMSP5 was marginally lower than that of the 3D7 wild-type line (data not shown). Consequentially, the growth rate assay was extended over a 2-week period whereby parasitemia levels were recorded in triplicate on a 24-h basis. Following 14 days in culture under identical conditions, no significant difference in growth rates was observed between the 3D7-ΔMSP5 line and 3D7 wild-type parasites (Fig. 6A).
FIG. 6.
Disruption of MSP-5 does not affect parasite growth or result in a switch in invasion pathways. (A) Results of an extended growth rate assay comparing 3D7 wild-type parasites with the 3D7-ΔMSP5 line over a 14-day period. Parasites were subcultured 1 in 5 every 48 h. Here, parasitemia levels are compared on days 1, 7, and 14. (B) Invasion into enzyme-treated erythrocytes by 3D7 wild-type and 3D7-ΔMSP5 parasites. Erythrocyte treatments include NaHCO3 (0.2%)-buffered RPMI-HEPES as a control or buffered media supplemented with 0.066 U/ml neuraminidase (Nm), 0.9 mg/ml trypsin (Trp), 0.9 mg/ml chymotrypsin (CTrp), 0.066 U/ml neuraminidase plus 0.9 mg/ml trypsin (Nm+Trp), and 0.5 mg/ml trypsin inhibitor (Trp Inh) as a control.
Furthermore, loss of MSP-5 expression did not result in a switch in erythrocyte invasion pathways distinct from those for 3D7 parental parasites. This was investigated by comparing invasion rates into erythrocytes treated with enzymes known to cleave erythrocyte receptors involved in parasite invasion. No significant difference in invasion rates into erythrocytes treated with trypsin, neuraminidase, chymotrypsin, and neuraminidase plus trypsin was observed between the 3D7-ΔMSP5 line and the 3D7 wild type (Fig. 6B). It has previously been reported that 3D7 utilizes a trypsin-dependent and neuraminidase- and chymotrypsin-independent invasion pathway (22). This appears unaltered by the loss of MSP-5.
DISCUSSION
In this paper, we attempted to disrupt genes encoding seven GPI-anchored proteins of P. falciparum merozoites. Although drug-resistant lines possessing episomally replicating forms of the transfected plasmid were obtained in each case, we were unable to target six of these seven genes, with only the gene encoding MSP-5 successfully disrupted. The MSP-5 knockout line represents the first and only GPI-anchored Plasmodium merozoite protein deletion mutant that has been generated. Loss of MSP-5 expression in these parasites was confirmed by Western blot analysis and IFA, approaches that validated the specificity of the anti-MSP-5 antibodies used and that appeared to confirm a surface localization of this protein in parental 3D7 parasites. The absence of MSP-5 did not affect blood-stage growth rate over a 14-day period of continuous in vitro culture. Moreover, parasite invasion assays with enzymatically treated erythrocytes, a process designed to expose a change in invasion pathways, also failed to reveal any change in invasion profile attributable to the loss of MSP-5.
It is thought that MSP-4 and MSP-5 arose due to a genetic duplication event given their similar gene organizations, structural features, and adjacent locations on chromosome 2 (37). MSP-5 has been reported to lack sequence variation between P. falciparum isolates from various geographical locations, indicating that it is not subject to significant immune selection (60). In addition, greater sequence diversity has been reported for MSP-4 than for MSP-5 (5, 59). In the syntenic chromosomal region of rodent malaria, there is a single gene homologous to P. falciparum msp-4 and msp-5 (7, 32). Despite studies confirming the expression of MSP-5 on P. falciparum merozoites, the ability to genetically disrupt the gene encoding this protein without any significant phenotypic outcome, along with its apparent lack of immune selection, indicates that it is quite likely that MSP-5 either plays a minor role in late-blood-stage parasites or has been made functionally redundant by the expression of MSP-4. The ability to ablate MSP-5 expression in P. falciparum blood-stage parasites indicates that MSP-5 is not essential for the growth of this particular parasite stage in vitro. With respect to the six genes that we were unable to disrupt, it is recognized that the inability to knock out any individual P. falciparum gene is not conclusive with respect to whether the protein in question is essential to parasite growth or invasion. In a given circumstance, additional transfections in other lines may yet yield a knockout. However, the general observation that only one of seven (14%) GPI-anchored proteins was disrupted is in stark contrast to observations with the other merozoite antigen classes, the apical and peripheral proteins. With some notable exceptions, including AMA-1 (58) and several SERA proteins (40), knockouts of most genes attempted in these classes have been achieved with relative ease. As mentioned in the introduction, this includes all members of the erythrocyte binding antigen and reticulocyte binding protein homologue families as well as peripheral proteins, such as MSP-3 (41), MSP-6 (J. A. Pearce and A. F. Cowman, unpublished data), H101 (46), H103 (46), MSP-7 (R. A. O'Donnell and B. S. Crabb, unpublished data), and some members of the low-molecular-weight (RAP-1 and RAP-3 [2, 3]) and high-molecular-weight (RhopH1/Clag9 [57]) rhoptry complexes. In some instances, these knockout lines have provided important functional insight into these proteins. However, the ability to generate these mutant lines also indicates that their roles are not obligatory to invasion and to in vitro blood-stage development. Whether susceptibility or resistance to genetic deletion in blood-stage culture is relevant to the vaccine potential of the antigen in question remains to be seen. In the absence of an effective blood-stage vaccine, it remains just one consideration, along with all other unvalidated parameters, for prioritizing blood-stage vaccine targets.
Assessing the biological function of these “essential” proteins requires further technical development. The development of an inducible expression or conditional knockout system has been sought for some time since the establishment of transfection in P. falciparum and in the rodent parasite Plasmodium berghei. Recently, the adaptation of the Flp/FRT site-specific recombination system in P. berghei parasites has enabled the targeted deletion of sequences (9). This system has clear potential for the deletion of essential blood-stage genes, especially in rodent malaria, where all life cycle stages are more readily maintained. Similarly, the more recently developed tetracycline-regulated expression system designed for use in blood-stage P. falciparum (38) could be adapted to generate dominant-negative mutant parasites or conditional “knockout” lines as with T. gondii (39, 42). Greater insight into the molecular basis of primary recognition of erythrocytes by merozoites is probably heavily dependent on the further improvement and use of such approaches.
Acknowledgments
We are grateful to the Australian Red Cross Blood Service for the provision of human blood and serum.
This work was supported by the NHMRC of Australia, the National Institutes of Health grant DK 32094, and the Wellcome Trust, United Kingdom. P.R.S. is the recipient of an Australian Postgraduate Research Award, D.R.D. and R.A.O. are recipients of Peter Doherty training awards from the NHMRC, and B.S.C. and R.L.C. are International Research Scholars of the Howard Hughes Medical Institute.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Adams, J. H., B. K. Sim, S. A. Dolan, X. Fang, D. C. Kaslow, and L. H. Miller. 1992. A family of erythrocyte binding proteins of malaria parasites. Proc. Natl. Acad. Sci. USA 89:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldi, D. L., K. T. Andrews, R. F. Waller, D. S. Roos, R. F. Howard, B. S. Crabb, and A. F. Cowman. 2000. RAP1 controls rhoptry targeting of RAP2 in the malaria parasite Plasmodium falciparum. EMBO J. 19:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldi, D. L., R. Good, M. T. Duraisingh, B. S. Crabb, and A. F. Cowman. 2002. Identification and disruption of the gene encoding the third member of the low-molecular-mass rhoptry complex in Plasmodium falciparum. Infect. Immun. 70:5236-5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannister, L. H., G. H. Mitchell, G. A. Butcher, E. D. Dennis, and S. Cohen. 1986. Structure and development of the surface coat of erythrocytic merozoites of Plasmodium knowlesi. Cell Tissue Res. 245:281-290. [DOI] [PubMed] [Google Scholar]
- 5.Benet, A., L. Tavul, J. C. Reeder, and A. Cortes. 2004. Diversity of Plasmodium falciparum vaccine candidate merozoite surface protein 4 (MSP4) in a natural population. Mol. Biochem. Parasitol. 134:275-280. [DOI] [PubMed] [Google Scholar]
- 6.Black, C., L. Wang, T. Wu, and R. Coppel. 2003. Apical location of a novel EGF-like domain-containing protein of Plasmodium falciparum. Mol. Biochem. Parasitol. 127:59-68. [DOI] [PubMed] [Google Scholar]
- 7.Black, C. G., L. Wang, A. R. Hibbs, E. Werner, and R. L. Coppel. 1999. Identification of the Plasmodium chabaudi homologue of merozoite surface proteins 4 and 5 of Plasmodium falciparum. Infect. Immun. 67:2075-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black, C. G., T. Wu, L. Wang, A. E. Topolska, and R. L. Coppel. 2005. MSP8 is a non-essential merozoite surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 144:27-35. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho, T. G., S. Thiberge, H. Sakamoto, and R. Menard. 2004. Conditional mutagenesis using site-specific recombination in Plasmodium berghei. Proc. Natl. Acad. Sci. USA 101:14931-14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, J. A., L. T. Cooper, and A. J. Saul. 1992. Mapping of the region predominantly recognized by antibodies to the Plasmodium falciparum merozoite surface antigen MSA 1. Mol. Biochem. Parasitol. 51:301-312. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran, L. M., K. P. Forsyth, A. E. Bianco, G. V. Brown, and D. J. Kemp. 1986. Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell 44:87-95. [DOI] [PubMed] [Google Scholar]
- 12.Cowman, A. F., D. L. Baldi, J. Healer, K. E. Mills, R. A. O'Donnell, M. B. Reed, T. Triglia, M. E. Wickham, and B. S. Crabb. 2000. Functional analysis of proteins involved in Plasmodium falciparum merozoite invasion of red blood cells. FEBS Lett. 476:84-88. [DOI] [PubMed] [Google Scholar]
- 13.Cowman, A. F., and B. S. Crabb. 2005. Genetic manipulation of Plasmodium falciparum, p. 50-67. In I. W. Sherman (ed.), Molecular approaches to malaria. ASM Press, Washington, D.C.
- 14.Cowman, A. F., and B. S. Crabb. 2002. The Plasmodium falciparum genome—a blueprint for erythrocyte invasion. Science 298:126-128. [DOI] [PubMed] [Google Scholar]
- 15.Crabb, B. S., and A. F. Cowman. 1996. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 93:7289-7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabb, B. S., M. Rug, T. W. Gilberger, J. K. Thompson, T. Triglia, A. G. Maier, and A. F. Cowman. 2004. Transfection of the human malaria parasite Plasmodium falciparum. Methods Mol. Biol. 270:263-276. [DOI] [PubMed] [Google Scholar]
- 17.Crabb, B. S., T. Triglia, J. G. Waterkeyn, and A. F. Cowman. 1997. Stable transgene expression in Plasmodium falciparum. Mol. Biochem. Parasitol. 90:131-144. [DOI] [PubMed] [Google Scholar]
- 18.de Koning-Ward, T. F., R. A. O'Donnell, D. R. Drew, R. Thomson, T. P. Speed, and B. S. Crabb. 2003. A new rodent model to assess blood-stage immunity to the Plasmodium falciparum antigen MSP-119 reveals a protective role for invasion inhibitory antibodies. J. Exp. Med. 198:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drew, D. R., P. R. Sanders, and B. S. Crabb. 2005. Plasmodium falciparum merozoite surface protein 8 is a ring-stage membrane protein that localizes to the parasitophorous vacuole of infected erythrocytes. Infect. Immun. 73:3912-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duraisingh, M., A. Maier, T. Triglia, and A. Cowman. 2003. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 100:4796-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duraisingh, M. T., T. Triglia, and A. F. Cowman. 2002. Negative selection of Plasmodium falciparum reveals targeted gene deletion by double crossover recombination. Int. J. Parasitol. 32:81-89. [DOI] [PubMed] [Google Scholar]
- 22.Duraisingh, M. T., T. Triglia, S. A. Ralph, J. C. Rayner, J. W. Barnwell, G. I. McFadden, and A. F. Cowman. 2003. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 22:1047-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fidock, D. A., and T. E. Wellems. 1997. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA 94:10931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galinski, M. R., A. R. Dluzewski, and J. W. Barnwell. 2005. A mechanistic approach to merozoite invasion of red blood cells: merozoite biogenesis, rupture, and invasion of erythrocytes, p. 113-168. In I. W. Sherman (ed.), Molecular approaches to malaria. ASM Press, Washington, D.C.
- 25.Gaur, D., D. C. Mayer, and L. H. Miller. 2004. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int. J. Parasitol. 34:1413-1429. [DOI] [PubMed] [Google Scholar]
- 26.Gerloff, D. L., A. Creasey, S. Maslau, and R. Carter. 2005. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 102:13598-13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilberger, T., J. Thompson, T. Triglia, R. Good, M. Duraisingh, and A. Cowman. 2003. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J. Biol. Chem. 278:14480-14486. [DOI] [PubMed] [Google Scholar]
- 28.Goel, V., X. Li, H. Chen, S. Liu, A. Chishti, and S. Oh. 2003. Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc. Natl. Acad. Sci. USA 100:5164-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodder, A. N., D. R. Drew, V. C. Epa, M. Delorenzi, R. Bourgon, S. K. Miller, R. L. Moritz, D. F. Frecklington, R. J. Simpson, T. P. Speed, R. N. Pike, and B. S. Crabb. 2003. Enzymic, phylogenetic, and structural characterization of the unusual papain-like protease domain of Plasmodium falciparum SERA5. J. Biol. Chem. 278:48169-48177. [DOI] [PubMed] [Google Scholar]
- 30.Holder, A. A., M. J. Lockyer, K. G. Odink, J. S. Sandhu, V. Riveros-Moreno, S. C. Nicholls, Y. Hillman, L. S. Davey, M. L. Tizard, R. T. Schwarz, and R. R. Freeman. 1985. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature 317:270-273. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko, O., D. A. Fidock, O. M. Schwartz, and L. H. Miller. 2000. Disruption of the C-terminal region of EBA-175 in the Dd2/Nm clone of Plasmodium falciparum does not affect erythrocyte invasion. Mol. Biochem. Parasitol. 110:135-146. [DOI] [PubMed] [Google Scholar]
- 32.Kedzierski, L., C. G. Black, and R. L. Coppel. 2000. Characterization of the merozoite surface protein 4/5 gene of Plasmodium berghei and Plasmodium yoelii. Mol. Biochem. Parasitol. 105:137-147. [DOI] [PubMed] [Google Scholar]
- 33.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 34.Langreth, S. G., J. B. Jensen, R. T. Reese, and W. Trager. 1978. Fine structure of human malaria in vitro. J. Protozool. 25:443-452. [DOI] [PubMed] [Google Scholar]
- 35.Maier, A. G., M. T. Duraisingh, J. C. Reeder, S. S. Patel, J. W. Kazura, P. A. Zimmerman, and A. F. Cowman. 2003. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat. Med. 9:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall, V. M., A. Silva, M. Foley, S. Cranmer, L. Wang, D. J. McColl, D. J. Kemp, and R. L. Coppel. 1997. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect. Immun. 65:4460-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall, V. M., W. Tieqiao, and R. L. Coppel. 1998. Close linkage of three merozoite surface protein genes on chromosome 2 of Plasmodium falciparum. Mol. Biochem. Parasitol. 94:13-25. [DOI] [PubMed] [Google Scholar]
- 38.Meissner, M., E. Krejany, P. R. Gilson, T. F. de Koning-Ward, D. Soldati, and B. S. Crabb. 2005. Tetracycline analogue-regulated transgene expression in Plasmodium falciparum blood-stages using Toxoplasma gondii transactivators. Proc. Natl. Acad. Sci. USA 102:2980-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meissner, M., D. Schluter, and D. Soldati. 2002. Role of Toxoplasma gondii myosin A in powering parasite gliding and host cell invasion. Science 298:837-840. [DOI] [PubMed] [Google Scholar]
- 40.Miller, S. K., R. T. Good, D. R. Drew, M. Delorenzi, P. R. Sanders, A. N. Hodder, T. P. Speed, A. F. Cowman, T. F. de Koning-Ward, and B. S. Crabb. 2002. A subset of Plasmodium falciparum SERA genes are expressed and appear to play an important role in the erythrocytic cycle. J. Biol. Chem. 277:47524-47532. [DOI] [PubMed] [Google Scholar]
- 41.Mills, K. E., J. A. Pearce, B. S. Crabb, and A. F. Cowman. 2002. Truncation of merozoite surface protein 3 disrupts its trafficking and that of acidic-basic repeat protein to the surface of Plasmodium falciparum merozoites. Mol. Microbiol. 43:1401-1411. [DOI] [PubMed] [Google Scholar]
- 42.Mital, J., M. Meissner, D. Soldati, and G. E. Ward. 2005. Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol. Biol. Cell 16:4341-4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikodem, D., and E. Davidson. 2000. Identification of a novel antigenic domain of Plasmodium falciparum merozoite surface protein-1 that specifically binds to human erythrocytes and inhibits parasite invasion, in vitro. Mol. Biochem. Parasitol. 108:79-91. [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell, R. A., P. R. Preiser, D. H. Williamson, P. W. Moore, A. F. Cowman, and B. S. Crabb. 2001. An alteration in concatameric structure is associated with efficient segregation of plasmids in transfected Plasmodium falciparum parasites. Nucleic Acids Res. 29:716-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Donnell, R. A., A. Saul, A. F. Cowman, and B. S. Crabb. 2000. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat. Med. 6:91-95. [DOI] [PubMed] [Google Scholar]
- 46.Pearce, J. A., K. Mills, T. Triglia, A. F. Cowman, and R. F. Anders. 2005. Characterisation of two novel proteins from the asexual stage of Plasmodium falciparum, H101 and H103. Mol. Biochem. Parasitol. 139:141-151. [DOI] [PubMed] [Google Scholar]
- 47.Perkins, M., and L. J. Rocco. 1988. Sialic acid-dependent binding of Plasmodium falciparum merozoite surface antigen, Pf200, to human erythrocytes. J. Immunol. 141:3190-3196. [PubMed] [Google Scholar]
- 48.Rayner, J., E. Vargas-Serrato, C. Huber, M. Galinski, and J. Barnwell. 2001. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J. Exp. Med. 194:1571-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rayner, J. C., M. R. Galinski, P. Ingravallo, and J. W. Barnwell. 2000. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc. Natl. Acad. Sci. USA 97:9648-9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed, M. B., S. R. Caruana, A. H. Batchelor, J. K. Thompson, B. S. Crabb, and A. F. Cowman. 2000. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc. Natl. Acad. Sci. USA 97:7509-7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed, M. B., K. J. Saliba, S. R. Caruana, K. Kirk, and A. F. Cowman. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906-909. [DOI] [PubMed] [Google Scholar]
- 52.Sanders, P. R., P. R. Gilson, G. T. Cantin, D. C. Greenbaum, T. Nebl, D. J. Carucci, M. J. McConville, L. Schofield, A. N. Hodder, J. R. Yates III, and B. S. Crabb. 2005. Distinct protein classes including novel merozoite surface antigens in raft-like membranes of Plasmodium falciparum. J. Biol. Chem. 280:40169-40176. [DOI] [PubMed] [Google Scholar]
- 53.Smythe, J. A., R. L. Coppel, G. V. Brown, R. Ramasamy, D. J. Kemp, and R. F. Anders. 1988. Identification of two integral membrane proteins of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 85:5195-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stubbs, J., K. M. Simpson, T. Triglia, D. Plouffe, C. J. Tonkin, M. T. Duraisingh, A. G. Maier, E. A. Winzeler, and A. F. Cowman. 2005. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science 309:1384-1387. [DOI] [PubMed] [Google Scholar]
- 55.Topolska, A. E., A. Lidgett, D. Truman, H. Fujioka, and R. L. Coppel. 2004. Characterization of a membrane-associated rhoptry protein of Plasmodium falciparum. J. Biol. Chem. 279:4648-4656. [DOI] [PubMed] [Google Scholar]
- 56.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 57.Trenholme, K. R., D. L. Gardiner, D. C. Holt, E. A. Thomas, A. F. Cowman, and D. J. Kemp. 2000. clag9: a cytoadherence gene in Plasmodium falciparum essential for binding of parasitized erythrocytes to CD36. Proc. Natl. Acad. Sci. USA 97:4029-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Triglia, T., J. Healer, S. R. Caruana, A. N. Hodder, R. F. Anders, B. S. Crabb, and A. F. Cowman. 2000. Apical membrane antigen 1 plays a central role in erythrocyte invasion by Plasmodium species. Mol. Microbiol. 38:706-718. [DOI] [PubMed] [Google Scholar]
- 59.Wang, L., C. G. Black, V. M. Marshall, and R. L. Coppel. 1999. Structural and antigenic properties of merozoite surface protein 4 of Plasmodium falciparum. Infect. Immun. 67:2193-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, T., C. G. Black, L. Wang, A. R. Hibbs, and R. L. Coppel. 1999. Lack of sequence diversity in the gene encoding merozoite surface protein 5 of Plasmodium falciparum. Mol. Biochem. Parasitol. 103:243-250. [DOI] [PubMed] [Google Scholar]