Abstract
A current or previous schistosome infection might compromise the efficacy of a schistosome vaccine administered to humans. We have therefore investigated the influence of infection on vaccination, using the baboon as the model host and irradiated Schistosoma mansoni cercariae as the vaccine. Protection, determined from worm burdens in test and controls, was not diminished when vaccination was superimposed on a chronic infection, nor was it diminished when it followed a primary infection terminated by chemotherapy. Protection was also assessed indirectly based on fecal egg output and circulating antigen levels, as would be the case in human vaccine trials. In almost all instances, these methods overestimated protection, sometimes with discrepancies of >20%. The overwhelming immune response to egg deposition in infected animals made it difficult to discern a contribution from vaccination. Nevertheless, the well-documented immunomodulation of immune responses that follows egg deposition did not appear to impede the protective mechanisms elicited by vaccination with attenuated cercariae.
The development of a vaccine against schistosomiasis mansoni would provide a powerful tool for the control of this important parasitic disease. It is inevitable that such a vaccine would be tested in, and ultimately administered to, people in areas of endemicity that either harbor a schistosome infection or have previously been infected and received drug treatment. Given the well-documented downregulation of specific immune responses associated with chronic schistosome infections (12), it is unclear whether vaccination would succeed in the situations described above. Furthermore, the modulating effects of current helminth infections on vaccination with purified tetanus toxoid have been recorded (3, 20). Drug treatment of a chronic infection could impair efficacy, as recently observed in vaccine trials for malaria (10), or boost it by release of parasite antigens upon worm death. Treatment is also known to alter schistosome-specific immune responses, affecting antibody levels and isotype switching (14).
The radiation-attenuated (RA) schistosome vaccine is highly effective under laboratory conditions but, for ethical and practical reasons, cannot be used in humans. Nevertheless, it serves as a compelling model for the development of a recombinant vaccine (4, 27). The protective capabilities of the RA vaccine were originally established in rodents (6) and subsequently extended to primates (8, 21, 28, 29), with up to 86% protection obtained after five vaccinations of the olive baboon (Papio anubis) (11). The baboon has the capacity to harbor a substantial schistosome infection long term, unlike mice, which succumb to egg-induced pathology even with low worm burdens (26). This makes it an ideal host in which to investigate the interaction of infection and vaccination. Another advantage of baboons is that they are closer phylogenetically and in body scale to humans, so that experimental results are likely to have a greater relevance (15). Furthermore, protective immunity can be assessed not only by comparison of worm burdens recovered from test and control animals but also via indirect estimates of infection, namely, fecal egg output and the level of schistosome antigens circulating in the bloodstream. These last two are the measures of efficacy that would be used in field trials of a human vaccine.
We report here two experiments designed to discover whether vaccine efficacy is compromised when either prior infection is terminated by drug treatment or the vaccination regime is superimposed on a chronic infection. The results reveal that the dominant immune responses to adult worm and especially egg antigens elicited by a schistosome infection do not compromise those to the RA vaccine that parallel the development of protection.
MATERIALS AND METHODS
Hosts and parasites.
Male and female juvenile olive baboons, obtained from a schistosome-free high altitude area, were kept under quarantine for 3 months at the Institute of Primate Research (IPR), Nairobi, Kenya and their negative infection status was confirmed by the absence of schistosome antibodies (by using the enzyme-linked immunosorbent assay [ELISA]), and fecal eggs (by using a KatoKatz smear and the miracidial hatching test). A human isolate of S. mansoni was maintained in chronically infected baboons and Biomphalaria pfeifferi snails. Animals were housed according to international standards and guidelines for primates, and all experimental procedures were approved by independent scientific and ethical committees at the Institute of Primate Research and the University of York.
Parasitology.
Groups of baboons (n = 5 to 7) were exposed to cercariae as previously described (22, 28). They were vaccinated percutaneously with 9,000 attenuated cercariae (30-krad gamma radiation) on five occasions at 4-week intervals (11, 28). A dose of 1,000 normal cercariae was used for the initial infection, while all animals received 800 cercariae at challenge. The health of the animals was monitored throughout the experiments, and rehydration therapy was given to those that experienced diarrhea during the acute phase of the infection. As an indirect measure of worm burden, mean eggs per gram (epg) was estimated from three 50-mg fresh fecal samples collected at weekly intervals from 4 weeks after primary or challenge infections, using the Kato/Katz smear technique (11, 23). The degree of vaccine-induced protection was determined by recovery of adult worms from baboons at 10 weeks postchallenge by perfusion of the hepatic portal vasculature (22, 28). The percent protection was calculated from the mean worm burdens, epg, or circulating antigen levels, using the formula: % protection = [(control − test)/control] × 100. In order to eliminate the primary infection, anesthetized animals, prior to vaccination or challenge, were given two doses of Praziquantel (PZQ; Biltricide, Bayer East Africa, Nairobi, Kenya), 1 week apart, by oral gavage at a dose of 60 mg/kg. Treatment was judged successful when egg-negative fecal samples were obtained on two consecutive weeks.
Assays.
Blood for serum was obtained from each animal at baseline and at regular intervals during the 47-week period. The parasite antigen preparations used for assay of antibody responses were material released during cercarial transformation into schistosomula (0-3hRAP), soluble proteins from adult worms (SWAP), and soluble proteins from eggs (SEA) (1, 13). Antigen-specific immunoglobulin M (IgM) and IgG antibodies in serum were measured by ELISA (11). Plates were coated with 0-3hRAP (0.25 μg/ml), SWAP (10 μg/ml), or SEA (5 μg/ml), and sera were diluted as follows: 0-3hRAP, 1/3,200; SWAP, 1/400; and SEA, 1/12,000. As a second indirect estimate of worm burden the glycans, circulating anodic antigen (CAA) and circulating cathodic antigen (CCA), which are released into the bloodstream from the parasites' gut, were measured by antigen-capture ELISA with specific monoclonal antibodies (7, 17).
Pathology.
A subjective assessment of gross pathology was made at 6 and 10 weeks postchallenge. At the earlier time point a laparotomy was performed on anesthetized animals to permit visual inspection of the intestines and liver (9). After taking a wedge biopsy (approximately 10 g), the edges of the liver were opposed and sutured, and the abdominal incision was closed by standard surgical procedures. At the 10-week time point, liver samples were obtained immediately after vascular perfusion. All samples were fixed in phosphate-buffered formalin prior to sectioning and staining with hematoxylin and eosin, prior to histopathological examination and evaluation of granulomata where present.
Experimental design.
For logistical reasons, the study was divided into two independent experiments (Table 1). In experiment A we determined whether a preceding schistosome infection terminated by drug treatment had any impact on the protection elicited by subsequent exposures to the RA vaccine. The experimental groups were identified as ITV (for infected, treated, vaccinated), IT (for infected, treated), TV (for treated, vaccinated), and control (C1). In experiment B we determined whether the superimposition of the RA vaccine on a chronic schistosome infection affected the level of protection induced. The experimental groups were identified as IVT (for infected, vaccinated, treated), VT (for vaccinated, treated), and control (C2). The infection was terminated by PZQ treatment at weeks 12 and 13 in experiment A and at weeks 32 and 33 in experiment B; in the latter case, this was necessary so that only challenge worms would be counted. Irrespective of the infection and/or vaccination regime, animals were challenged at 37 weeks with normal cercariae and worm burdens were determined 10 weeks later.
TABLE 1.
Experimental plan
| Expt | Group (no. of animals)a | Treatment at wkb:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 12-13 | 18 | 22 | 26 | 30 | 34 | 37 | 47 | ||
| A | ITV (7) | I | T T | V | V | V | V | V | Ch | P |
| IT (6) | I | T T | Ch | P | ||||||
| TV (5) | T T | V | V | V | V | V | Ch | P | ||
| C1 (5) | Ch | P | ||||||||
| B | IVT (7) | I | V* | V | V | V | V | T T† | Ch | P |
| VT (5) | V* | V | V | V | V | T T† | Ch | P | ||
| C2 (5) | Ch | P | ||||||||
The experimental groups received a combination of regimes over a period of 47 weeks as indicated.
I, infection with normal cercariae; T, treatment with PZQ; V, vaccination with RA cercariae; Ch, challenge with normal cercariae; P, portal perfusion. *, week 14; †, weeks 32 to 33.
Statistical analysis.
Data were analyzed by using the two-tailed Student t test; differences between groups were considered not significant (NS) when P > 0.05. All values depicted are means ± the standard error of the mean (SEM) (n = 5 to 7 animals). A regression analysis of fecal eggs, CAA and CCA on time was performed on the IVT group data between weeks 12 and 32 after primary infection.
RESULTS
Fecal egg and circulating antigen profile infection status.
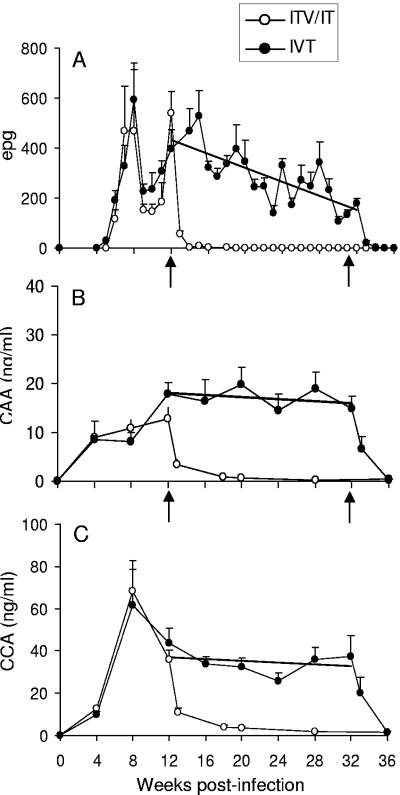
Three separate measures were used to chart infection status, and the effects of drug treatment, up to challenge. Eggs were detected in the feces at week 5 after primary infection, with numbers rising to a peak at week 8 and declining rapidly to a trough at weeks 9 to 11, before rebounding (Fig. 1A). In drug-treated ITV and IT groups the egg output was essentially zero by week 14 (except in one animal in the ITV group which was removed from the study). The IVT group showed a gradual decline in fecal egg output over the period from 12 to 32 weeks, coinciding with the five exposures to the RA vaccine, which regression analysis showed to be highly significant (P < 0.005). After treatment at weeks 32 and 33, egg output returned to zero (again, except in one animal which was removed). The concentration of both circulating antigens was similar at week 4 (Fig. 1B and C) but, whereas CAA levels rose only slowly thereafter, CCA showed a large increment at week 8 (four times the level of CAA) before declining. In ITV and IT groups, there was a sharp initial fall in levels after drug treatment and then a more gradual convergence with the baseline. In contrast to fecal eggs the levels of both CAA and CCA in the IVT group, although differing in magnitude, remained constant until treatment (regression analyses, NS), when they declined rapidly to baseline. At challenge, on the basis of the three indirect measures, few worms of the primary infection remained in any group.
FIG. 1.
Infection status of baboons between primary exposure and challenge at 37 weeks, determined by three independent measures: eggs/gm feces (epg) (A), CAA (B), and CCA (C). Experimental groups were ITV and IT plotted as combined data and IVT. PZQ treatment of the ITV and IT groups was at weeks 12 and 13, and the IVT group was treated at weeks 32 and 33 (arrows in panels A and B). Regression analysis was performed on IVT data between weeks 12 and 32: y = −14.1x + 603 (P < 0.005) (A); y = 0.029x + 14.33 (NS) (B); and y = −0.214x + 39.7 (NS) (C). Values are means + the SEM (n = 5 to 7).
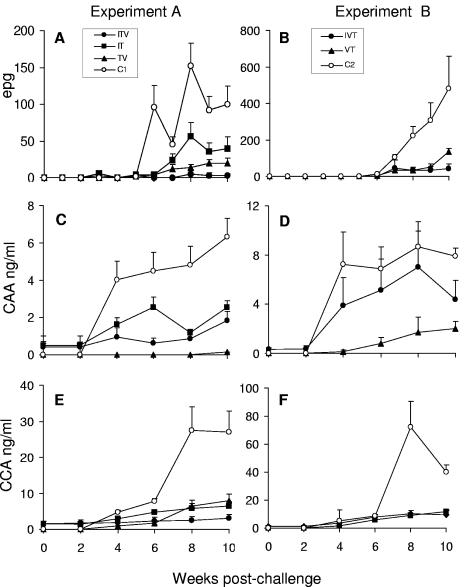
After challenge, the parasitological profiles of the control groups were broadly similar to those observed after primary infection except that fecal eggs were not detected until 6 weeks and there was no marked decline at weeks 9 and 10 (Fig. 2A and B; mean values at weeks 8, 9, and 10 not significantly different from each other). CAA levels rose rapidly between weeks 2 and 4 to a plateau, while those for CCA rose later, between weeks 6 and 8 (Fig. 2C to F; CAA means for weeks 6, 8, and 10, and CCA means for weeks 8 and 10 not significantly different from each other). In comparison, all test groups in both experiments showed more muted parasitological profiles, a finding consistent with a lower worm burden. The one exception was the IVT group where CAA levels, but not CCA, were close to the controls.
FIG. 2.
Infection status of baboons from challenge to perfusion, determined by epg (A and B), CAA (C and D), and CCA (E and F). Groups in experiment A (infection, treatment, vaccination, challenge) are plotted in panels A, C, and E, and groups in experiment B (infection, vaccination, treatment, challenge) are plotted in panels B, D, and F. Values are means + SEM (n = 5 to 7).
The efficacy of the RA vaccine is not compromised by a schistosome infection.
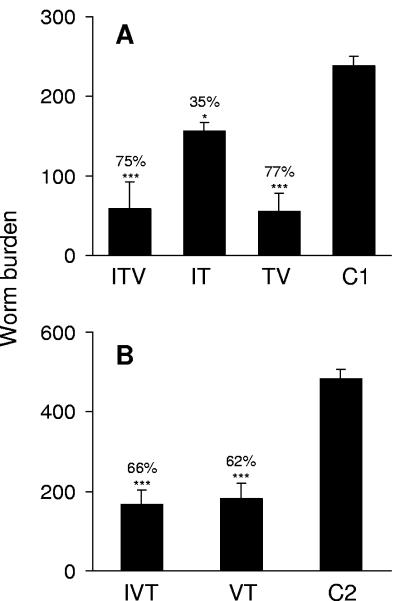
In experiment A, the standard vaccination schedule given to the positive control (TV) group elicited a highly significant reduction in mean worm burden relative to the C1 group, which equated to 77% protection (Fig. 3A). The ITV group also showed a substantial reduction in worm burden, equating to 75% protection. The IT group that did not receive the vaccine had a 35% reduction in worm burden. In experiment B, animals in the positive control (VT) group showed a highly significant reduction in worm burden relative to the C2 group; this equated to 62% protection (Fig. 3B). The infected, vaccinated (IVT) group, which was treated shortly before challenge, also showed a significant reduction in worm numbers, equating to 66% protection.
FIG. 3.
Worm burdens after challenge and perfusion for experiment A (infection, treatment, vaccination, challenge) (A) and experiment B (infection, vaccination, treatment, challenge) (B). The percent protection relative to controls is indicated above each bar: ✽✽✽, P < 0.001; ✽✽, P < 0.01; ✽, P < 0.05. Values are means + the SEM (n = 5 to 7).
Indirect measures of worm burden overestimate protection.
From the data on fecal eggs and circulating antigens, at steady state, we calculated apparent protection for comparison with that based on actual worm burden (Table 2). Using an arbitrary difference of 5% or more, fecal egg counts overestimated protection on five of five, CAA on three of five, and CCA on four of five occasions. Overall, in 7 of these 12 instances the discrepancy was >20%. For fecal eggs, an immunologically driven reduction in female fecundity is a potential contributory factor to the overestimation. The fecal egg output per mature female (mean of weeks 8, 9, and 10) provides a crude indicator of reproductive fitness. The combined data for control groups and vaccine-only groups gave values of 69 and 72 eggs/female/day, respectively, while those for the infected and vaccinated groups had a mean of 42 (or 34 if one outlier was removed).
TABLE 2.
Percent protection measured by worm burden, eggs per gram of feces, and circulating antigen levels (CAA and CCA)
| Expt | Group | % Protectiona
|
|||
|---|---|---|---|---|---|
| WB | epg | CAA | CCA | ||
| A | ITV | 75 | 97 | 74b | 89 |
| TV | 77 | 84 | 99 | 74 | |
| IT | 35 | 62 | 67 | 78 | |
| B | IVT | 66 | 89 | 61 | 85 |
| VT | 62 | 79 | 77 | 82 | |
Numbers in boldface are protection values ≥ 5% than those based on worm burden (WB).
Estimates of protection are based on means derived from time points before perfusion that were not significantly different from each other (epg, weeks 8, 9, and 10; CAA, weeks 6, 8, and 10; CCA, weeks 8 and 10). All datum points from which protection was calculated were significantly lower than their respective controls (P at least <0.05).
Antibody responses to eggs greatly exceed those to vaccination.
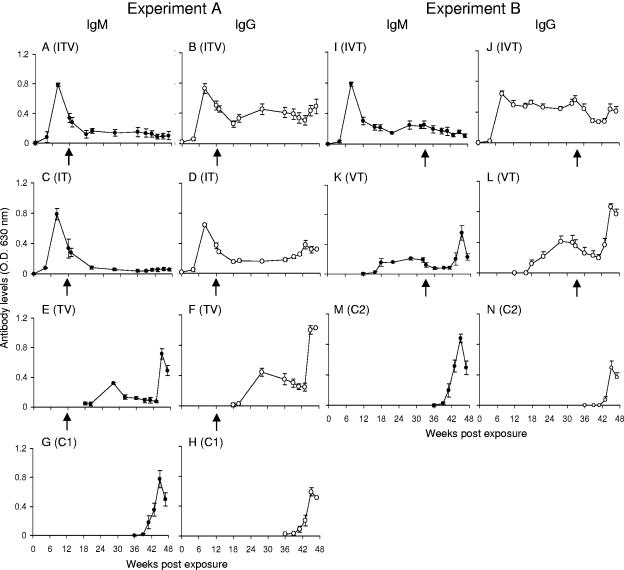
The IgM and IgG profiles detected by using three crude antigen preparations were essentially similar, so only the responses to 0-3hRAP are displayed. In baboons exposed to normal cercariae (ITV, IT, and IVT), IgM levels rose sharply after patency to a peak at 8 weeks before falling equally rapidly, irrespective of when animals received treatment (Fig. 4A, C, and I). Furthermore, there was no rise in IgM in the weeks after treatment, in response to antigen released from dying worms. Only modest levels of IgM were induced by vaccination alone (TV and VT; Fig. 4E and K), but these rose dramatically 6 to 8 weeks after challenge, as did those of the controls (C1 and C2; Fig. 4G and M), again in response to egg deposition; in all four groups IgM was already declining at perfusion. No such rise in IgM occurred after challenge in the three previously infected groups (Fig. 4A, C, and I). The IgG profiles throughout were broadly similar to those of IgM except in the following specifics. There was a perceptible response to the vaccinations in the ITV group (Fig. 4B [cf. Fig. 4D]). A small recall response to egg deposition occurred in ITV, IT, and IVT groups (Fig. 4B, D, and J). There was a sustained IgG response in the IVT group due to the continued presence of the initial infection, which obscured any potential response to the vaccine (Fig. 4J). The magnitude of the IgG response at week 8 postchallenge in the TV and VT groups (Fig. 4F, and L) was approximately double that of their challenge controls (Fig. 4H and N), although the latter had much higher worm burdens. There was no significant correlation between IgG levels at challenge and percentage protection in TV and VT groups (the only ones where comparisons are valid as there was no previous infection).
FIG. 4.
Antibody profiles over the whole time course probed by using 0-3hRAP (cercarial secretions). For experiment A (infection, treatment, vaccination, challenge) the IgM levels are plotted in panels A, C, E, and G, and the IgG levels are plotted in panels B, D, F, and H for groups ITV, IT, TV, and C1, respectively. For experiment B (infection, vaccination, treatment, challenge) the IgM levels are plotted in panels I, K, and M and the IgG levels are plotted in panels J, L, and N for groups IVT, VT, and C2, respectively. Five vaccinations were given at 4-week intervals starting at week 18 in experiment A and week 14 in experiment B. PZQ treatment (arrow below x axis) was at weeks 12 and 13 in experiment A and at weeks 32 and 33 in experiment B. Values are means + the SEM (n = 5 to 7).
Infection but not vaccination diminishes hepatic inflammation.
Animals receiving a primary infection exhibited acute symptoms between weeks 8 and 12, characterized by bloody diarrhea, loss of appetite, and general malaise. Groups treated with PZQ at weeks 12 and 13 rapidly resolved their diarrhea, but even in the absence of treatment the acute symptoms gradually disappeared. Repeated vaccinations were not associated with detectable change in health status in any experimental group. After challenge, no symptoms were apparent before week 7, when general malaise was observed in all groups. The challenge controls developed persistent diarrhea, which was not apparent in any of the vaccinated groups (with the exception of three of six IVT animals where diarrhea was recorded on a single occasion).
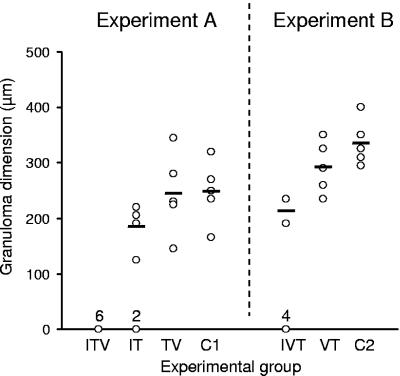
At 6 weeks postchallenge, all previously infected and/or vaccinated animals displayed mild to moderate hepatic pathology at the gross level, which was manifested as periportal inflammatory cell infiltration but with no classical granulomata that warranted measurement. In contrast, all of the challenge controls had florid granulomatous inflammation that was graded as moderate or severe. The distinction at the gross level between previously exposed and control animals persisted at 10 weeks. Nevertheless, granulomata were present in all animals exposed to the vaccine only as well as the challenge controls, with no significant difference in mean granuloma size (Fig. 5). However, no measurable granulomata were evident around eggs in the majority of previously infected animals (all six ITV, four of six IVT, and two of six IT).
FIG. 5.
Hepatic egg granuloma dimensions at 10 weeks postchallenge for experiment A (infection, treatment, vaccination, challenge) and experiment B (infection, vaccination, treatment, challenge). Circles and solid bars represent individual and mean values, respectively (n = 5 to 7). The number of animals in each group with zero granulomas is depicted above the x axis.
DISCUSSION
We used worm burden as the unequivocal measure to determine whether the protective capacity of the RA vaccine was compromised by a previous or ongoing schistosome infection. Vaccine efficacy and reproducibility was confirmed by the protection it elicited in the two vaccinated-only groups. With a 3-week interval between the last vaccination and challenge, the level was comparable to that in our previous study (11), while extending it to 7 weeks resulted in diminished protection, again in line with our earlier data. Where vaccination followed a primary infection terminated by drug treatment just as it reached the chronic stage, we saw no difference in the level of protection relative to the vaccine-only group. Infection and treatment alone also elicited modest protection (35%), but its combination with vaccination was not additive. A comparable report recorded 41% protection in baboons with a primary infection terminated by PZQ between 15 and 5 weeks before challenge (16). Where the vaccine was superimposed on the chronic phase of a schistosome infection, the level of protection was virtually identical to that after vaccination alone. Unfortunately, in the absence of an IT group, we cannot rule out a contribution to protection from the long-term infection itself or of boosting by the drug treatment, but the latter seems unlikely in view of our failure to detect any associated increase in antibody level in the weeks after treatment.
Human trials of recombinant vaccines against schistosomiasis mansoni will have to rely on indirect assays to estimate the intensity of the infection. The circulating antigen assays are used for diagnosis but sometimes also to estimate the intensity of infection (18, 24). Unfortunately, it is difficult to envisage how the mathematical form of the relationship between each indirect measure and worm burden or the absolute sensitivity (i.e., the minimum number of worms detectable) could be established for humans. However, it is clear that the three assays have limitations for evaluating differences in the intensity of infection, and hence protection. Based on the data from this and our previous study (11), fecal egg output overestimated protection on nine of nine, CAA on six of nine, and CCA on four of five occasions, making CAA the best predictor. Unlike our previous study, we cannot ascribe overestimation by fecal egg counts to the delayed arrival of parasites in the portal tract since the 10-week interval to perfusion was selected to provide an adequate margin after patency. While the lower egg output per female in the infected and vaccinated groups relative to the controls suggests an anti-fecundity effect, which would partly account for the overestimation, no such disparity was observed in the vaccine-only groups. In the former groups other factors deriving from the prior infection status, such as greater retention of eggs in the intestine or proportionally greater deposition in the liver, might explain the lower egg output, but histological analyses of intestinal and liver tissues did not reveal a discrepancy (unpublished data).
We can be confident that the initial cercarial exposures resulted in substantial primary infections, thus providing the necessary immunological background for the vaccination experiments. On the basis of the indirect assays, parasite development was virtually identical in the two experiments up to 13 weeks. The trough in egg output between weeks 9 and 11 coincided with the acute phase of infection when all animals experienced diarrhea and is likely to be an artifact since eggs are difficult to discern in smears made from loose stools. Although the early patterns of CAA and CCA production differed, both circulating antigens quickly reached equilibrium, albeit at different absolute levels. In animals receiving later treatment, there was a significant decline in egg output during the chronic phase, which could reflect either worm attrition or reduced fecundity. Since the uniform circulating antigen levels between weeks 12 and 32 indicate normal feeding patterns without worm death, we suggest that the decline in fecal egg output represents an antifecundity effect. The absence of a parallel infection-only group does not allow us to attribute this phenomenon to the superimposed vaccinations. However, in an earlier study no decline in fecal egg output was observed over a 30-week period after exposure of baboons to 1,000 cercariae (22), suggesting the vaccinations are responsible.
Since we had found antibody responses to be more informative than those of peripheral blood mononuclear cells in our previous study (11), we used changes in parasite-specific IgM and IgG levels as indicators of host immune reactivity to infection and vaccination. In animals given a primary infection, the most prominent feature before challenge was the overwhelming response to parasite egg deposition, which made it difficult to discern a contribution from vaccination. One explanation for the intense response to eggs relative to attenuated larvae is the enormous disparity in antigen load, such that the egg production in a single day was greater than the entire biomass of five doses of the vaccine. In the previously infected and treated baboons (ITV group), it was possible to detect an IgG response to the vaccine above the background provided by the IT group. In both experiments drug treatment failed to boost antibody levels, which is surprising given the amount of antigen that would be released into the bloodstream following worm death. This was not the experience with responses in human populations, where there was a significant rise in antibody levels 5 weeks after drug treatment of schoolchildren (2, 19). It seems unlikely that the discrepancy can be attributed to differences in worm burden between the cited human study and our own because fecal egg output was comparable.
After challenge, the impact of the vaccination regimes is clearly visible when we examine the parasitological and immunological parameters. In all but one instance, the vaccinated groups had depressed profiles of egg output and circulating antigen levels relative to their respective controls. The latter groups mirrored both the pattern of egg output and the circulating antigen production after the primary infection except that no decline in fecal eggs was observed at the 9- and 10-week sampling times. Potential explanations include less severe diarrhea due to the smaller worm burden postchallenge or slower maturation of worms. Characterization of antibody profiles after challenge revealed that previously infected animals lacked a secondary IgM response and showed only a muted IgG response compared to both vaccinated-only and control groups. These observations provide the best evidence that immune responses to eggs had downmodulated irrespective of whether the animals had been exposed to a 12- or a 32-week primary infection. In contrast, after the onset of egg deposition, the two vaccinated-only groups showed a marked antibody response. In spite of lower worm burdens, the IgM levels were equivalent to those of their respective controls, whereas the IgG levels were higher. It would appear that the antigens from vaccinating larvae are qualitatively or quantitatively insufficient to cause immune downmodulation. Indeed, the stronger secondary IgG response of vaccinated-only animals after egg deposition implies that exposure to larvae primes for egg antigens.
Overall, our observations suggest that the protective immune responses elicited by the RA vaccine are distinct from those made to the egg and imply that current or previous schistosome infections are not interfering with the vaccination protocol. By extension, this situation should pertain to the antigenic targets that mediate protection elicited by the RA vaccine, provided that they can be identified, expressed as recombinants, and administered to replicate the same immunological mechanisms. Recent developments in analysis of the S. mansoni genome, transcriptome, and proteome should facilitate the identification of these antigenic targets (5, 25). Simultaneously, and before any S. mansoni recombinant antigen vaccines are taken forward to human trials, the need for more sensitive predictors of worm burden should be addressed. The baboon appears to be the ideal animal in which to authenticate such tools for determining vaccine efficacy.
Acknowledgments
This study was supported by an INCO-DEV Program, Research for Development EC contract ICA4-CT99-10006. T.M.K. received financial assistance from UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
We thank S. Kiarie and S. Kisara for help with parasitological assays and portal perfusions; F. Nyundo and K. Kithome for technical support in immunological assays; D. Chai and M. Ndungu for surgical procedures; the animal care staff at IPR, Nairobi, Kenya; and D. Kornelis at LUMC, Leiden, The Netherlands for performing the circulating antigen assays.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Ashton, P. D., R. S. Curwen, and R. A. Wilson. 2001. Linking proteome and genome: how to identify parasite proteins. Trends Parasitol. 17:198-202. [DOI] [PubMed] [Google Scholar]
- 2.Butterworth, A. E., P. R. Dalton, D. W. Dunne, M. Mugambi, J. H. Ouma, B. A. Richardson, T. K. Siongok, and R. F. Sturrock. 1984. Immunity after treatment of human schistosomiasis mansoni. I. Study design, pretreatment observations, and the results of treatment. Trans. R. Soc. Trop. Med. Hyg. 78:108-123. [DOI] [PubMed] [Google Scholar]
- 3.Cooper, P. J., I. Espinel, M. Wieseman, W. Paredes, M. Espinel, R. H. Guderian, and T. B. Nutman. 1999. Human onchocerciasis and tetanus vaccination: impact on the postvaccination antitetanus antibody response. Infect. Immun. 67:5951-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulson, P. S. 1997. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv. Parasitol. 39:271-336. [DOI] [PubMed] [Google Scholar]
- 5.Curwen, R. S., P. D. Ashton, D. A. Johnston, and R. A. Wilson. 2004. The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages. Mol. Biochem. Parasitol. 138:57-66. [DOI] [PubMed] [Google Scholar]
- 6.Dean, D. A. 1983. Schistosoma and related genera: acquired resistance in mice. Exp. Parasitol. 55:1-104. [DOI] [PubMed] [Google Scholar]
- 7.Deelder, A. M., N. De Jonge, O. C. Boerman, Y. E. Fillie, G. W. Hilberath, J. P. Rotmans, M. J. Gerritse, and D. W. Schut. 1989. Sensitive determination of circulating anodic antigen in Schistosoma mansoni infected individuals by an enzyme-linked immunosorbent assay using monoclonal antibodies. Am. J. Trop. Med. Hyg. 40:268-272. [DOI] [PubMed] [Google Scholar]
- 8.Eberl, M., J. A. Langermans, P. A. Frost, R. A. Vervenne, G. J. van Dam, A. M. Deelder, A. W. Thomas, P. S. Coulson, and R. A. Wilson. 2001. Cellular and humoral immune responses and protection against schistosomes induced by a radiation-attenuated vaccine in chimpanzees. Infect. Immun. 69:5352-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farah, I. O., M. Nyindo, M. A. Suleman, J. Nyaundi, T. M. Kariuki, R. E. Blanton, L. H. Elson, and C. L. King. 1997. Schistosoma mansoni: development and modulation of the granuloma after single or multiple exposures in the baboon (Papio cynocephalus anubis). Exp. Parasitol. 86:93-101. [DOI] [PubMed] [Google Scholar]
- 10.Genton, B., I. Betuela, I. Felger, F. Al-Yaman, R. F. Anders, A. Saul, L. Rare, M. Baisor, K. Lorry, G. V. Brown, D. Pye, D. O. Irving, T. A. Smith, H. P. Beck, and M. P. Alpers. 2002. A recombinant blood-stage malaria vaccine reduces Plasmodium falciparum density and exerts selective pressure on parasite populations in a phase 1-2b trial in Papua New Guinea. J. Infect. Dis. 185:820-827. [DOI] [PubMed] [Google Scholar]
- 11.Kariuki, T. M., I. O. Farah, D. S. Yole, J. M. Mwenda, G. J. Van Dam, A. M. Deelder, R. A. Wilson, and P. S. Coulson. 2004. Parameters of the attenuated schistosome vaccine evaluated in the olive baboon. Infect. Immun. 72:5526-5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King, C. L. 2001. Initiation and regulation of disease in schistosomiasis, p. 213-264. In A. A. F. Mahmoud (ed.), Schistosomiasis, vol. 3. Imperial College Press, London, England. [Google Scholar]
- 13.Mountford, A. P., R. Harrop, and R. A. Wilson. 1995. Antigens derived from lung-stage larvae of Schistosoma mansoni are efficient stimulators of proliferation and gamma interferon secretion by lymphocytes from mice vaccinated with attenuated larvae. Infect. Immun. 63:1980-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutapi, F. 2001. Heterogeneities in anti-schistosome humoral responses following chemotherapy. Trends Parasitol. 17:518-524. [DOI] [PubMed] [Google Scholar]
- 15.Nyindo, M., and I. O. Farah. 1999. The baboon as a non-human primate model of human schistosome infection. Parasitol. Today 15:478-482. [DOI] [PubMed] [Google Scholar]
- 16.Nyindo, M., T. M. Kariuki, P. W. Mola, I. O. Farah, L. Elson, R. E. Blanton, and C. L. King. 1999. Role of adult worm antigen-specific immunoglobulin E in acquired immunity to Schistosoma mansoni infection in baboons. Infect. Immun. 67:636-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polman, K., M. M. Diakhate, D. Engels, S. Nahimana, G. J. Van Dam, S. T. Falcao Ferreira, A. M. Deelder, and B. Gryseels. 2000. Specificity of circulating antigen detection for schistosomiasis mansoni in Senegal and Burundi. Trop. Med. Int. Health 5:534-537. [DOI] [PubMed] [Google Scholar]
- 18.Polman, K., F. F. Stelma, S. Le Cessie, S. J. De Vlas, S. T. Falcao Ferreira, I. Talla, A. M. Deelder, and B. Gryseels. 2002. Evaluation of the patterns of Schistosoma mansoni infection and reinfection in Senegal, from faecal egg counts and serum concentrations of circulating anodic antigen. Ann. Trop. Med. Parasitol. 96:679-689. [DOI] [PubMed] [Google Scholar]
- 19.Roberts, S. M., R. A. Wilson, J. H. Ouma, H. C. Kariuki, D. Koech, T. K. arap Siongok, R. F. Sturrock, and A. E. Butterworth. 1987. Immunity after treatment of human schistosomiasis mansoni quantitative and qualitative antibody responses to tegumental membrane antigens prepared from adult worms. Trans. R. Soc. Trop. Med. Hyg. 81:786-793. [DOI] [PubMed] [Google Scholar]
- 20.Sabin, E. A., M. I. Araujo, E. M. Carvalho, and E. J. Pearce. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 173:269-272. [DOI] [PubMed] [Google Scholar]
- 21.Soisson, L. A., G. D. Reid, I. O. Farah, M. Nyindo, and M. Strand. 1993. Protective immunity in baboons vaccinated with a recombinant antigen or radiation-attenuated cercariae of Schistosoma mansoni is antibody-dependent. J. Immunol. 151:4782-4789. [PubMed] [Google Scholar]
- 22.Sturrock, R. F., A. E. Butterworth, and V. Houba. 1976. Schistosoma mansoni in the baboon (Papio anubis): parasitological responses of Kenyan baboons to different exposures of a local parasite strain. Parasitology 73:239-252. [DOI] [PubMed] [Google Scholar]
- 23.Sturrock, R. F., A. E. Butterworth, V. Houba, S. D. Karamsadkar, and R. Kimani. 1978. Schistosoma mansoni in the Kenyan baboon (Papio anubis): the development and predictability of resistance to homologous challenge. Trans. R. Soc. Trop. Med. Hyg. 72:251-261. [DOI] [PubMed] [Google Scholar]
- 24.Van Lieshout, L., K. Polman, B. Gryseels, and A. M. Deelder. 1998. Circulating anodic antigen levels in two areas endemic for schistosomiasis mansoni indicate differences in worm fecundity. Trans. R. Soc. Trop. Med. Hyg. 92:115-119. [DOI] [PubMed] [Google Scholar]
- 25.Verjovski-Almeida, S., R. DeMarco, E. A. Martins, P. E. Guimaraes, E. P. Ojopi, A. C. Paquola, J. P. Piazza, M. Y. Nishiyama, Jr., J. P. Kitajima, R. E. Adamson, P. D. Ashton, M. F. Bonaldo, P. S. Coulson, G. P. Dillon, L. P. Farias, S. P. Gregorio, P. L. Ho, R. A. Leite, L. C. Malaquias, R. C. Marques, P. A. Miyasato, A. L. Nascimento, F. P. Ohlweiler, E. M. Reis, M. A. Ribeiro, R. G. Sa, G. C. Stukart, M. B. Soares, C. Gargioni, T. Kawano, V. Rodrigues, A. M. Madeira, R. A. Wilson, C. F. Menck, J. C. Setubal, L. C. Leite, and E. Dias-Neto. 2003. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nat. Genet. 35:148-157. [DOI] [PubMed] [Google Scholar]
- 26.Warren, K. S., and E. G. Berry. 1972. Induction of hepatosplenic disease by single pairs of the Philippine, Formosan, Japanese, and Chinese strains of Schistosoma japonicum. J. Infect. Dis. 126:482-491. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, R. A., and P. S. Coulson. 1999. Strategies for a schistosome vaccine: can we manipulate the immune response effectively? Microbes Infect. 1:535-543. [DOI] [PubMed] [Google Scholar]
- 28.Yole, D. S., R. Pemberton, G. D. Reid, and R. A. Wilson. 1996. Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology 112:37-46. [DOI] [PubMed] [Google Scholar]
- 29.Yole, D. S., G. D. Reid, and R. A. Wilson. 1996. Protection against Schistosoma mansoni and associated immune responses induced in the vervet monkey Cercopithecus aethiops by the irradiated cercaria vaccine. Am. J. Trop. Med. Hyg. 54:265-270. [DOI] [PubMed] [Google Scholar]