Abstract
Vancomycin and gentamicin act synergistically against penicillin-resistant pneumococci in vitro and in experimental rabbit meningitis. The aim of the present study was to investigate the underlying mechanism of this synergism. The intracellular concentration of gentamicin was measured by using the following experimental setting. Bacterial cultures were incubated with either gentamicin alone or gentamicin plus vancomycin for a short period (15 min). The gentamicin concentration was determined before and after grinding of the cultures by using the COBAS INTEGRA fluorescence polarization system (Roche). The grinding efficacies ranged between 44 and 54%, as determined by viable cell counts. In the combination regimen the intracellular concentration of gentamicin increased to 186% compared to that achieved with gentamicin monotherapy. These data suggest that the synergy observed in vivo and in vitro is based on an increased intracellular penetration of the aminoglycoside, probably due to the effect of vancomycin on the permeability of the cell wall.
Before the emergence of penicillin-resistant pneumococci, penicillin was usually the first-line antibiotic used in the treatment of pneumococcal infections. The increasing spread of resistant strains has changed this situation. In meningitis, where the penetration of antibiotics is limited, penicillin is ineffective even against strains with intermediate resistance, and penicillin resistance is often associated with resistance to other β-lactam antibiotics. Because treatment failures in meningitis have been observed with cephalosporin monotherapy, a combination of vancomycin and an extended broad-spectrum cephalosporin (ceftriaxone or cefotaxime) is usually recommended for the treatment of meningitis caused by resistant strains (1, 7). Especially in the case of β-lactam allergy, it is compelling for infectious disease specialists to elaborate new therapeutic strategies. We have recently investigated the role of gentamicin against penicillin-resistant pneumococci in the rabbit meningitis model (4). Although gentamicin monotherapy was only marginally effective, addition of vancomycin produced killing rates comparable to those achieved by the standard treatment based on ceftriaxone in combination with vancomycin. The synergy between the two antibiotic groups was also demonstrated in vitro in time-killing experiments. The aim of this work was to study the underlying mechanism of the synergism between gentamicin and vancomycin in vitro.
(This study was partially presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., December 2001.)
MATERIALS AND METHODS
Pneumococcal strain.
The serotype 6 penicillin-resistant strain (penicillin G MIC, 4 mg/liter; ceftriaxone MIC, 0.5 mg/liter; cefepime MIC, 0.5 mg/liter; vancomycin MIC, 0.25 mg/liter, gentamicin MIC, 4 mg/liter) used in this study was isolated from a patient with pneumonia at the University Hospital of Bern. MICs were determined by the broth macrodilution method.
In vitro assays.
The penicillin-resistant strain was grown in C+Y medium (8) to an optical density at 590 nm of 0.3 and was then diluted 40-fold to 106 CFU/ml, which corresponds to the bacterial titer in the cerebrospinal fluid of rabbits before the initiation of therapy. Gentamicin was added at concentrations corresponding to one-fourth, one-half, one, and two times the MIC, alone or in combination with vancomycin at the MIC. Bacterial titers were determined at hours 0, 2, 4, 6, and 8 by serial dilution of samples; plated on agar plates containing 5% sheep blood; and incubated at 37°C for 24 h. The experiments were performed in triplicate, and results are expressed as means ± standard deviations.
Measurement of intracellular gentamicin levels.
The penicillin-resistant strain was grown in C+Y medium to an optical density at 590 nm of 0.3. Then, antibiotics were added at the following concentrations: gentamicin at the MIC, gentamicin at two times the MIC, vancomycin at the MIC, and the combination of gentamicin (at the MIC) and vancomycin (at the MIC).
After a short incubation (15 min), the cultures were centrifuged at 3,600 rpm in a Hettich Universal 30RF washed with saline (three times), and ground with glass beads (diameter, 212 to 300 μm) for 15 min. The numbers of CFU were determined after 15 min of incubation, after each washing step, and after grinding. Then, the cultures were resuspended in a small volume of saline. The gentamicin concentration was determined before and after grinding by using the COBAS INTEGRA fluorescence polarization system (Roche) (5). In brief, probes were incubated with a mouse monoclonal antibody, and then a tracer reagent was added. The light emission was measured at 515 nm. The light emission is proportional to the gentamicin concentration of the probe. The gentamicin levels were expressed in micromoles per liter. The sensitivity and specificity of the method are excellent, the detection level for gentamicin is 0.08 μg/ml. No cross-reactivity has been observed with vancomycin at concentrations up to 400 μg/ml. The gentamicin concentrations obtained with the COBAS INTEGRA system have been compared to those determined by a commercially available fluorescence polarization immunoassay method with an excellent correlation (correlation coefficient, 0.992; slope, 0.955; intercept, −0.015 μg/ml [Roche, personal communication]).
Grinding efficacy was determined by plating an aliquot of the surviving microorganisms on blood agar plates overnight at 37°C. The grinding efficacies ranged between 44 and 55% for all groups. Data have been statistically analyzed by the nonparametric Mann-Whitney test.
The gentamicin level measurements were kindly performed by U. Schilt, MCL Laboratories, Bern, Switzerland.
Checkerboard method.
Checkerboards and fractional inhibitory concentration (FIC) indices were determined by the method of Eliopoulos and Moellering (6). Checkerboard runs were repeated three times, and the FIC index was calculated. Synergy was defined as an FIC index of ≤0.5, indifference was defined as an FIC index of >0.5 and ≤4, and antagonism was defined as an FIC index of >4 (11).
RESULTS AND DISCUSSION
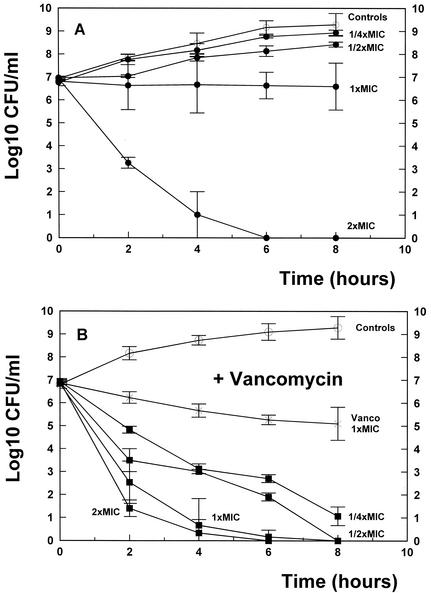
The permanent increase in the numbers of penicillin- and cephalosporin-resistant pneumococci has jeopardized the successful treatment of pneumococcal meningitis, underlining the need for alternative therapy. As a potential alternative, a combination of gentamicin and vancomycin showed antibacterial activity similar to that of the standard regimen based on ceftriaxone and vancomycin in the rabbit meningitis model (4). The antibacterial activities of the different regimens in time-killing assays over 8 h are shown in Fig. 1A and B. By using concentrations under the MIC (one-fourth and one-half the MIC), gentamicin monotherapy did not show antibacterial activity. Gentamicin at the MIC, which corresponded to the level achieved in the cerebrospinal fluid of rabbits during experimental meningitis (4), led to a negligible decrease in the viable cell counts (0.3 log10 CFU/ml over 8 h). On the other hand, a concentration over the MIC (two times the MIC) produced activity that was highly bactericidal and that managed to sterilize the cultures after 6 h (Fig. 1A).
FIG. 1.
(A) Killing rates (•) of monotherapy with gentamicin at different concentrations (one-fourth, one-half, one, and two times the MIC) over 8 h. ○, untreated controls. The experiments were performed in triplicate, and the results are expressed as means ± standard deviations. (B) Killing rates (▪) of different concentrations of gentamicin in combination with vancomycin (Vanco; at the MIC) over 8 h. ×, monotherapy with vancomycin (at the MIC); ○, untreated controls. The experiments were performed in triplicate, and the results are expressed as means ± standard deviations.
The vancomycin concentration was chosen in order to produce a minimal intrinsic activity, thus avoiding masking of a potential synergy between the two antibiotics. Although the bactericidal activity was more pronounced than that of gentamicin, vancomycin (at the MIC) produced only modest bactericidal activity (1.8 log10 CFU/ml over 8 h). Surprisingly the combination therapies (Fig. 1B) were highly synergistic, even when each drug was used at a concentration below the MIC (one-fourth and one-half the MIC), and managed to sterilize the majority of the cultures after 8 h. The synergy was less pronounced for the combination of gentamicin (at two times the MIC) and vancomycin because of the highly bactericidal activity of gentamicin monotherapy (Fig. 1A).
On the other hand, the results of the checkerboard technique were less evident. In this experimental setting the combination was repeatedly tested and was marginally synergistic (FIC index ≤ 0.5) or indifferent (FIC index = 1), underlining the fact that the time-killing assays in vitro correspond more closely to the situation in vivo.
In a further step the intracellular gentamicin concentrations were calculated for all regimens.
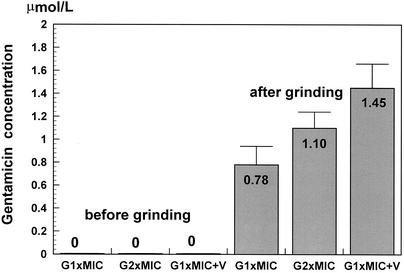
Due to the limits of the method used in this experimental setting, we were not able to localize precisely the intrabacterial compartment in which gentamicin accumulated. Therefore, we use the term “cell-associated gentamicin” to describe intrabacterial gentamicin accumulation. In order to avoid technical bias due to the intrinsic bacteriolytic activity of vancomycin, the cultures were incubated with the different regimens only for a short period (15 min) and were then washed three times with saline and extensively ground with glass beads. This procedure did not influence the viable cell count for any of the regimens except the combination regimen (gentamicin with vancomycin; reduction, 0.5 log10 CFU/ml). The grinding efficacies, estimated by counting the surviving microorganisms, were similar for all groups and ranged between 44 and 55% of the starting inoculum. The results are presented in Fig. 2. After washout and before grinding of the cultures, no gentamicin could be detected in any of the groups. After grinding of the cultures, a relative concentration of 0.78 ± 0.16 μmol of gentamicin per liter was measured when the cultures were incubated with gentamicin alone. The addition of vancomycin drastically increased the cell-associated level of gentamicin to 186% (1.45 ± 0.21 μmol/liter), even higher than the levels obtained with higher doses of gentamicin (1.10 ± 0.14 μmol/liter). It is interesting that the cell-associated gentamicin level was probably underestimated in the group that received the combination due to the reduction in the number of CFU after as soon as 15 min (0.5 log10 CFU/ml); the reduction corresponded to an approximately threefold decrease in the cell population. This decrease in the numbers of viable cells was due to cell lysis and was confirmed by a decrease in the optical density for the group treated with the combination (optical density decrease, 0.330 to 0.280). Thus, the real concentration in the group treated with the combination (vancomycin at the MIC plus gentamicin at the MIC) was probably three times higher, i.e., 4.35 μmol/liter. One-fourth of that value would approximately correspond to the gentamicin concentration obtained with two times the MIC. This could explain why the activity of gentamicin at a lower concentration (one-fourth the MIC) in combination with vancomycin was still synergistic and why gentamicin showed pronounced bactericidal activity (Fig. 1B).
FIG. 2.
Relative gentamicin (G) concentration (in micromoles per liter) in the supernatant before and after grinding of the cultures, as measured by using the COBAS INTEGRA fluorescence polarization system (Roche). The experiments were performed in triplicate, and the results are expressed as means ± standard deviations. Due to the small number of probes used (three for each group), the differences were not significant (by the nonparametric Mann-Whitney test).
These findings may explain the mechanism of the synergy between gentamicin and vancomycin in pneumococci. The underlying mechanism is not completely understood, but these data strongly suggest that the inhibition of cell wall synthesis by vancomycin alters the permeability of the cell wall, which probably acts as a natural barrier to aminoglycosides, and facilitates the penetration of gentamicin into the cell.
These results are reminiscent of the synergism between streptomycin and penicillin against enterococci observed by Moellering and colleagues (9, 10) in the 1970s. Although the experimental setting was completely different, the synergy was explained by increased levels of uptake of radiolabeled streptomycin by enterococci (9, 10).
These data might provide a plausible explanation for the similar synergism observed with either vancomycin (3, 13) or β-lactam antibiotics and quinolones (2, 12) and might open the avenue to new therapeutic options for pneumococcal diseases.
REFERENCES
- 1.Bradley, J., and W. M. Scheld. 1997. Penicillin-resistant Streptococcus pneumoniae meningitis: current antibiotic therapy in the 1990s. Clin. Infect. Dis. 24(Suppl. 2):213-217. [DOI] [PubMed] [Google Scholar]
- 2.Cottagnoud, P., F. Acosta, M. Cottagnoud, K. M. Neftel, and M. G. Täuber. 2000. Synergy between trovafloxacin and ceftriaxone against penicillin-resistant pneumococci in the rabbit meningitis model and in vitro. Antimicrob. Agents Chemother. 44:2179-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cottagnoud, P., J. M. Entenza, M. Cottagnoud, Y.-A. Que, P. Moreillon, and M. G. Täuber. 2001. Sub-inhibitory concentrations of vancomycin prevent quinolone-resistance in a penicillin-resistant isolate of Streptococcus pneumoniae. BioMed Central Microbiol. 1:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottagnoud, P., M. C. Gerber, M. Cottagnoud, and M. G. Täuber. 2002. Gentamicin increases the efficacy of vancomycin against penicillin-resistant pneumococci in the rabbit meningitis model. Antimicrob. Agents Chemother. 46:188-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandliker, W. B. 1961. Quantification of the antigen-antibody reaction by polarization of fluorescence. Biochem. Res. Commun. 5:299-304. [DOI] [PubMed] [Google Scholar]
- 6.Eliopoulos, G. M., and R. C. Moellering. 1991. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 7.Kaplan, S. L., and E. O. Mason. 1998. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin. Microbiol. Rev. 11:628-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lack, S., and R. D. Hotchkiss. 1960. A study of genetic material determining an enzyme activity in pneumococcus. Biochim. Biophys. Acta 39:508-518. [DOI] [PubMed] [Google Scholar]
- 9.Moellering, R. C., and A. N. Weinberg. 1971. Studies on antibiotic synergism against enterococci. J. Clin. Investig. 50:2580-2584.5001959 [Google Scholar]
- 10.Moellering, R. C., S. Wennersten, and A. N. Weinberg. 1971. Studies on antibiotic synergism against enterococci. J. Lab. Clin. Med. 77:821-827. [PubMed] [Google Scholar]
- 11.Nicolau, D. P., P. M. Tessier, R. Quintiliani, and C. H. Nightingale. 1998. Synergistic activity of trovafloxacin and ceftriaxone or vancomycin against Streptococcus pneumoniae with various penicillin susceptibilities. Antimicrob. Agents Chemother. 42:991-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perrig, M., F. Acosta, M. Cottagnoud, C. M. Gerber, M. G. Täuber, and P. Cottagnoud. 2001. Efficacy of gatifloxacin alone and in combination with cefepime against penicillin-resistant Streptococcus pneumoniae in a rabbit meningitis model and in vitro. J. Antimicrob. Chemother. 47:701-704. [DOI] [PubMed] [Google Scholar]
- 13.Rodoni, D., F. Hänni, C. M. Gerber, M. Cottagnoud, K. Neftel, M. G. Täuber, and P. Cottagnoud. 1999. Trovafloxacin in combination with vancomycin against penicillin-resistant pneumococci in the rabbit meningitis model. Antimicrob. Agents Chemother. 43:963-965. [DOI] [PMC free article] [PubMed] [Google Scholar]