Abstract
Vaccination against Actinobacillus pleuropneumoniae is hampered by the lack of vaccines inducing reliable cross-serotype protection. In contrast, pigs surviving natural infection are at least partially protected from clinical symptoms upon reinfection with any serotype. Thus, we set out to construct an attenuated A. pleuropneumoniae live vaccine allowing the differentiation of vaccinated from infected animals (the DIVA concept) by successively deleting virulence-associated genes. Based on an A. pleuropneumoniae serotype 2 prototype live negative marker vaccine (W. Tonpitak, N. Baltes, I. Hennig-Pauka, and G.-F. Gerlach, Infect. Immun. 70:7120-7125, 2002), genes encoding three enzymes involved in anaerobic respiration and the ferric uptake regulator Fur were deleted, resulting in a highly attenuated sixfold mutant; this mutant was still able to colonize the lower respiratory tract and induced a detectable immune response. Upon a single aerosol application, this mutant provided significant protection from clinical symptoms upon heterologous infection with an antigenically distinct A. pleuropneumoniae serotype 9 challenge strain and allowed the serological discrimination between infected and vaccinated groups.
Actinobacillus pleuropneumoniae is the causative agent of porcine pleuropneumonia, a highly contagious, often fatal disease encountered worldwide (14). The pathogen is transmitted by aerosol or direct contact with infected pigs (32, 34, 50); the course of disease can range from peracute to chronic, with asymptomatic carrier pigs being a major source for introduction into previously uninfected herds (8). Based on capsular and lipopolysaccharide antigens, 15 serotypes are recognized which have a regionally variable distribution (7). Virulence of A. pleuropneumoniae is caused by several factors, such as capsular polysaccharide (25), lipopolysaccharide (51), outer membrane proteins (42, 43), iron uptake proteins (1, 5), and Apx toxins (15). Also, enzymes involved in anaerobic respiration appear to play an important role in A. pleuropneumoniae infection (2, 29).
Due to an increasing consumer demand concerning food safety, vaccination is an adequate way to decrease the use of antibiotic drugs by decreasing morbidity and mortality in infected herds (52, 54). Vaccination against A. pleuropneumoniae infection is hampered by the occurrence of different serotypes and the finding that commonly used whole-cell bacterin vaccines neither induce cross-serotype immunity nor prevent development of the carrier state. Furthermore, the differentiation between vaccinated and infected animals is not possible (14, 24).
Since pigs surviving natural or experimental infections with A. pleuropneumoniae are at least partially protected from clinical symptoms upon infection with another serotype (10, 22, 36, 37), Tonpitak et al. (49) proposed the use of an A. pleuropneumoniae serotype 2 prototype live marker vaccine constructed by deletion of apxIIA and ureC genes. This double mutant protected pigs from homologous challenge upon a single aerosol application. Furthermore, it follows the differentiating infected and vaccinated animals (DIVA) concept (53), which is based on the absence of one immunogenic protein (ApxII) in the vaccine strain. However, this prototype marker vaccine strain was still able to cause clinical disease in a small proportion of pigs.
In the study presented here, we set out to gradually increase the attenuation of the prototype live negative marker vaccine strain by deleting newly identified virulence-associated genes using an established single-step transconjugation system (39). We initially focused on enzymes involved in anaerobic respiration to impair the survival of the mutant strain under conditions found in sequestered lung tissue and on epithelial surfaces (2, 3, 29) and subsequently on the ferric uptake regulator protein Fur, which is known to play an important role in A. pleuropneumoniae virulence (28). Furthermore, we investigated the properties of the resulting sixfold mutant strain as a live negative marker vaccine to induce a protective immune response upon challenge with a heterologous A. pleuropneumoniae serotype 9 strain.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains, plasmids, and primers used in this work are listed in Table 1. Escherichia coli strains were cultured in Luria-Bertani medium supplemented with the appropriate antibiotics (ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml); for cultivation of E. coli β2155 (ΔdapA), diaminopimelic acid (1 mM; Sigma-Aldrich, Munich, Germany) was added. A. pleuropneumoniae strains were cultured in PPLO medium (Difco GmbH, Augsburg, Germany) supplemented with nicotinamide dinucleotide (NAD; 10 μg/ml; Merck, Darmstadt, Germany), l-cysteine-hydrochloride (260 μg/ml; Sigma-Aldrich), l-cystine-dihydrochloride (10 μg/ml; Sigma-Aldrich), dextrose (1 mg/ml), and Tween 80 (0.1%) at 37°C in a shaking incubator at 180 rpm. A. pleuropneumoniae transconjugants (single crossovers) and transformants were grown in supplemented PPLO medium containing chloramphenicol (5 μg/ml) or kanamycin (25 μg/ml), and the medium for counterselection was prepared as described previously (49). Iron restriction was induced by addition of diethylentriamine-pentaacetic acid calcium trisodium salt hydrate (Na3CaDTPA; Fluka Chemika and BioChemika, Buchs, Switzerland) at a final concentration of 150 μM. Anaerobic cultures used for determination of aspartase activity and DmsA expression were first cultured to an optical density at 600 nm of 0.3 under aerobic conditions and then placed into an anaerobic jar without shaking at 37°C for 3 h.
TABLE 1.
Characteristics of bacterial strains, plasmids, primers, and sera used in this study
| Strain, plasmid, primer, and serum | Characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5αF′ | F′ endA1 hsdR17(rK− mK+) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacZYA-argF) U169 deoR Φ80dlacΔ(lacZ)M15 | 41 |
| E. coli β2155 | thrB1004 pro thi hsdS lacZΔM15 (F′ lacZΔM15 lacIqtraD36 proA+proB+) Δdap::erm (Ermr) | 12 |
| A. pleuropneumoniae C5934 | A. pleuropneumoniae serotype 2 clinical isolate from the lung of a diseased pig in northern Germany | 49 |
| A. pleuropneumoniae C5934 ΔapxIIA ΔureC | Unmarked apxIIA- and ureC-negative mutant of A. pleuropneumoniae C5934 | 49 |
| A. pleuropneumoniae C5934 ΔapxIIA ΔureC ΔdmsA | Unmarked dmsA-negative mutant of A. pleuropneumoniae C5934 ΔapxIIA ΔureC | This work |
| A. pleuropneumoniae C5934 ΔapxIIA ΔureC ΔdmsA ΔhybB | Unmarked hybB-negative mutant of A. pleuropneumoniae C5934 ΔapxIIA ΔureC ΔdmsA | This work |
| A. pleuropneumoniae C5934 ΔapxIIA ΔureC ΔdmsA ΔhybB ΔaspA | Unmarked aspA-negative mutant of A. pleuropneumoniae C5934 ΔapxIIA ΔureC ΔdmsA ΔhybB | This work |
| A. pleuropneumoniae C5934 ΔapxIIA ΔureC ΔdmsA ΔhybB ΔaspA Δfur | Unmarked fur-negative mutant of A. pleuropneumoniae C5934 ΔapxIIA ΔureC ΔdmsA ΔhybB ΔaspA | This work |
| A. pleuropneumoniae C1269 | A. pleuropneumoniae serotype 9 field isolate, obtained from a pig with symptoms of pneumonia in our laboratory | 21 |
| Plasmids | ||
| pBluescript SK | E. coli cloning vector carrying an ampicillin resistance determinant | Stratagene Europe, Amsterdam, The Netherlands |
| pCR 2.1-TOPO | Topoisomerase I-“enhanced” E. coli cloning vector carrying ampicillin and kanamycin resistance determinants as well as a lacZ gene for blue-white selection | TOPO TA Cloning; Invitrogen, Karlsruhe, Germany |
| pEMOC2 | Transconjugation vector based on pBluescipt SK with mobRP4, polycloning site, Cmr, and transcriptional fusion of the omlA promoter with the sacB gene | 6 |
| pDM800 | pBMK1 carrying the dmsA gene of A. pleuropneumoniae serotype 7 AP76 with an internal SwaI-NdeI deletion | 2 |
| pHYB603 | pBMK1 carrying the hybB gene of A. pleuropneumoniae serotype 7 AP76 with a 169-bp deletion between the HindIII and NarI restriction sites | 3 |
| pHYB700 | Transconjugation plasmid, containing a PspOMI/NotI fragment with the truncated hybB gene from pHYB603 cloned into pEMOC2 | This work |
| pAS110 | pBMK1 carrying the aspA gene of A. pleuropneumoniae serotype 7 strain AP76 with an internal Acc65I/SnaBI deletion | 29 |
| pAS700 | Transconjugation plasmid, containing a PspOMI/NotI fragment with the truncated aspA gene from pAS110 cloned into pEMOC2 | This work |
| pFUR802 | PCR products obtained with primers oFUR7 and oFUR7intb as well as PCR products obtained with primers oFUR8 and oFUR8int were cut with BsmBI and ligated; the ligation product, which represents the fur gene with a 153-bp deletion, was used as template for a PCR with primers oFUR7 and oFUR8, and the obtained PCR product was cloned into pCR 2.1 TOPO resulting in pFUR802 | This work |
| pFUR102 | Ligation of an XbaI fragment of pFUR802 into pBluescript SK cut with XbaI | This work |
| pFUR702 | Ligation of a PspOMI/NotI fragment of pFUR102 into pEMOC2 cut with PspOMI/NotI | This work |
| Primers | ||
| M13 forward | 5′ CAG GAA ACA GCT ATG AC 3′ | Amersham Bioscience |
| M13 reverse | 5′ GTA AAA CGA CGG CCA G 3′ | |
| oDMSAdel1 | 5′ TTG AAA TAT CCG ATG AAA CGT 3′, downstream primer comprising positions 327-348 of the dmsA homologue | 2 |
| oDMSAdel2 | 5′ TCA TAT TGG CGA CAT AAG CAT C 3′, upstream primer comprising positions 1593-1614 of the dmsA homologue | 2 |
| o34-1f | 5′ GCC AGC TTA TTC GGA TAT ACC 3′, upstream primer comprising positions 290-310 of the hybB gene | 3 |
| o34-1r | 5′ AAT AGC GTG TAC CGT CGT ACA 3′, downstream primer comprising positions 1399-1419 of the hybB gene | 3 |
| oASPX | 5′ TGG GCC GTA CTC AGT TAC AA 3′, upstream primer comprising positions 556-575 of the aspA gene | This work |
| oASPY | 5′ GGG CCT GAT GAA AGT AAA CG 3′, downstream primer comprising positions 891-910 of the aspA gene | This work |
| oFUR7 | 5′ GTCG TCT AGA GGA GTA ACA CGC GGA CAG TT 3′, upstream primer with internal XbaI site (underlined) comprising positions 654-625 upstream of the fur gene start codon | This work |
| oFUR7intb | 5′ TTAA CGT CTC GTA AAC CGT TGC CAA ACC GAT A 3′, downstream primer with internal BsmBI site (underlined) comprising positions 155-186 of the fur gene | This work |
| oFUR8 | 5′ CGAT TCT AGA CAA TAC TGC CCA CCG GTA CT 3′, downstream primer with internal XbaI site (underlined) comprising positions 693 to 722 downstream of the fur gene stop codon | This work |
| oFUR8int | 5′ TAAA CGT CTC GTT TAC GAA CGC CGT CAG CGT GAA ATC A 3′, upstream primer with internal BsmBI site (underlined) comprising positions 314-351 of the fur gene | This work |
| oFURX | 5′ GAA CGT GTA AAC CGT TGG TG 3′, forward primer situated 91-72 bp upstream of the start codon of the fur gene | This work |
| oFURY | 5′ GCC TGC AAA ACC TTC GGT AT 3′, reverse primer situated 32-51 bp upstream of the stop codon of the fur gene | This work |
| oAPX2A1 | 5′ GCT ATG ATT CGG GTC AAG GA 3′, forward primer situated 166 bp downstream of the start codon within the apxIIA gene | This work |
| oAPX2A2 | 5′ TCA TTA CCG GTT CCT CCA AC 3′, reverse primer situated 2332 bp downstream of the start codon within the apxIIA gene | This work |
| Sera | ||
| anti-DmsA | Antibodies raised against recombinant DmsA protein from A. pleuropneumoniae serotype 7 | 2 |
| anti-TbpB7 | Antibodies raised against recombinant TbpB protein from A. pleuropneumoniae serotype 7 | 20 |
Manipulation of DNA.
DNA-modifying enzymes were purchased from New England Biolabs (Bad Schwalbach, Germany) and used according to the manufacturer's instructions. Taq polymerase was purchased from Gibco-BRL Life Technologies (Karlsruhe, Germany). Chromosomal DNA for PCR and Southern blotting as well as plasmid DNA were prepared by standard protocols (45). PCR, Southern blotting, transformation, and gel electrophoresis were done by standard procedures (45), and pulsed-field gel electrophoresis (PFGE) was performed as described previously (38).
Cloning of plasmids and construction of unmarked isogenic mutants.
Constructions of transconjugation plasmids were performed as described in Table 1. Plasmid pDM800 was used to introduce the dmsA deletion into A. pleuropneumoniae ΔapxIIAΔureC via the single-step transconjugation system as described previously (6, 39), resulting in A. pleuropneumoniae ΔapxIIAΔureCΔdmsA. This threefold mutant provided the basis for the construction of the fourfold mutant using transconjugation plasmid pHYB700, resulting in A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybB. For the construction of the fivefold mutant A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybBΔaspA, pAS700 was used to delete the aspA gene in the fourfold mutant A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybB. For generation of the sixfold mutant strain A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybBΔaspAΔfur, transconjugation plasmid pFUR702 was used.
Preparation of whole-cell lysates.
Bacteria were cultured, centrifuged (7,000 × g, 5 min), resuspended in 50 mM Tris (pH 7.3), and stored at −70°C overnight. Cells were thawed and then ruptured using the Fast Prep Instrument (Qbiogene, Heidelberg, Germany) three times for 40 s on intensity setting 5.0. Protein concentration was determined using a MicroBC assay (Uptima Interchim, Montlucon Cedex, France).
Aspartase assay.
Aspartase activity was measured spectrophotometrically at 240 nm by determination of fumarate formation (48) as described previously (29). Briefly, the assay buffer contained 3 mM MgCl2, 0.1 M L-aspartate (Sigma), and 0.1 M Tris-HCl (pH 9.0). The reaction was initiated by addition of 100 μg protein of whole-cell lysates of cultures grown under anaerobic conditions.
Western blot analysis.
For Western blot analysis of the DmsA and TbpB protein content, whole-cell lysates were analyzed by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% or 10.8% acrylamide and 0.3% bisacrylamide) according to standard procedures (45) using a Protean II Minigel system (Bio-Rad, Munich, Germany) as described previously (2, 5). The sera used are listed in Table 1.
Serology.
For serological examinations, different enzyme-linked immunosorbent assays (ELISAs) were used. In the ApxII-ELISA, the recombinant ApxII protein functions as the solid-phase antigen as described previously (35). The response is quantified in ELISA units (EU) based on an external standard. For this standardized ELISA, activities of ≤10 EU in the sera are considered negative, 11 to 25 EU are intermediate, and >25 EU are positive. In the detergent extract ELISA (deELISA), a detergent extract of A. pleuropneumoniae ΔapxIIAΔureC containing outer membrane-associated proteins was used as a solid-phase antigen (21), and the immune response was quantified by determining the antibody titer in comparison to that of an internal negative control. The negative control consisted of an equal mixture of all serum samples taken at the arrival of the pigs, and the positive control consisted of an equal mixture of all serum samples of infected pigs taken 21 days postinfection. The titer in the deELISA was defined as the highest serum dilution resulting in an optical density twice as high as that of the negative control serum at a dilution of 1:100. To quantify the humoral immune response against the TbpB protein, recombinant TpbB of A. pleuropneumoniae serotype 7 was used as a solid-phase antigen (20), and the titer was determined in comparison to an internal negative control as described for the deELISA. Finally, a commercial ApxIV-ELISA was employed detecting antibodies directed against the ApxIV toxin, which is produced only in vivo by all A. pleuropneumoniae serotypes (13). In this standardized ELISA, activities of ≤30% compared to a positive control are considered negative, 30% to 40% are intermediate, and activities of ≥40% are positive.
Blood samples were taken 1 week prior to infection to confirm the absence of A. pleuropneumoniae-specific antibodies and at necropsy on day 7 or day 21 postinfection to determine the serological response to challenge with the different A. pleuropneumoniae mutant strains.
Virulence studies.
For virulence studies, outbred pigs (8 to 9 weeks of age) were purchased from an A. pleuropneumoniae-free herd (no clinical symptoms and no serological responses in the ApxII-ELISA and the deELISA) and randomly assigned to different groups. They were cared for in accordance with the principles outlined in the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (European Treaty Series, no. 123; http://conventions.coe.int/treaty/EN/V3menutraites.asp; permit nos. 01/471 and 05/984). Virulence of the A. pleuropneumoniae mutant strains was assessed in an aerosol infection model that has been described previously (5, 31). Clinical examinations were performed daily or as needed. Body temperature and clinical symptoms were recorded daily for each individual pig in the 2 days before and 7 days after infection or as needed. A clinical scoring system based on the directive in the European Pharmacopoeia for testing A. pleuropneumoniae vaccines (porcine actinobacillosis vaccine [inactivated]) was employed to assess the clinical condition of each individual animal as follows. A score of 1 was given for each case of coughing, dyspnea, and vomitus, resulting in a minimum clinical score of 0 and a maximum score of 3 per day; pigs dying from the disease were assigned a score of 4. The added daily clinical scores of days 1 to 7 were designated the total clinical score. Statistical analysis of the total clinical score was performed using the Wilcoxon signed-rank test. Pigs were euthanized by intravenous injection of pentobarbital. Post mortem analysis was performed as described previously (5). Briefly, lung lesion scores were determined as described by Hannan et al. (23) and statistically analyzed using the Wilcoxon signed-rank test.
The bacteriological examination included surface swabs of palatine tonsils, of bronchial lymph nodes, and of defined positions located in the outer third of each of the seven lung lobes; an additional swab of diseased lung tissue was taken if it was not covered by any of the defined lung locations. Plating was done on Columbia sheep blood agar to exclude other bacterial infections, as well as on selective meat blood agar (30), and fractionated twice. Due to growth deficiencies of A. pleuropneumoniae Δfur deletion mutants, the sixfold mutant was cultured on modified selective meat blood agar lacking bacitracin (28). A score for reisolation of 0 was given for either no growth or if A. pleuropneumoniae colonies grew only in the directly swabbed area; a score of 1 was given if colonies were present in the fractionated streaks. The reisolation score was determined by adding these numbers for each pig in the respective group, and the arithmetic means and standard deviations were determined. Several individual A. pleuropneumoniae-like colonies were subcultured on supplemented PPLO agar and confirmed by CAMP test and PCR analyses using primers oAPX2A1 and oAPX2A2.
In a preliminary experiment performed in order to assess the necessity of introducing additional mutations, nine pigs (German Landrace and Pietrain) were infected with the threefold mutant (A. pleuropneumoniae ΔapxIIAΔureCΔdmsA); pigs were euthanized and necropsied on day 21 postinfection. Due to high residual virulence of the threefold mutant, controlled infection experiments were performed with the fivefold (A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybBΔaspA) and the sixfold (A. pleuro-pneumoniae ΔapxIIAΔureCΔdmsAΔhybBΔaspAΔfur) mutant strains in comparison to the A. pleuropneumoniae parent strain. Nine pigs (German Landrace) were infected with the fivefold mutant and the parent strain, respectively, and five pigs were infected with the sixfold mutant. In the groups infected with the fivefold mutant and the parent strain, four pigs each were randomly assigned and euthanized 7 days postinfection, and all remaining pigs as well as pigs infected with the sixfold mutant strain were euthanized 21 days postinfection.
Protection studies.
Protection experiments were performed by using the A. pleuropneumoniae sixfold mutant as a live vaccine in a single aerosol immunization. Fifteen pigs 7 weeks of age were used. They were randomly assigned to two groups of 10 and 5 pigs, respectively. The 10 pigs in group 1 were vaccinated in a single aerosol application of the sixfold mutant, and the 5 pigs in group 2 were given a NaCl solution (150 mM) by aerosol application (control group). Three weeks after immunization, all pigs were challenged with a heterologous A. pleuropneumoniae serotype 9 strain in the aerosol chamber with five pigs at a time. Clinical examinations were performed as described above. Four vaccinated pigs were euthanized on day 7 postinfection, and the remaining pigs as well as the control pigs were euthanized on day 21 postinfection. Mortality was compared using Fisher′s exact test, and post mortem analysis as well as serological and bacteriological examinations were performed as described above.
RESULTS
Construction and verification of unmarked isogenic mutants.
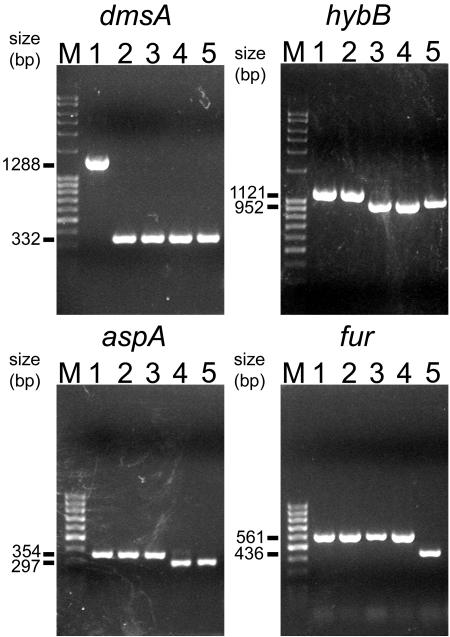
Based on the A. pleuropneumoniae ΔapxIIAΔureC strain (49), a threefold mutant was constructed using transconjugation plasmid pDM800. In the next step an isogenic fourfold mutant was constructed using plasmid pHYB700. Transconjugation plasmid pASP700 was then used to delete the aspA gene in the fourfold mutant A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybB, resulting in the fivefold mutant. Finally, a sixfold mutant was constructed by deleting the fur gene with transconjugation plasmid pFUR702 in the fivefold mutant. All mutant strains were verified using PCR (Fig. 1) as well as Southern blotting, pulsed-field gel electrophoresis, and nucleotide sequencing (data not shown).
FIG. 1.
PCR analyses of A. pleuropneumoniae wild-type and isogenic mutant strains. Lanes: 1, A. pleuropneumoniae serotype 2 wild type; 2, A. pleuropneumoniae ΔapxIIAΔureCΔdmsA; 3, A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybB; 4, A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybBΔaspA; 5, A. pleuropneumoniae ΔapxIIAΔureCΔdmsAΔhybBΔaspAΔfur. Lanes M, size marker. The numbers to the left indicate the size of the PCR products obtained. Primers used were oDMSAdel1 and oDMSAdel2 (for the dmsA gene), o34-1f and 034-1r (for the hybB gene), oASPX and oASPY (for the aspA gene), and oFURX and oFURY (for the fur gene).
The lack of expression of the DmsA protein was shown by Western blot analyses of whole-cell lysates of the fivefold mutant grown under aerobic and anaerobic conditions using antibodies raised against the DmsA protein (data not shown). The lack of aspartase activity in the fivefold mutant was confirmed by an aspartase assay of whole-cell lysates grown under anaerobic conditions (data not shown). The constitutive expression of Fur-repressed proteins due to deletion of the fur gene was demonstrated by Western blot analysis of whole-cell lysates of the sixfold mutant grown under standard culture conditions and iron-restrictive conditions using antibodies raised against the TbpB protein (data not shown).
Virulence studies.
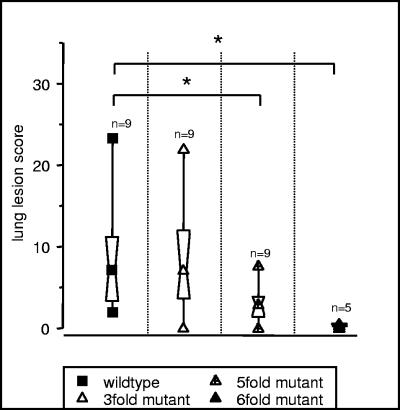
In order to investigate the residual virulence of the threefold mutant, pigs were infected with an aerosolized dose of 1.2 × 105 CFU per five pigs. All pigs developed fever (body temperature, >40.5°C) 1 day postinfection, accompanied by anorexia, lethargy, and vomiting in some pigs. One pig died 2 days postinfection, and a second pig died 9 days postinfection. At necropsy, these pigs had severe lung lesions. The remaining pigs survived infection and, at necropsy on day 21 postinfection, all but one pig also showed lung lesions (Fig. 2). As this residual virulence is unacceptable for a live vaccine, five- and sixfold mutants were constructed as described above.
FIG. 2.
Lung lesion score upon challenge with different mutant strains of A. pleuropneumoniae serotype 2. The central symbol within the hourglass shape represents the geometric mean, the hinges present the values in the middle of each half of data, and the top and bottom symbols mark the minimum and maximum value. The asterisk denotes statistical significance (P < 0.05) in the Wilcoxon signed-rank test.
In the experiment that followed, pigs were challenged with the wild-type strain (9.1 × 104 CFU aerosolized per five pigs), the fivefold mutant (1.5 × 105 CFU aerosolized per five pigs), and the sixfold mutant (1.1 × 105 CFU aerosolized per five pigs). In the group infected with the fivefold mutant, six of nine pigs showed increased body temperatures (>40.5°C) on day 1 postinfection and three pigs refused to feed. Three days postinfection, all pigs had body temperatures below 40.5°C, and by 7 days postinfection only one pig developed coughing. In the group infected with the sixfold mutant, four of five pigs had an increase of body temperature to above 40.5°C on the first day postinfection. No other clinical symptoms were observed. In the wild-type group all pigs infected developed severe disease; eight of nine pigs had body temperatures above 40.5°C on the first day after infection, accompanied by anorexia, dyspnea, lethargy, and vomiting in some pigs. The clinical score in this group was significantly higher (P < 0.05) than that of the fivefold and sixfold mutant group (data not shown). Necropsies performed on days 7 and 21 postinfection revealed a significantly higher lung lesion score in the group infected with the wild-type strain compared to those of the groups infected with the mutant strains (P < 0.05; Wilcoxon test; Fig. 2), and pigs infected with the sixfold mutant had only minimal pathological alterations.
No apparent differences were observed with respect to the reisolation of the challenge strains, determined as the reisolation score between the groups infected with the parent strain and the fivefold mutant strain on day 7 postinfection. On day 21 postinfection, reisolation of the fivefold and the sixfold mutant from intact lung tissue was reduced (isolation in one of five animals) compared to that of the wild-type strain (four of five animals). Looking at intact and altered lung tissue, the sixfold mutant could only be reisolated from two of five pigs in small numbers (2 to 10 colonies in the directly swabbed area of the plate), whereas the wild-type strain could be isolated from all pigs in large numbers (confluent growth in the first fractionation and single colonies in the second fractionation) 21 days postinfection.
Pigs infected with either of the mutant strains had no titer in the ApxII-ELISA at any time after infection but showed a detectable response in the deELISA 21 days postinfection. Four of five pigs infected with the wild-type strain were positive in the ApxII-ELISA 21 days postinfection, and all had titers in the deELISA comparable to those of pigs infected with the fivefold mutant (Table 2).
TABLE 2.
Virulence of A. pleuropneumoniae mutant strains
| Challenge strain | Challenge dose (CFU aerosolized per 5 pigs)a | Necropsy time (day) | Serological response to:
|
No. of animals with lung lesions/ total no.d | Arithmetic mean ± SD of lung lesion score | No. of animals with reisolation of A. pleuropneumoniae at post mortem analysis in:
|
Arithmetic mean ± SD of reisolation score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Detergent washb | ApxIIAc | Tonsil | Lymph node | Lung
|
|||||||
| Pneumonic | Intact | ||||||||||
| Serotype 2 wild type | 9.1 × 104 | 7 | 1,125 ± 1,412 | 1 ± 0.5 | 4/4 | 8.2 ± 4.8 | 1/4 | 3/4 | 4/4 | 4/4 | 3.5 ± 2.4 |
| 21 | 6,400 ± 3,919 | 27 ± 20 | 5/5 | 9.1 ± 8.4 | 3/5 | 2/5 | 5/5 | 4/5 | 1.2 ± 1.8 | ||
| Fivefold mutant | 1.5 × 105 | 7 | 475 ± 754 | 1 ± 0.5 | 3/4 | 2.9 ± 2.7 | 1/4 | 3/4 | 4/4 | 4/4 | 1 ± 0.8 |
| 21 | 3,000 ± 2,476 | 1 | 4/4e | 2.1 ± 1.1 | 0/4 | 0/4 | 3/4 | 1/4 | 0 | ||
| Sixfold mutant | 1.1 × 105 | 21 | 880 ± 438 | 1 | 4/5 | 0.3 ± 0.2 | 1/5 | 1/5 | 1/4 | 1/5 | 0.2 ± 0.4 |
Bacteria were grown to an optical density of approximately 0.45 at 600 nm and then diluted 1:30,000 with sterile 0.9% NaCl solution; 13 ml of this dilution was aerosolized in the chamber. The numbers given are the CFU in 13 ml.
The solid-phase antigen was prepared as described previously (21); the number given is the arithmetic mean of the highest serum dilution resulting in an optical density twice as high as the negative control serum at a dilution of 1:100.
Recombinant ApxIIA protein was used as solid-phase antigen as described previously (35); the number given is the arithmetic mean of the serum activity in ELISA units.
The lung lesion score was determined as described by Hannan et al. (23).
One pig was euthanized on day 10 because of a backhand paresis and could not be added to any group. This pig had titers in the deELISA of 3,200 and in the ApxII-ELISA of 1. The lung lesion score was 7.66. The challenge strain was reisolated in the lymph node and pneumonic and intact lung.
Protection studies.
Since pigs immunized with the sixfold mutant had a reasonable humoral immune response and the immune response of a serotype 2 double mutant is known to be protective against homologous challenge (49), pigs were directly subjected to a heterologous challenge using an A. pleuropneumoniae serotype 9 strain. On day 1 after aerosol immunization with the sixfold mutant, 5 of 10 vaccinated pigs had an increase of body temperature above 40.5°C, and in the next 6 days 2 pigs developed mild coughing for 1 to 2 days. Other clinical symptoms were not observed. Control pigs aerosolized with NaCl solution had no clinical symptoms or alterations of body temperature. Upon challenge with A. pleuropneumoniae serotype 9, five immunized pigs had an increased body temperature (>40.5°C) on the day after infection. During the next 6 days, all but one pig had no or only mild clinical symptoms. All control pigs developed anorexia, severe dyspnea, and depression, and three out of five pigs died within 48 h, whereas none of the vaccinated pigs died (P < 0.05; Fisher′s exact test). At necropsy, 7 of 10 vaccinated pigs had lung lesions (Table 3). At necropsy, the vaccine strain could be reisolated sporadically from vaccinated pigs; no difference was observed in the reisolation frequency of the challenge strain between the vaccinated and the control group (Table 3).
TABLE 3.
Protective effect of live negative marker vaccine upon A. pleuropneumoniae serotype 9 challenge
| Group | Challenge dose (CFU aerosolized per 5 pigs)a | Necropsy time (day) | No. of animals with lung lesions/total no.b | Arithmetic mean ± SD of lung lesion score | No. of animals with reisolation of A. pleuropneumoniae at post mortem analysis in:
|
Arithmetic mean ± SD of reisolation score | |||
|---|---|---|---|---|---|---|---|---|---|
| Tonsil | Lymph node | Lung
|
|||||||
| Pneumonic | Intact | ||||||||
| Vaccinatedc | 1.6 × 105 | 7 | 3/4 | 4.9 ± 5.4 | 1/4 | 4/4 | 3/3 | 3/4 | 1.5 ± 1 |
| 21 | 4/6 | 3 ± 5.6 | 2/6 | 1/6 | 2/4 | 2/6 | 1.4 ± 2.8 | ||
| Unvaccinated control groupd | 1.7 × 105 | 2 | 3/3 | 24.9 ± 3.1 | 3/3 | 3/3 | 3/3 | 3/3 | 7 |
| 21 | 2/2 | 3 ± 1.6 | 1/2 | 0/2 | 1/2 | 1/2 | 0.5 ± 0.7 | ||
A. pleuropneumoniae serotype 9 was grown to an optical density of 0.43 at 600 nm and then diluted 1:30,000 with sterile 0.9% NaCl solution; 13 ml of this dilution was aerosolized in the chamber. The numbers given are the CFU in 13 ml.
The lung lesion score was determined as described by Hannan et al. (23).
A. pleuropneumoniae sixfold mutant was grown to an optical density of 0.45 at 600 nm and diluted 1:30,000 with sterile 0.9% NaCl solution; 13 ml of this dilution was aerosolized in the chamber.
Three nonvaccinated control pigs died within 48 h after infection.
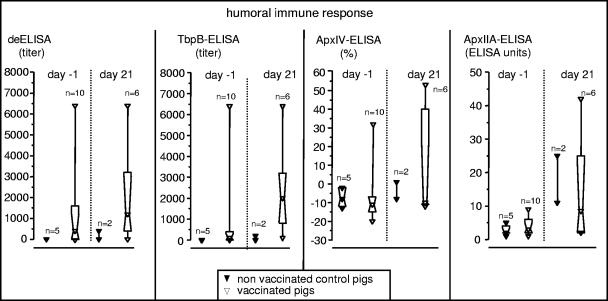
Three weeks after aerosol immunization, all pigs were tested in the deELISA as well as the TbpB-, ApxIV-, and the ApxII-ELISA (DIVA function); control pigs had no detectable titer in either ELISA, whereas some of the vaccinated pigs had detectable antibody titers in the deELISA (7 of 10) and in the TbpB-ELISA (6 of 10) but not in the ApxIV- and ApxII-ELISA (Fig. 3). Three weeks postchallenge, the two surviving control pigs had antibody titers in the deELISA and the TbpB-ELISA, and one pig was positive in the ApxII-ELISA. Vaccinated pigs had strong titers in the deELISA (5 of 6) and TbpB-ELISA (6 of 6), two were positive in the ApxIV-ELISA, and two were positive in the ApxII-ELISA.
FIG. 3.
Antibody titer upon heterologous challenge. Shown are humoral immune responses of control and vaccinated pigs on the day before and 21 days after infection, assessed using a detergent extract (deELISA), recombinant TbpB protein of A. pleuropneumoniae serotype 7 (TbpB-ELISA), recombinant ApxIV protein (ApxIV-ELISA), and the recombinant ApxIIA protein (ApxII-ELISA) as solid-phase antigen. The immune response was expressed in ELISA units (based on an external standard) for the standardized ApxII-ELISA, with activities of ≥25 ELISA units considered positive. Using the standardized ApxIV-ELISA, activities of ≥40% in comparison to an external control were considered positive; for the deELISA and the TbpB-ELISA, the immune response was expressed as a serum titer in comparison to that of an internal negative control.
DISCUSSION
An ideal vaccine for livestock is inexpensive to produce, easy to use (requiring only a single application), and highly protective, and it facilitates the differentiation of infected and vaccinated animals (DIVA principle). With respect to an A. pleuropneumoniae infection, this goal is particularly difficult to meet due to the occurrence of 15 serotypes, with only limited cross-protection occurring upon the use of bacterin vaccines. However, it has been reported that pigs surviving infection with one serotype are at least partially protected from clinical symptoms upon reinfection with other serotypes (10, 22, 36, 37).
Therefore, we set out to construct a highly attenuated defined multiple A. pleuropneumoniae mutant with residual colonizing ability, enabling it on the one hand to consistently induce an immune response upon a single aerosol application but, on the other hand, rendering it unable to cause clinical disease. As starting material, we chose a double mutant constructed previously which already fulfills the DIVA principle but which was still able to cause disease (49). In order to further attenuate this strain, we initially focused on enzymes involved in anaerobic respiration which have been shown to facilitate the pathogen's persistence in the reducing environment of the epithelial lining fluid as well as in necrotic lung tissue with reduced oxygen tension (4). To avoid problems with antibiotic resistance and undefined mutations present in experimental live attenuated A. pleuropneumoniae vaccines described previously (17, 27, 40), the single-step transconjugation system was employed, as it allows the construction of strains with multiple isogenic mutations not containing foreign DNA as shown in this study.
Since the sole deletion of the dmsA gene coding for the DMSO reductase had only slightly attenuating effects (2), we deleted two additional enzymes involved in anaerobic metabolism, namely the aspartate-ammonia lyase and the [NiFE] hydrogenase, which were shown to be associated with A. pleuropneumoniae virulence (3, 29). The resulting fivefold mutant was significantly attenuated but was still able to cause disease. Based on the findings of Baltes et al. (4) that a complete impairment of the anaerobic metabolism by deletion of the global anaerobic regulator HlyX renders A. pleuropneumoniae unable to reliably colonize the respiratory tract (4), we saw the necessity of impairing a second metabolic pathway and decided on the iron uptake pathway. As iron uptake-associated proteins, e.g., TbpA and TbpB, are important protective antigens (1, 19, 20, 44), we set out to delete the fur gene, thereby causing impairment by constitutive expression of highly immunogenic proteins (28, 47). Following the hypothesis that impairment of both anaerobic and iron uptake pathways should render A. pleuropneumoniae very highly attenuated but still able to colonize, we constructed a sixfold mutant lacking a functional fur gene.
Since an ideal live vaccine should still be able to colonize the respiratory tract but should show only limited survival within the host to prevent unwanted spread of the vaccine strain, we thoroughly investigated the ability of the sixfold mutant to persist in the host. We took seven swab samples from defined localizations of each lung as well as from tonsils and bronchial lymph nodes at necropsy and used a semiquantitative reisolation score for the lung samples to compare the quantity of reisolated bacteria. The results of this study and the comparison with previous studies using single and double mutants of A. pleuropneumoniae serotype 7 (2, 28, 29) indicate that the two major metabolic functionalities impaired (the anaerobic metabolism with deletion of dmsA, hybB, and aspA and the ferric uptake pathway with deletion of fur) are equally important for the high degree of attenuation and the decreased persistence within the host. Thus, a fur single mutant as well as the fivefold mutant are still able to cause clinical disease, whereas the sixfold mutant does not cause clinical disease but is still able to persist in intact lung tissue over a period of 6 weeks in small numbers.
Since pigs asymptomatically carrying A. pleuropneumoniae on the tonsils do not generally develop measurable antibody titers, a colonization of the lower respiratory tract appears to be required for a humoral immune response (8, 9). Consequently, an A. pleuropneumoniae vaccine strain devised for single aerosol application must be able to colonize the lower respiratory tract and express protective antigens. Due to the ability of the sixfold mutant to colonize and consistently induce a humoral immune response without occurrence of severe clinical disease, we used it as a vaccine strain in a protection experiment, although the short rise in body temperature observed upon vaccination is not in accordance with current licensing rules for commercial vaccines. In previous studies using highly attenuated A. pleuropneumoniae single mutants as experimental live vaccines, a certain degree of cross-serotype protection could be observed, but at least two applications of a high dose (∼109 CFU) were required (27, 40). Since it is known that animals convalescent from A. pleuropneumoniae infection with one serotype are at least partially protected from clinical disease upon infection with other serotypes (10), we investigated the protective efficacy of the sixfold mutant upon challenge with an A. pleuropneumoniae serotype 9 strain. This strain is antigenically highly distinct from A. pleuropneumoniae serotype 2. Thus, the serotype 9 strain belongs to the 1, 9, and 11 groups (26), and A. pleuropneumoniae serotype 2 is not assigned to any group (37) expressing other Apx toxins (16) and a different OmlA protein (18) than that of A. pleuropneumoniae serotype 9. However, A. pleuropneumoniae serotypes 2 and 9 carry the same tbpBA operon (11), and immunization with both recombinant TbpB and TbpA proteins has been shown to be protective (20, 55).
Pigs vaccinated with the sixfold mutant were significantly protected from clinical disease upon infection with A. pleuropneumoniae serotype 9, thereby supporting the concept of an attenuated A. pleuropneumoniae live vaccine providing cross-serovar protection. Here, antibodies directed against the TbpB protein might be one important factor. Thus, although not all animals had detectable TbpB-specific titers prior to infection, vaccinated animals, in contrast to the nonvaccinated controls, all had detectable titers after infection, thereby supporting the occurrence of a booster reaction upon infection (Fig. 3). Further, a cellular Th1-type immune response causing cross-serovar protection may have been induced by the live vaccine strain. This, as well as the possible role of additional antigens possibly expressed only upon entry of the host, needs to be determined in future studies.
Vaccination via aerosol is currently not being used in pigs. However, in the past vaccination of pigs via aerosol has been used successfully in the field (33, 46). Also, the principle feasibility of live aerosol vaccines under current legislation has been documented recently by the licensing of a Mycoplasma gallisepticum live vaccine for hens (Nobilis MG 6/85; Intervet, Unterschleissheim, Germany) to be applied via spray aerosolization. Thus, although the efficacy of conventional application strategies like intramuscular injection should be elucidated, licensing of a live A. pleuropneumoniae aerosol vaccine might be feasible if the initial rise in temperature observed upon vaccination can be reduced. Even the apparent lack of protection from airway colonization might be acceptable, since this disadvantage would be compensated for by the advantage of a single application and the discrimination between infected and vaccinated-plus-infected groups (DIVA function).
Acknowledgments
This study was supported by the Bioprofile project PTJ-BIO/0313037 and by Sonderforschungsbereich 587 (project A4) of the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany.
Editor: J. N. Weiser
REFERENCES
- 1.Baltes, N., I. Hennig-Pauka, and G.-F. Gerlach. 2002. Both transferrin binding proteins are virulence factors in Actinobacillus pleuropneumoniae serotype 7 infection. FEMS Microbiol. Lett. 209:283-287. [DOI] [PubMed] [Google Scholar]
- 2.Baltes, N., I. Hennig-Pauka, I. Jacobsen, A. D. Gruber, and G.-F. Gerlach. 2003. Identification of dimethyl sulfoxide reductase in Actinobacillus pleuropneumoniae and its role in infection. Infect. Immun. 71:6784-6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baltes, N., S. Kyaw, I. Hennig-Pauka, and G.-F. Gerlach. 2004. Lack of influence of the anaerobic [NiFe] hydrogenase and L-1,2 propanediol oxidoreductase on the outcome of Actinobacillus pleuropneumoniae serotype 7 infection. Vet. Microbiol. 102:67-72. [DOI] [PubMed] [Google Scholar]
- 4.Baltes, N., M. N′Diaye, I. D. Jacobsen, A. Maas, F. F. Buettner, and G.-F. Gerlach. 2005. Deletion of the anaerobic regulator HlyX causes reduced colonization and persistence of Actinobacillus pleuropneumoniae in the porcine respiratory tract. Infect. Immun. 73:4614-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltes, N., W. Tonpitak, G.-F. Gerlach, I. Hennig-Pauka, A. Hoffmann-Moujahid, M. Ganter, and H. J. Rothkotter. 2001. Actinobacillus pleuropneumoniae iron transport and urease activity: effects on bacterial virulence and host immune response. Infect. Immun. 69:472-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baltes, N., W. Tonpitak, I. Hennig-Pauka, A. D. Gruber, and G.-F. Gerlach. 2003. Actinobacillus pleuropneumoniae serotype 7 siderophore receptor FhuA is not required for virulence. FEMS Microbiol. Lett. 220:41-48. [DOI] [PubMed] [Google Scholar]
- 7.Blackall, P. J., H. L. Klaasen, B. H. Van Den, P. Kuhnert, and J. Frey. 2002. Proposal of a new serovar of Actinobacillus pleuropneumoniae: serovar 15. Vet. Microbiol. 84:47-52. [DOI] [PubMed] [Google Scholar]
- 8.Chiers, K., E. Donne, I. van Overbeke, R. Ducatelle, and F. Haesebrouck. 2002. Actinobacillus pleuropneumoniae infections in closed swine herds: infection patterns and serological profiles. Vet. Microbiol. 85:343-352. [DOI] [PubMed] [Google Scholar]
- 9.Chiers, K., E. Donne, I. van Overbeke, R. Ducatelle, and F. Haesebrouck. 2002. Evaluation of serology, bacteriological isolation and polymerase chain reaction for the detection of pigs carrying Actinobacillus pleuropneumoniae in the upper respiratory tract after experimental infection. Vet. Microbiol. 88:385-392. [DOI] [PubMed] [Google Scholar]
- 10.Cruijsen, T. L., L. A. van Leengoed, M. Ham-Hoffies, and J. H. Verheijden. 1995. Convalescent pigs are protected completely against infection with a homologous Actinobacillus pleuropneumoniae strain but incompletely against a heterologous-serotype strain. Infect. Immun. 63:2341-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Puente-Redondo, V. A., N. G. del Blanco, C. B. Gutierrez-Martin, J. N. Mendez, and E. F. Rodriquez Ferri. 2000. Detection and subtyping of Actinobacillus pleuropneumoniae strains by PCR-RFLP analysis of the tbpA and tbpB genes. Res. Microbiol. 151:669-681. [DOI] [PubMed] [Google Scholar]
- 12.Dehio, C., and M. Meyer. 1997. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J. Bacteriol. 179:538-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreyfus, A., A. Schaller, S. Nivollet, R. P. Segers, M. Kobisch, L. Mieli, V. Soerensen, D. Hussy, R. Miserez, W. Zimmermann, F. Inderbitzin, and J. Frey. 2004. Use of recombinant ApxIV in serodiagnosis of Actinobacillus pleuropneumoniae infections, development and prevalidation of the ApxIV ELISA. Vet. Microbiol. 99:227-238. [DOI] [PubMed] [Google Scholar]
- 14.Fenwick, B., and S. Henry. 1994. Porcine pleuropneumonia. J. Am. Vet. Med. Assoc. 204:1334-1340. [PubMed] [Google Scholar]
- 15.Frey, J. 1995. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 3:257-261. [DOI] [PubMed] [Google Scholar]
- 16.Frey, J., J. T. Bosse, Y. F. Chang, J. M. Cullen, B. Fenwick, G.-F. Gerlach, D. Gygi, F. Haesebrouck, T. J. Inzana, and R. Jansen. 1993. Actinobacillus pleuropneumoniae RTX-toxins: uniform designation of haemolysins, cytolysins, pleurotoxin and their genes. J. Gen. Microbiol. 139:1723-1728. [DOI] [PubMed] [Google Scholar]
- 17.Fuller, T. E., B. J. Thacker, C. O. Duran, and M. H. Mulks. 2000. A genetically-defined riboflavin auxotroph of Actinobacillus pleuropneumoniae as a live attenuated vaccine. Vaccine 18:2867-2877. [DOI] [PubMed] [Google Scholar]
- 18.Gerlach, G.-F., C. Anderson, S. Klashinsky, A. Rossi-Campos, A. A. Potter, and P. J. Willson. 1993. Molecular characterization of a protective outer membrane lipoprotein (OmlA) from Actinobacillus pleuropneumoniae serotype 1. Infect. Immun. 61:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerlach, G.-F., C. Anderson, A. A. Potter, S. Klashinsky, and P. J. Willson. 1992. Cloning and expression of a transferrin-binding protein from Actinobacillus pleuropneumoniae. Infect. Immun. 60:892-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlach, G.-F., S. Klashinsky, C. Anderson, A. A. Potter, and P. J. Willson. 1992. Characterization of two genes encoding distinct transferrin-binding proteins in different Actinobacillus pleuropneumoniae isolates. Infect. Immun. 60:3253-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goethe, R., O. F. Gonzales, T. Lindner, and G.-F. Gerlach. 2000. A novel strategy for protective Actinobacillus pleuropneumoniae subunit vaccines: detergent extraction of cultures induced by iron restriction. Vaccine 19:966-975. [DOI] [PubMed] [Google Scholar]
- 22.Haesebrouk, F., K. A. Van de, P. Dom, K. Chiers, and R. Ducatelle. 1996. Cross-protection between Actinobacillus pleuropneumoniae biotypes-serotypes in pigs. Vet. Microbiol. 52:277-284. [DOI] [PubMed] [Google Scholar]
- 23.Hannan, P. C., B. S. Bhogal, and J. P. Fish. 1982. Tylosin tartrate and tiamutilin effects on experimental piglet pneumonia induced with pneumonic pig lung homogenate containing mycoplasmas, bacteria and viruses. Res. Vet. Sci. 33:76-88. [PubMed] [Google Scholar]
- 24.Higgins, R., S. Lariviere, K. R. Mittal, G. P. Martineau, P. Rousseau, and J. Cameron. 1985. Evaluation of a killed vaccine against porcine pleuropneumonia due to Haemophilus pleuropneumoniae. Can. Vet. J. 26:86-89. [PMC free article] [PubMed] [Google Scholar]
- 25.Inzana, T. J., J. Ma, T. Workman, R. P. Gogolewski, and P. Anderson. 1988. Virulence properties and protective efficacy of the capsular polymer of Haemophilus (Actinobacillus) pleuropneumoniae serotype 5. Infect. Immun. 56:1880-1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inzana, T. J., J. Todd, C. Koch, and J. Nicolet. 1992. Serotype specificity of immunological assays for the capsular polymer of Actinobacillus pleuropneumoniae serotypes 1 and 9. Vet. Microbiol. 31:351-362. [DOI] [PubMed] [Google Scholar]
- 27.Inzana, T. J., J. Todd, and H. P. Veit. 1993. Safety, stability, and efficacy of noncapsulated mutants of Actinobacillus pleuropneumoniae for use in live vaccines. Infect. Immun. 61:1682-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobsen, I., J. Gerstenberger, A. D. Gruber, J. T. Bosse, P. R. Langford, I. Hennig-Pauka, J. Meens, and G.-F. Gerlach. 2005. Deletion of the ferric uptake regulator Fur impairs the in vitro growth and virulence of Actinobacillus pleuropneumoniae. Infect. Immun. 73:3740-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsen, I., I. Hennig-Pauka, N. Baltes, M. Trost, and G.-F. Gerlach. 2005. Enzymes involved in anaerobic respiration appear to play a role in Actinobacillus pleuropneumoniae virulence. Infect. Immun. 73:226-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen, M. J., and J. P. Nielsen. 1995. Development and evaluation of a selective and indicative medium for isolation of Actinobacillus pleuropneumoniae from tonsils. Vet. Microbiol. 47:191-197. [DOI] [PubMed] [Google Scholar]
- 31.Jacobsen, M. J., J. P. Nielsen, and R. Nielsen. 1996. Comparison of virulence of different Actinobacillus pleuropneumoniae serotypes and biotypes using an aerosol infection model. Vet. Microbiol. 49:159-168. [DOI] [PubMed] [Google Scholar]
- 32.Jobert, J. L., C. Savoye, R. Cariolet, M. Kobisch, and F. Madec. 2000. Experimental aerosol transmission of Actinobacillus pleuropneumoniae to pigs. Can. J. Vet. Res. 64:21-26. [PMC free article] [PubMed] [Google Scholar]
- 33.Kaden, V., and J. Beer. 1982. Aerogenic immunization against red murrain and swine fever in fatting units. Monatsh. Vetmed. 37:380-384. [Google Scholar]
- 34.Lechtenberg, K. F., T. R. Shryock, and G. Moore. 1994. Characterization of an Actinobacillus pleuropneumoniae seeder pig challenge-exposure model. Am. J. Vet. Res. 55:1703-1709. [PubMed] [Google Scholar]
- 35.Leiner, G., B. Franz, K. Strutzberg, and G.-F. Gerlach. 1999. A novel enzyme-linked immunosorbent assay using the recombinant Actinobacillus pleuropneumoniae ApxII antigen for diagnosis of pleuropneumonia in pig herds. Clin. Diagn. Lab. Immunol. 6:630-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen, R. 1979. Haemophilus parahaemolyticus pathogenicity and cross immunity in pigs. Nord. Vetmed. 31:407-413. [PubMed] [Google Scholar]
- 37.Nielsen, R. 1984. Haemophilus pleuropneumoniae serotypes-cross protection experiments. Nord. Vetmed. 36:221-234. [PubMed] [Google Scholar]
- 38.Oswald, W., D. V. Konine, J. Rohde, and G.-F. Gerlach. 1999. First chromosomal restriction map of Actinobacillus pleuropneumoniae and localization of putative virulence-associated genes. J. Bacteriol. 181:4161-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oswald, W., W. Tonpitak, G. Ohrt, and G.-F. Gerlach. 1999. A single-step transconjugation system for the introduction of unmarked deletions into Actinobacillus pleuropneumoniae serotype 7 using a sucrose sensitivity marker. FEMS Microbiol. Lett. 179:153-160. [DOI] [PubMed] [Google Scholar]
- 40.Prideaux, C. T., C. Lenghaus, J. Krywult, and A. L. Hodgson. 1999. Vaccination and protection of pigs against pleuropneumonia with a vaccine strain of Actinobacillus pleuropneumoniae produced by site-specific mutagenesis of the ApxII operon. Infect. Immun. 67:1962-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raleigh, F. A., K. Lech, and R. Brent. 1989. Selected topics from classical bacterial genetics, p. 1.4.1-1.4.14. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, I. G. Seidman, I. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 14. Publishing Associates and Wiley Interscience, New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 42.Rapp, V. J., R. S. Munson, Jr., and R. F. Ross. 1986. Outer membrane protein profiles of Haemophilus pleuropneumoniae. Infect. Immun. 52:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapp, V. J., and R. F. Ross. 1986. Antibody response of swine to outer membrane components of Haemophilus pleuropneumoniae during infection. Infect. Immun. 54:751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi-Campos, A., C. Anderson, G.-F. Gerlach, S. Klashinsky, A. A. Potter, and P. J. Willson. 1992. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine 10:512-518. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 46.Schulz, V. 1972. Aerogenic immunization against red murrain in facilities for industrialized animal production. Monatsh. Vetmed. 27:818-820. [PubMed] [Google Scholar]
- 47.Sheehan, B. J., J. T. Bosse, A. J. Beddek, A. N. Rycroft, J. S. Kroll, and P. R. Langford. 2003. Identification of Actinobacillus pleuropneumoniae genes important for survival during infection in its natural host. Infect. Immun. 71:3960-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokushige, M. 1985. Aspartate ammonia-lyase. Methods Enzymol. 113:618-627. [DOI] [PubMed] [Google Scholar]
- 49.Tonpitak, W., N. Baltes, I. Hennig-Pauka, and G.-F. Gerlach. 2002. Construction of an Actinobacillus pleuropneumoniae serotype 2 prototype live negative-marker vaccine. Infect. Immun. 70:7120-7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torremorell, M., C. Pijoan, K. Janni, R. Walker, and H. S. Joo. 1997. Airborne transmission of Actinobacillus pleuropneumoniae and porcine reproductive and respiratory syndrome virus in nursery pigs. Am. J. Vet. Res. 58:828-832. [PubMed] [Google Scholar]
- 51.Udeze, F. A., K. S. Latimer, and S. Kadis. 1987. Role of Haemophilus pleuropneumoniae lipopolysaccharide endotoxin in the pathogenesis of porcine Haemophilus pleuropneumonia. Am. J. Vet. Res. 48:768-773. [PubMed] [Google Scholar]
- 52.van Oirschot, J. T. 1994. Vaccination in food animal populations. Vaccine 12:415-418. [DOI] [PubMed] [Google Scholar]
- 53.van Oirschot, J. T. 2001. Present and future of veterinary viral vaccinology: a review. Vet. Q. 23:100-108. [DOI] [PubMed] [Google Scholar]
- 54.White, D. G., S. Zhao, S. Simjee, D. D. Wagner, and P. F. McDermott. 2002. Antimicrobial resistance of foodborne pathogens. Microbes Infect. 4:405-412. [DOI] [PubMed] [Google Scholar]
- 55.Wilke, M., B. Franz, and G.-F. Gerlach. 1997. Characterization of a large transferrin-binding protein from Actinobacillus pleuropneumoniae serotype 7. J. Vet. Med. 44:73-86. [DOI] [PubMed] [Google Scholar]