Abstract
Microbial interactions with host cell signaling pathways are key determinants of the host cell response to infection. Many toxins secreted by bacterial type III secretion systems either stimulate or inhibit the host inflammatory response. We investigated the role of type III secreted toxins of the lung pathogen Pseudomonas aeruginosa in the inflammatory response of human respiratory epithelial cells to infection. Using bacteria with specific gene deletions, we found that interleukin-8 production by these cells was almost entirely dependent on bacterial type III secretion of exotoxin U (ExoU), a phospholipase, although other bacterial factors are involved. ExoU activated the c-Jun NH2-terminal kinase pathway, stimulating the phosphorylation and activation of mitogen-activated kinase kinase 4, c-Jun NH2-terminal kinase, and c-Jun. This in turn increased levels of transcriptionally competent activator protein-1. Although this pathway was dependent on the lipase activity of ExoU, it was independent of cell death. Activation of mitogen-activated kinase signaling by ExoU in this fashion is a novel mechanism by which a bacterial product can initiate a host inflammatory response, and it may result in increased epithelial permeability and bacterial spread.
The gram-negative human pathogen Pseudomonas aeruginosa is an important opportunist bacterium, infecting those with cystic fibrosis, ventilated patients on intensive care units, the immunosuppressed, and those with burns (1, 5, 39, 49, 53, 64). Infection is common and is associated with a poor outcome (59). The respiratory system is the main site of infection, from where the microbe can invade deeper tissue and become disseminated into the bloodstream, producing sepsis and septic shock (38).
In common with a number of different gram-negative pathogens, P. aeruginosa uses a type III secretion system to introduce toxins into cells. These toxins differ between microbes and in P. aeruginosa are termed exotoxin S (ExoS), ExoT, ExoU, and ExoY (14, 17, 65). Type III secreted toxins typically mimic a eukaryotic activity, subverting signaling within the infected cell and contributing importantly to virulence (6, 12, 26). Infections with P. aeruginosa strains that are able to translocate one or more of these toxins are associated with poor outcome both in natural infection and in animal models (50, 54). The molecular characteristics of these toxins have been determined by a number of groups. ExoS and ExoT are bifunctional, possessing both GTPase-activating protein activity and an ADP-ribosyl transferase domain (17). ExoY is an adenylate cyclase (65), and ExoU has recently been shown to be a lipase (53). ExoU confers a potent cytotoxic phenotype that in vitro produces marked cell death and epithelial damage and in vivo is associated with invasive pneumonia and death (14, 57). However, it is clear that well-differentiated pulmonary epithelial sheets are relatively resistant to the deleterious effects of these secreted toxins (8, 16, 29, 30) and that epithelial damage greatly enhances the ability of P. aeruginosa to infect and invade the respiratory epithelium (47).
Infection of the lungs with P. aeruginosa initiates an intense inflammatory response in the host. Epithelial cells are important in this process, as they are the first cell to encounter inhaled microbes and are able to secrete a number of inflammatory cytokines, such as interleukin-8 (IL-8) (CXCL8) and IL-1 (9). This is evidenced by increased levels of inflammatory cytokines in bronchoalveolar lavage fluid following infection, which are detected in both experimental animal models and patients (3, 10). Associated with this is marked epithelial damage, leading to leakage of inflammatory cytokines into the general circulation and wide dissemination of bacteria, often resulting in severe sepsis and septic shock (35).
Toxins produced by other bacteria with type III secretion systems often produce profound alterations in the intracellular signaling associated with inflammation. For example, following infection with Yersinia spp., the secreted Yersinia toxin YopJ produces a dramatic down-regulation of the inflammatory response (43), due to its ubiquitin-like cysteine protease activity. This strategy allows the bacterium to limit the immune response to infection, increase cell death, and enhance bacterial virulence. In other circumstances, type III secreted toxins can be proinflammatory, such as the Salmonella SipB and Shigella flexneri IpaB toxins, which enhance intracellular IL-1 production by activating caspase-1 (21, 23). Other proinflammatory type III secreted products include the Salmonella protein SipA (37) and the yersinial protein translocase YopB (62). This inflammation is part of a protective immune response mounted by the host that recruits phagocytic cells to the site of infection. It may, however, result in enhanced dissemination of microbes such as Salmonella enterica serovar Typhimurium (41) or in increased epithelial damage such as seen with gastrointestinal infection with Shigella flexneri (45).
The effects of the type III secreted toxins of P. aeruginosa on signaling pathways within mammalian cells are not known. Given the importance of the response of the respiratory epithelium to infection, we set out to examine the effects of these toxins on signaling following infection of respiratory epithelial cells with this bacterium. We hypothesized that the type III secreted toxins would influence intracellular signaling following infection. We chose to examine in particular the production of the chemokine IL-8 from respiratory epithelial cells following P. aeruginosa infection, since this is an important mediator of neutrophil influx and a robust proinflammatory response of these cells following infection (34, 46). We found that type III secretion is required for effective production of IL-8 following infection of respiratory epithelial cells with P. aeruginosa. This effect was largely dependent on the secreted ExoU toxin, in a mechanism that did not produce cytotoxic effects. We demonstrate that ExoU produces activation of the c-Jun NH2-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) pathway and that this is required for efficient IL-8 production following infection. This is a novel mechanism by which a bacterial product can initiate a host inflammatory response.
MATERIALS AND METHODS
Bacteria and construction of mutants.
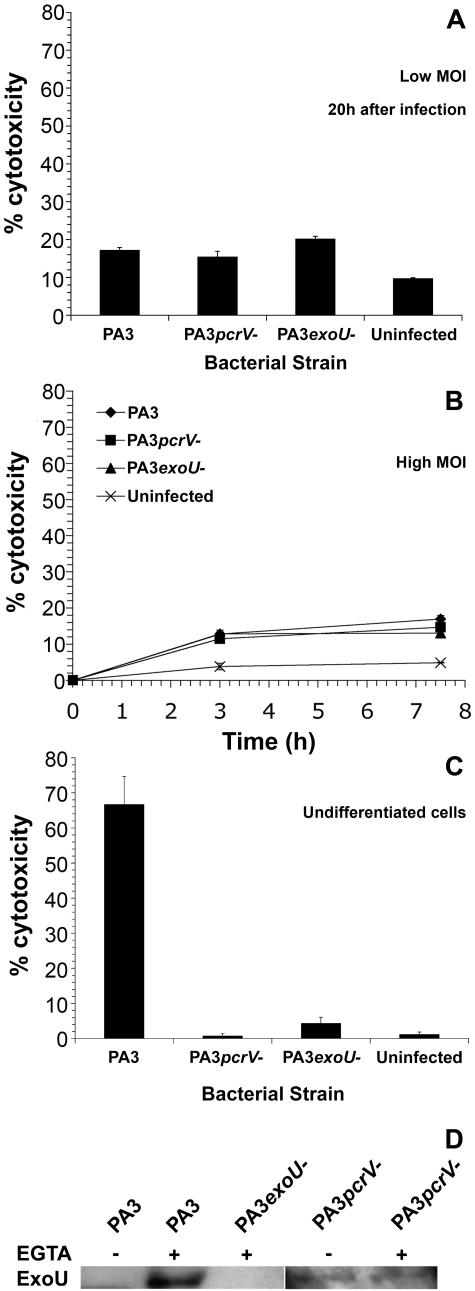
Strains of Pseudomonas aeruginosa were obtained from clinical isolates at the Hammersmith Hospital, London, United Kingdom, as described previously (7). PA103ΔUΔT was a kind gift from Dara Frank, University of Wisconsin. The strains were grown on LB agar or broth with agitation at 37°C. Construction of nonpolar single gene disruptions in P. aeruginosa was performed using the pEX100T plasmid and sacB counterselection as described previously (56). The pcrV gene was cloned from pseudomonal genomic DNA (PA3 strain) by PCR using the primers CGC AGG ACG CTG TCG TAT TTC and GGA ATC ACG ATG GAA GTC AGA AAC and cloned into the SmaI site of pEX100T. A blunt-ended DraI fragment from pUCP22 containing the Gmr gene was then cloned into the unique SfoI site within pcrV, disrupting the gene 65 bp downstream from the initiator methionine. Ampicillin (100 μg · ml−1)- and gentamicin (30 μg · ml−1)-resistant colonies containing this plasmid were isolated in TOP10 Escherichia coli (Invitrogen, United Kingdom). Plasmids from these colonies were then used to transform the SM10 mobilizer strain of E. coli. This was used to introduce the construct into pseudomonal strains by conjugation, using filter mating. After growth of transconjugates and SM10 donor strain on LB agar, transconjugant P. aeruginosa strains were selected on Vogel-Bonner medium with gentamicin (100 μg · ml−1). Double crossovers were isolated by streaking single colonies on LB containing 30 μg · ml−1 gentamicin and 5% sucrose to select against the vector sacB. Colonies were then propagated on LB agar with 100 μg · ml−1 gentamicin. Correct replacement of the pcrV gene was confirmed by Southern blotting. Phenotypic confirmation of disruption was confirmed by using antisera raised against type III secretion products to demonstrate calcium-blind secretion of ExoU (Fig. 1D).
FIG. 1.
Cytotoxicity following infection of 16HBE14o− cells. A. Differentiated cells were infected at a low MOI (1 CFU per 5 × 103 cells) as detailed in Materials and Methods, and cytotoxicity was measured at 20 h after infection for the strains indicated using LDH release. Noninternalized bacteria were removed by washing at 6 h following infection, and gentamicin or colistin was added for the remainder of the experiment to kill extracellular bacteria. Results are the means of triplicate determinations; error bars show standard errors of the means. B. Cells were infected at a high MOI (1 CFU per 1 cell) and cytotoxicity measured at various times and with various strains as shown by LDH release. Results are the means of triplicate observations. C. Undifferentiated cells plated at 80% confluence in 24-well tissue culture dishes were infected at an MOI of 1 CFU per 1 cell and assayed for cell death 6 h after infection by trypan blue staining. Results are the means of triplicate determinations; error bars show standard errors of the means. D. Levels of secreted ExoU produced by the indicated bacterial strains. EGTA was added to induce type III secretion as indicated. Levels of ExoU produced by the PA3 ΔpcrV strain were lower than those produced by the wild type, so the panel showing secreted proteins from the PA3 ΔpcrV strain is from a longer exposure of the immunoblot.
Disruption of the exoU gene was performed in a similar fashion. exoU was cloned by PCR using the primers CACC ATG CAT ATC CAA TCG TTG GGG and TCA TGT GAA CTC CTT ATT CCG CCA. The product was cloned into the SmaI site of pEX100T and the Gmr gene from pUCP22 introduced into the unique SmaI site of exoU as described above. Conjugal transfer of this construct into pseudomonal strains and verification of disruption were exactly as described above, by both genetic and phenotypic analyses (Fig. 1).
Epithelial cell culture and infection model.
16HBE14o− (18), a simian virus 40-transformed human bronchial epithelial cell line (a kind gift from Diener Gruenert, University of Vermont), was grown in RPMI plus 10% fetal calf serum. For all infection experiments, cells were grown on Anapore membranes and cultured as described previously (7, 8). For infection at a low multiplicity of infection (MOI) (1 bacterium per 5 × 103 cells), experiments were carried out as described previously (7, 8). Because some of the gene deletion strains carried gentamicin resistance as a selectable marker, we added colistin (10 μg · ml−1) instead of gentamicin after washing loosely associated bacteria from the epithelial sheet at 6 h after infection. This concentration of colistin effectively killed all noninternalized bacteria from the epithelial cells (data not shown). Importantly, the antibiotic on its own did not induce IL-8 secretion and was added to control infections as well as to strains with specific gene deletions. For infection at a high MOI (1 bacterium per 1 cell), the infection was allowed to proceed in all cases for up to 7.5 h after infection with no added antibiotics.
Analysis of phosphorylated MAPK components.
For experiments analyzing changes in the phosphorylation status of signaling molecules, cells were prepared exactly as described above but were infected at a higher multiplicity of infection with 1 CFU per cell. At various times after infection, epithelial monolayers were washed three times with ice-cold phosphate-buffered saline and then lysed directly into 400 μl of sodium dodecyl sulfate sample buffer. Cell debris was scraped up, collected into a microcentrifuge tube, and frozen prior to analysis by Western blotting. The following antibodies were used to detect the various signaling intermediates studied: p38, phospho-p38 (Thr180/Tyr182), phospho-extracellular signal-regulated kinase (ERK) 1/2 (Thr202/Tyr204), phospho-stress-activated protein kinase (SAPK)/JNK (Thr183/Tyr185), phospho-SAPK kinase 1/MAPK kinase 4 (SEK1/MKK4) (Ser257/Thr261), phospho-c-Jun (Ser63), c-Jun, and nonphosphorylated SEK1/MKK4 (all from Cell Signaling Technology, Hitchin, United Kingdom). Inhibitor of NF-κBα (IκBα) was from Santa Cruz Biotechnology, Calne, United Kingdom. Blots were developed using the Amersham ECL kit according to the manufacturer's instructions (Amersham Biosciences, Amersham, United Kingdom).
Assay of AP-1.
Activator protein-1 (AP-1) binding to its DNA recognition sequence was determined using a TransAM enzyme-linked immunosorbent assay-based kit from Active Motif, Rixensart, Belgium. This kit provides the specific AP-1 binding site immobilized in plastic 96-well plates, to which AP-1 proteins bind. We detected bound AP-1 using antibodies to phospho-c-Jun within the kit. Competition with specific oligonucleotides reduced binding to background levels, which were subtracted from the levels seen with no added competitor oligonucleotide. No significant effect was observed using irrelevant competing oligonucleotides.
IL-8 assay.
IL-8 was assayed using a DuoSet immunoassay kit for human IL-8 according to the manufacturer's instructions (R&D Systems Europe Ltd., Abingdon, United Kingdom). Where indicated, apical and basal measurements were performed using tissue culture medium retrieved from the indicated compartment of the Anapore membrane culture system. For some graphs, total IL-8 was calculated as the weighted mean of the concentrations of IL-8 in the basal and apical compartments. The assay uses specific antibodies to capture and detect IL-8; absolute values are obtained by comparison against standard concentrations of human IL-8.
Recombinant ExoU.
Recombinant ExoU was prepared from E. coli expressing His-tagged ExoU exactly as described previously (58).
Statistics.
Differences between groups were analyzed by either the t test or analysis of variance (ANOVA), as indicated. P values of <0.05 were considered significant.
Cytotoxicity.
Cell cytotoxicity was measured by lactate dehydrogenase (LDH) release, using a kit from Promega (Southampton, United Kingdom) according to the manufacturer's instructions. Percent cytotoxicity was calculated relative to the total LDH released from cells lysed with Triton X-100. In some experiments, cytotoxicity was calculated by the percentage of cells showing trypan blue staining.
Plasmids and constructs.
Complementation of mutant strains of P. aeruginosa was performed with the exoU and spcU genes cloned into the pUCP19 vector, kindly supplied by Dara Frank, University of Wisconsin. Site-directed mutagenesis of the exoU gene was performed with the QuickChange II mutagenesis kit (Stratagene, Amsterdam, The Netherlands) according to the manufacturer's instructions. The S142A mutation was constructed using the oligonucleotides GTCCGGTTCGGCCGCTGGCGGCA and TGCCGCCAGCGGCCGAACCGGAC. Mutations were verified by DNA sequencing of the whole construct. Because the PA3 strain is intrinsically resistant to ampicillin/carbenicillin, we cloned the Tetr gene from pALTER into pUCP19 containing exoU to convert it to the pUCP27 vector as described previously (63). This was selected using tetracycline at 10 μg · ml−1 for E. coli and 100 μg · ml−1 for P. aeruginosa.
ExoU secretion.
Bacteria were grown in Trypticase soy broth supplemented with 1% glycerol, 100 mM monosodium glutamate, and antibiotics at the concentrations indicated above. For induction of type III secreted proteins, the medium was supplemented with 2 mM EGTA. Bacteria were grown for >16 h to an optical density at 600 nm of >4.0 with vigorous aeration. Cells were pelleted by centrifugation at 16,000 × g for 2.5 min, and proteins within supernatants were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, with loading adjusted to bacterial density. Gels were electroblotted and the amount of ExoU assayed by the binding of a polyclonal rabbit antiserum to ExoU prepared as described previously (58).
RESULTS
Model of respiratory epithelium infection with Pseudomonas aeruginosa: infection of differentiated epithelial cells does not result in acute cytotoxicity.
In order to study the signaling pathways induced following P. aeruginosa infection of respiratory epithelial cells, we developed a tissue culture model of infection that aimed to reproduce key features of natural infection. We have used this model previously in a number of studies (7, 8). Briefly, this model uses the respiratory epithelial cell line 16HBE14o− differentiated at an air interface and infected at a very low MOI (1 CFU per 5 × 103 cells), using an inoculum of minimal volume (2 μl). Infection is allowed to proceed for 6 h, after which time the epithelial sheet is washed and then treated with an extracellular antibiotic (either gentamicin or colistin), which will kill any extracellular bacteria. Under these conditions, bacteria increase in number about 1,000-fold during this 6-h period, corresponding to about 10 doublings (7). However, although the bacteria adhere to the surface of the differentiated 16HBE14o− cells, there is no internalization in assays that could detect as few as 1 internalized bacterium per 500 cells (7). After this 6-h period, all extracellular bacteria are killed by antibiotics. We used this model to follow the changes produced by infection in respiratory epithelial cells.
In our initial studies, we used a cytotoxic clinical strain of P. aeruginosa, PA3, isolated from blood. This strain actively secretes the type III toxins, ExoT, ExoU, and ExoY. We constructed a number of isogenic mutants of this strain with disruptions in the pcrV gene, which controls all type III secretion (55), or in the exoU gene, encoding the principal type III secreted cytotoxin. Phenotypic analysis of these strains confirmed that the ΔexoU strain lacked production of the deleted type III gene product and that the ΔpcrV strain had a calcium-blind pattern of ExoU secretion (Fig. 1D).
In contrast to infection of undifferentiated cells at high multiplicities of infection, this model results in little cytotoxicity of the epithelial cells for up to 20 h after infection of the 16HBE14o− cell lines, using assays for necrosis (Fig. 1A) and also apoptosis by the use of annexin V binding, as previously described (7, 8). Even at higher multiplicities of infection, there was no dramatic increase in cell death following infection, as assayed by LDH release up to 7 h after infection (Fig. 1B). Importantly, we did not observe any significant difference in cell death in this infection model when epithelial cells were infected with the wild-type PA3 strain or with PA3 strains lacking pcrV or exoU (Fig. 1). This is in contrast to models of infection where cells are not polarized and infected at high multiplicities of infection. Under these conditions, there is extensive cell death which is dramatically abrogated by mutations in the pcrV and exoU genes (14) (Fig. 1C). In addition, in our model of infection there is no detectable internalization of bacteria after infection (7), even in the ΔexoU and ΔpcrV strains (data not shown).
Mutations preventing type III secretion or deletion of ExoU abrogate IL-8 secretion following infection.
As an important inflammatory response of the respiratory epithelium following infection is the production of the chemokine IL-8, we assayed its production following infection of respiratory epithelial cells with P. aeruginosa in this model. Previously we have demonstrated that infection of polarized CFBE41o− cells results in an increased production of IL-8 from the basal compartment (7), which was consistently increased 20 h after infection. We explored the production of IL-8 from 16HBE14o− epithelial cells further following infection with a wild-type cytotoxic strain of P. aeruginosa, PA3, and isogenic mutants of this strain with targeted disruption of the pcrV gene (ΔpcrV), which controls all type III secretion, or of exoU (ΔexoU), encoding the principal cytotoxic toxin. At 20 h after infection, we found that IL-8 production from both apical and basal compartments was dramatically reduced in 16HBE14o− cells infected with PA3 with the pcrV gene disrupted compared to the wild-type controls (Fig. 2). This was a significant reduction in IL-8 production in both compartments (P < 0.01). We consistently found that the cells produced more IL-8 from the apical compartment (Fig. 2; also see Fig. 3 and 4).
FIG. 2.
IL-8 release following infection of 16HBE14o− cells with strains as indicated. IL-8 production was measured from either the apical compartment or the basal compartment at 20 h following infection. Results are the means of triplicate determinations; error bars are standard errors of the means. Background values of IL-8 released from uninfected cells have been subtracted. The experiment was repeated five times with identical results. Significant differences for the corresponding values obtained with the wild-type PA3 strain are marked with an asterisk (P < 0.05, t test).
FIG. 3.
IL-8 production following infection of 16HBE14o− cells. Total IL-8 produced over time following infection with the different bacterial inocula of wild-type PA3 is shown. Results are means of triplicate determinations; error bars are standard errors of the means. The differences between IL-8 produced by the different bacterial inocula compared to no infection were significant as compared using two-way ANOVA (P < 0.05).
FIG. 4.
Complementation of ΔexoU strain with active exoU restores epithelial IL-8 secretion. A. Total IL-8 production (basal plus apical) was measured 20 h after infection at low MOI with wild-type PA3, the isogenic ΔexoU mutant, the ΔexoU mutant complemented with wild-type exoU (ΔexoU:exoU), and the ΔexoU mutant complemented with the lipase-inactive S142A mutant of ΔexoU (ΔexoU:S142AexoU). In each case, the plasmid used to complement the ΔexoU strains also carried the chaperone, spcU. Results are the means of two or three measurements expressed as percentages of the total IL-8 secretion produced by wild-type PA3; error bars are standard errors of the means. The asterisks indicate results significantly different from those for the wild-type PA3 strain (P < 0.05, t test). B. Total IL-8 production (basal plus apical) was measured 20 h after infection at low MOI with wild-type PA103ΔUΔT, PA103ΔUΔT complemented with wild-type exoU (PA103ΔUΔT:exoU), and PA103ΔUΔT complemented with the lipase-inactive S142A mutant of exoU (PA103ΔUΔT:S142AexoU). Control values for PA3 are also shown. Results are the means of triplicate determinations expressed as percentages of the total IL-8 secretion produced by wild-type PA3; error bars are standard errors of the means. Asterisks indicate values significantly different from those for the PA103 ΔUΔT strain (P < 0.05, t test). In all cases, background IL-8 values from uninfected cells have been subtracted. C. ExoU secretion from the indicated bacterial strains grown in the presence of EGTA and assayed by immunoblotting.
To determine which toxin of the type III secretion system was involved in the increased production of IL-8 in these cells, we constructed a number of other targeted mutations in individual toxins. We found that PA3 with a disruption in the exoU gene produced very little IL-8 following infection of 16HBE14o− cells (Fig. 2), with levels reduced to a level comparable to that seen in the ΔpcrV mutant. Again, it is important to emphasize that under the conditions used to perform this experiment, there was little cell death and no significant differences between the different strains in the cytotoxicity produced (Fig. 1). Importantly, cells infected with wild-type bacteria or the ΔpcrV or ΔexoU mutants were all treated identically with colistin after 6 h of infection, to limit further bacterial growth. In addition, uninfected cells treated with colistin did not produce IL-8 (data not shown). Thus, the differences between the different bacterial strains reflect the differences in the gene products they produce and not any other differences in experimental conditions.
Recently, it has been shown that exogenous ExoS can stimulate cytokine production when added to cells (11). In order to determine whether extracellular ExoU might be having a similar effect on IL-8 production, we tested the effect of recombinant ExoU alone added at either 0.1 or 1 μg/ml to differentiated 16HBE14o− cells. Under the same conditions in which bacteria secrete IL-8, the exogenous ExoU was without effect (data not shown). Bacterial viability was important for IL-8 production, since if the initial inoculum of P. aeruginosa was heat killed, again no IL-8 above background levels was produced (data not shown).
We repeated measurements of IL-8 at different times and with different inocula of bacteria in the 16HBE14o− cell line. Small amounts of IL-8 accumulate in uninfected cells (Fig. 3). Following infection, we found that the production of IL-8 increased steadily over time and with the dose of bacteria used in the original inoculum (Fig. 3). We used the 20-h time point after infection with the MOI of 1 CFU per 5 × 103 cells as the optimum time for assessing IL-8 secretion following infection, as the accumulated IL-8 at this time was consistently at least 5 times the background release with minimal cytotoxicity of the epithelial cells.
Complementation of ExoU deletions restores epithelial IL-8 secretion following infection.
The data presented above suggested that type III secretion of ExoU was largely responsible for the increased output of IL-8 from respiratory epithelial cells following infection in our model. In order to be sure that the differences observed in the mutants were due to the disruption of the exoU gene, we complemented the ΔexoU strain with an expression plasmid bearing exoU (with its cognate chaperone spcU, whose product is not secreted [15]). Bacteria complemented in this fashion produced significantly more IL-8 than the ΔexoU mutant (Fig. 4A), to levels greater than seen in the wild type. Complementation of PA3 ΔexoU with exoU alone without its chaperone also restored IL-8 secretion, but only to about 60% of the levels seen with wild-type PA3, suggesting that spcU is not fully functional in ΔexoU (data not shown). The significant elevation in IL-8 observed by complementation with exoU suggested that ExoU was indeed the factor responsible for the production of IL-8 in respiratory epithelial cells following infection with P. aeruginosa. Complementation of the PA3 ΔexoU strain with the lipase-inactive S142A exoU (53) did not result in a significant increase in IL-8 production, which remained at about the same level as seen with the PA3 ΔexoU mutant alone (Fig. 4A). This demonstrates that the increase in IL-8 produced by ExoU is dependent on the catalytic lipase activity of the protein and that spcU alone is unable to stimulate IL-8 secretion.
These experiments indicated that exoU was necessary for IL-8 production. In order to determine whether ExoU acted alone or required other secreted toxins of the type III system, we attempted to delete the exoT and exoY genes present in the PA3 strain. However, a lack of suitable antibiotic selection markers precluded this approach. As an alternative, we utilized the pseudomonal strain PA103ΔUΔT, which does not secrete any of the known type III cytotoxins but does possess a fully functional type III transport apparatus (60). This was complemented with either the active exoU gene or the lipase-inactive S142A exoU mutant. Figure 4B shows that following infection with PA103ΔUΔT there is detectable release of IL-8, but to much lower levels than seen with the PA3 strain. When the exoU gene was expressed in the PA103ΔUΔT strain, the amount of IL-8 produced following infection was significantly enhanced compared to that in the uncomplemented strain (Fig. 4B), although only to about 50% of the levels seen with PA3. The S142A exoU lipase-deficient mutant did not produce a significant rise in IL-8 secretion compared to the PA103ΔUΔT strain (Fig. 4B) and produced less than 50% of that of the PA103ΔUΔT:exoU strain, although this difference did not achieve statistical significance. Thus, ExoU alone is able to produce IL-8 secretion. However, combination with type III secreted toxins may modify this activity. Assay of the amounts of ExoU secreted by these various complemented strains showed that there was little difference between them (Fig. 4C). Thus, the different levels of IL-8 produced by the complemented PA3 ΔexoU and PA103ΔUΔT strains suggest that factors other than ExoU are also important in the induction of IL-8 following infection with P. aeruginosa.
Infection of differentiated epithelial cells with P. aeruginosa results in an ExoU-dependent activation of the JNK MAPK pathway.
IL-8 secretion in response to inflammation results from gene activation and mRNA stabilization and is dependent on three main signals: activation of the transcription factor NF-κB; activation of the JNK MAPK pathway, leading to activation of the AP-1 transcription factor; and activation of the p38 MAPK, which increases IL-8 mRNA stability (24). 16HBE14o− cells increase IL-8 mRNA levels following P. aeruginosa infection (46). In order to define the pathways potentially activated by ExoU in epithelial cells, we analyzed the activation of components of these pathways in 16HBE14o− cells following infection with the wild-type PA3 strain of P. aeruginosa and the isogenic ΔpcrV and ΔexoU strains. When we infected cells at the low MOI used in the studies of IL-8 secretion, we found that the kinetics of kinase activation were very variable and differed greatly between experiments (data not shown). We felt that this reflected the very low dose of bacteria used as a stimulus (only 200 CFU), which initially produced an asynchronous response. This resulted in clear differences in the accumulation of a stable mediator such as IL-8 but was not suitable for examining transient phosphorylation responses. In order to facilitate analysis of kinase activation, we infected cells at a higher multiplicity of infection than used in the IL-8 analyses, with 1 CFU per 1 cell. This provided a more synchronous stimulus to the epithelial sheet but over the course of the experiment still did not result in appreciable cell death (Fig. 1). Importantly, there were no significant differences in death between cells infected with the wild-type, ΔpcrV, and ΔexoU strains (Fig. 1B). We analyzed total cellular lysates for a number of factors at various times following infection, as shown in Fig. 5. This figure summarizes data obtained from three independent experiments, with essentially identical findings. First, we examined the phosphorylation (and hence activation) of the MAPK p38 and ERK 1/2. In resting, uninfected cells there was no detectable phosphorylated form of either kinase (Fig. 5). Between 1.5 and 3 h following infection, we observed striking phosphorylation of p38 and ERK 1/2. The degrees of activation of these MAPKs were indistinguishable between the wild-type PA3 and the ΔexoU mutant (Fig. 5). In a similar fashion, we examined the degradation of IκB, the inhibitory factor whose destruction leads to activation and nuclear localization of NF-κB. At 1.5 h after infection, there was marked degradation of IκB, such that between 4 and 6 h following bacterial inoculation this factor was virtually undetectable (Fig. 5B). There was no significant difference in this degradation between the wild-type PA3 and the ΔexoU mutant (Fig. 5B). Loading of the lanes was assayed by probing the blot for actin, total p38, MKK4, or c-Jun staining (Fig. 5). This confirmed that there were no gross changes in total protein loaded. Because of the limits in sensitivity in reprobing the same blot repeatedly, we separately determined levels of unmodified JNK and ERK 1/2 following infection and showed that these were unchanged and not different between the different mutant strains (data not shown).
FIG. 5.
Changes in phosphorylation status of signaling molecules following infection with wild-type PA3 and the isogenic ΔexoU mutant at high MOI (1 bacterium per 1 cell). No antibiotics were added. Results show levels of the specific proteins as indicated at various times (hours) after infection with the strains as shown. Results are representative from two separate experiments with slightly different sampling times (A and B); the experiment was repeated on two further occasions with the same results. The blots were stripped and blotted sequentially to obtain the results as shown.
Next, we examined the phosphorylation and activation of the SAPK/JNK pathway kinases, SEK1/MKK4 and SAPK/JNK, as well as phosphorylation of the transcription factor component c-Jun. Between 4.5 and 7.5 h after infection, we found that wild-type PA3 induced marked phosphorylation of SEK1/MKK4, SAPK/JNK, and c-Jun (Fig. 5), although at longer exposures, we could detect very small levels of increased activation of these factors at 3 h after infection (data not shown). This was consistently at a slightly later time point than the phosphorylation of ERK 1/2 and p38 and the degradation of IκB. Following infection with the ΔexoU mutant of PA3, the phosphorylation of both SEK1/MKK4 and SAPK/JNK was markedly reduced compared to that observed following infection with the wild-type strain (Fig. 5). In addition, the phosphorylation of c-Jun was also significantly diminished compared to that observed with the wild-type strain (Fig. 5B). Controls for protein loading and for levels of unmodified MKK4 and c-Jun showed that these changes were due to changes in phosphorylation of the kinases and not to differences in amounts of protein loaded (Fig. 5 and data not shown). Thus, ExoU is responsible for the activation of the SAPK/JNK MAPK pathway in these respiratory epithelial cells. A similar lack of SAPK/JNK MAPK activation was seen following infection with the PA3 ΔpcrV mutant (data not shown).
Requirement for SAPK/JNK in IL-8 production in respiratory epithelial cells following infection.
The data shown in Fig. 5 show that ExoU is responsible for the activation of the SAPK/JNK pathway following P. aeruginosa infection. This pathway has been shown to be important in IL-8 gene activation in a number of cell types (24). To confirm that the ExoU-induced activation of SAPK/JNK could be responsible for its ability to up-regulate IL-8 production in the infected respiratory epithelial cells used in these experiments, we tested the effects of the specific JNK inhibitor SP600125 (2) on the ability of 16HBE14o− cells to produce IL-8 following infection with P. aeruginosa strain PA3. SP600125 produced a dose-dependent reduction in IL-8 production following infection, with a 50% inhibitory concentration of approximately 0.2 μM (Fig. 6). Thus, activation of the SAPK/JNK is required for IL-8 production in this cell type. This supports the conclusion that ExoU activation of SAPK/JNK contributes to IL-8 production following infection in this model.
FIG. 6.
Effects of JNK inhibition with different doses of the inhibitor SP600125 on total IL-8 release following infection. 16HBE14o− cells treated with the indicated doses of SP600125 were infected at low MOI with wild-type PA3, and total IL-8 was determined 20 h after infection and expressed as a percentage of maximal IL-8 secretion in the absence of inhibitor. Results are means of triplicate determinations; error bars are standard errors of the means. The drug exerted a significant inhibitory effect on IL-8 secretion as determined by ANOVA (P < 0.05). Background values from uninfected cells were subtracted in all cases.
ExoU produces increased activity of the AP-1 transcription factor.
Activation of JNK increases IL-8 transcription by phosphorylation of c-Jun, resulting in increased activity of its transcriptional action as part of the AP-1 transcription factor. To confirm that ExoU produces functionally important activation of the SAPK/JNK pathway, we measured the production of the transcription factor AP-1 containing activated c-Jun following infection with PA3 and the isogenic ΔexoU strain. We used an assay that specifically measured AP-1 containing phospho-c-Jun bound to its consensus DNA binding sequence. Following infection with wild-type PA3, levels of active AP-1 containing phospho-c-Jun began to rise 1.5 h after infection, and they rose over threefold by 5 h after infection (Fig. 7). In the ΔexoU mutant, this rise was significantly abrogated (Fig. 7). This assay is more sensitive than the immunoblotting of phospho-c-Jun, which accounts for the slightly earlier rise in detection of phospho-c-Jun in this assay compared to that used for Fig. 5. We conclude, therefore, that ExoU leads to the production of active AP-1 containing phospho-c-Jun.
FIG. 7.
AP-1 induction following infection of 16HBE14o− cells with wild-type (WT) or ΔexoU strains of PA3. Results show the changes in induction of AP-1 containing phospho-c-Jun bound specifically to its DNA recognition site after infection at high MOI (1 bacterium per 1 cell with no added antibiotics). Results are expressed relative to the value observed at time zero and are the means of triplicate determinations; error bars are standard errors of the means. Specificity of the assay for AP-1 binding was gauged by using a fivefold molar excess of free AP-1 binding site oligonucleotide; this reduced the signal to less than 5% of the total and was subtracted in the calculations shown here. Irrelevant sequence oligonucleotide produced no significant change in signal. Significant differences from wild-type values are shown with an asterisk (t test, P < 0.05).
DISCUSSION
Knowledge of the signaling mechanisms activated following host interaction with a pathogen is central to understanding the outcome of the infection. We demonstrate here that efficient activation of the JNK MAPK pathway following infection of respiratory epithelial cells with P. aeruginosa is dependent on the type III secreted toxin ExoU. This toxin activates the kinases SEK1/MEKK4 and SAPK/JNK, resulting in phosphorylation of c-Jun and production of activated AP-1 transcription factor. This inflammatory cascade is initiated following translocation of ExoU into differentiated respiratory epithelial cells under conditions that do not produce cell death. ExoU also produces a markedly increased production of the proinflammatory cytokine IL-8 following infection, again independent of its cytotoxic effects. Since IL-8 production following infection is abrogated by JNK inhibition, we propose that the observed effects of ExoU on IL-8 production are as a result of its ability to activate the JNK MAPK pathway.
Many type III secreted proteins have profound effects on host cell signaling pathways following infection. Yersinia pestis, Yersinia enterocolitica, and Yersinia pseudotuberculosis possess a plasmid-encoded type III secretory system that can act to down-regulate inflammatory signaling following infection. This is through the actions of the secreted toxin YopJ/P, which via a cysteine protease-like activity can inhibit activation of both the MAPK and NF-κB pathways (43, 62). This counteracts inflammatory signaling mediated by bacterial components such as invasin and YopB, a component of the type III translocation machinery. A protein related to Yop J/P in Salmonella enterica serovar Typhimurium, termed AvrA, has a similar counterinflammatory role following Salmonella infection (20, 44).
Here we have shown that ExoU acts to increase proinflammatory signaling, through activation of the JNK MAPK pathway leading to increased epithelial cell IL-8 secretion. The control of IL-8 secretion is complex, with contributions from NF-κB, AP-1, and p38 MAPK pathways (24). Although NF-κB is essential for IL-8 production, the role of AP-1 is also very important in stimulus-induced IL-8 production following cytokine treatment (25, 32) or infection (19). Inhibition of SAP/JNK with SP600125 reduced IL-8 secretion by over 80%, showing the importance of this pathway in the production of IL-8 by these cells (Fig. 6). ExoU is involved in the activation of MKK4, SAP/JNK, and c-Jun (Fig. 5) and also produces increased activation of the AP-1 transcription factor following infection (Fig. 7). Taken together, these data show that ExoU contributes very significantly to activation of the SAPK/JNK MAPK pathway following infection and that this significantly augments epithelial IL-8 produced in infection. Other kinase pathways are activated in infection which are not dependent on ExoU, e.g., those leading to NF-κB and p38 activation (Fig. 5). These pathways have been demonstrated to be important in IL-8 secretion in other systems (25) and may well also be important in IL-8 production following pseudomonal infection, although this would require further investigation. ExoU alone is sufficient to stimulate IL-8 secretion in the PA103ΔUΔT strain (Fig. 4). The levels produced were lower than those achieved with the PA3 strain. This may reflect additive interactions with the type III secreted toxins ExoT and ExoY found in PA3 or other bacterial products. We stress that ExoU is not the only factor from P. aeruginosa that induces IL-8. Importantly, although PA3 ΔexoU produces little IL-8, it does produce some, as does the ExoU-lacking strain PAO1 (10). Clearly, additional bacterial factors such as pili and flagellin are able to produce IL-8 from respiratory epithelial cells (10), although the different cells and bacterial inocula used make it difficult to compare the absolute levels of IL-8 produced in different experimental settings. However, we have shown that ExoU clearly augments the increases in IL-8 from epithelial cells following infection with P. aeruginosa. Our experimental model produces IL-8 in the apparent absence of cytotoxicity. However, there could be some degree of cell death occurring that is below the limit of detection of our assays.
Increased levels of IL-8 may have a number of effects in infection. IL-8 is the principal chemoattractant for neutrophils, which might thus lead to increased bacterial clearance. However, neutrophils can also mediate tissue damage and increase epithelial permeability. This has been shown to be significant in intestinal epithelial infection with Shigella flexneri, where neutrophils mediate considerable epithelial damage following infection and allow microbial invasion through exposure of the basal epithelial surface (27, 45, 52). Infection of an intact epithelial surface by P. aeruginosa is similarly limited and much increased following epithelial damage and exposure of the basal surface. Thus, increased epithelial IL-8 secretion following pseudomonal infection might produce greater epithelial injury and allow invasion of the microbe. Certainly, ExoU-carrying strains of P. aeruginosa are associated with increased tissue invasion and septic shock. This may be mediated by the lipase action of ExoU directly on cell membranes, although as shown here, well-differentiated respiratory epithelial cells are relatively resistant to direct ExoU-mediated cytotoxic effects. The ability of ExoU to increase epithelial IL-8 secretion may augment its tissue-damaging effects through recruitment of neutrophils.
The kinetics of activation of the JNK pathways following infection described here differ from those of activation of the p38 MAPK and NF-κB pathways (Fig. 5 and 7). Activation of p38 and degradation of IκB can be detected 1.5 h after infection, while JNK pathway activation is not seen until 3 to 4.5 h (Fig. 5 and 7). Only the latter pathway is dependent on type III secreted ExoU, so this different time course may represent different signaling mechanisms. We speculate that immediate recognition of pathogen-associated molecular patterns may produce initial activation of p38 and NF-κB, while elaboration and transport of the type III secreted toxins requires the rather longer time interval observed. Translocation of type III effectors typically takes several hours, as shown in Xanthomonas (4) and in the translocation of ExoY by P. aeruginosa (65).
The lipolytic action of ExoU produces a number of potential signaling intermediates that may be responsible for the JNK MAPK pathway activation we describe here. One of these, lysophosphatidylcholine, has potent inflammatory action and activates JNK, producing elevated active AP-1 transcription factor levels (13, 28). Such a mechanism might account for the actions of ExoU observed here. Phospholipase action of ExoU may also release other signaling intermediates from cell membranes, such as inositol 1,4,5-triphosphate, diacylglycerol, and arachidonic acid intermediates, that in turn can initiate a number of intracellular signaling pathways, including activation of SAPK/JNK MAPK (33, 36, 51).
Considerable differences in signaling pathways exist in different tissues. We have used respiratory epithelial cell lines to study type III-dependent signaling following infection with P. aeruginosa because of the importance of the respiratory epithelium in the innate immune response to pulmonary infection (9). Epithelial cells express a variety of pathogen recognition pattern receptors, such as members of the Toll-like receptor family (22, 42, 48). Other signaling pathways, such as recognition by nucleotide oligomerization domain (Nod) family proteins, remain less well characterized. Recently, pseudomonal infection has been shown to activate the phosphoinositol-3-kinase protein kinase B/Akt pathway (31), although the bacterial product required for this activation was not defined. Activation of intracellular MAPK signaling by a type III-delivered lipase is a novel signaling mechanism and adds to other signaling events mediated by type III systems. Recently, a type IV secretion system in Helicobacter pylori was shown to mediate intracellular signaling to Nod1 via intracellular delivery of peptidoglycan (61). It is possible that type III secretion systems might deliver such bacterial components to intracellular sites, and these could synergize with type III secreted mediators such as ExoU in the initiation of an inflammatory response.
In conclusion, we have demonstrated that the type III secreted toxin ExoU produced by P. aeruginosa can activate the JNK MAPK pathway, leading to increased IL-8 secretion by respiratory epithelial cells. This agrees well with recent data suggesting that infection with P. aeruginosa containing ExoU leads to a preferential up-regulation of genes controlled by AP-1 (40). Increases in epithelial IL-8 production produced in this fashion might augment epithelial damage through recruitment of neutrophils and allow greater spread of the pathogen.
Acknowledgments
We are grateful to Dara Frank for her kind gift of exoU constructs in pUCP19.
This work was supported by Action Research and the Wellcome Trust.
Editor: J. T. Barbieri
REFERENCES
- 1.Bishara, J., L. Leibovici, S. Ashkenazi, Z. Samra, and S. Pitlik. 2000. Seven-year study of bacteraemic pneumonia in a single institution. Eur. J. Clin. Microbiol. Infect. Dis. 19:926-931. [DOI] [PubMed] [Google Scholar]
- 2.Bogoyevitch, M. A., I. Boehm, A. Oakley, A. J. Ketterman, and R. K. Barr. 2004. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim. Biophys. Acta 1697:89-101. [DOI] [PubMed] [Google Scholar]
- 3.Bonfield, T. L., J. R. Panuska, M. W. Konstan, K. A. Hilliard, J. B. Hilliard, H. Ghnaim, and M. Berger. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152:2111-2118. [DOI] [PubMed] [Google Scholar]
- 4.Casper-Lindley, C., D. Dahlbeck, E. T. Clark, and B. J. Staskawicz. 2002. Direct biochemical evidence for type III secretion-dependent translocation of the AvrBs2 effector protein into plant cells. Proc. Natl. Acad. Sci. USA 99:8336-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chastre, J., and J. Y. Fagon. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867-903. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 7.Darling, K. E., A. Dewar, and T. J. Evans. 2004. Role of the cystic fibrosis transmembrane conductance regulator in internalization of Pseudomonas aeruginosa by polarized epithelial cells. Cell. Microbiol. 6:521-533. [DOI] [PubMed] [Google Scholar]
- 8.Darling, K. E., and T. J. Evans. 2003. Effects of nitric oxide on Pseudomonas aeruginosa infection of epithelial cells from a human respiratory cell line derived from a patient with cystic fibrosis. Infect. Immun. 71:2341-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27-38. [DOI] [PubMed] [Google Scholar]
- 10.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epelman, S., T. F. Bruno, G. G. Neely, D. E. Woods, and C. H. Mody. 2000. Pseudomonas aeruginosa exoenzyme S induces transcriptional expression of proinflammatory cytokines and chemokines. Infect. Immun. 68:4811-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinosa, A., and J. R. Alfano. 2004. Disabling surveillance: bacterial type III secretion system effectors that suppress innate immunity. Cell Microbiol. 6:1027-1040. [DOI] [PubMed] [Google Scholar]
- 13.Fang, X., S. Gibson, M. Flowers, T. Furui, R. C. Bast, Jr., and G. B. Mills. 1997. Lysophosphatidylcholine stimulates activator protein 1 and the c-Jun N-terminal kinase activity. J. Biol. Chem. 272:13683-13689. [DOI] [PubMed] [Google Scholar]
- 14.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 15.Finck-Barbancon, V., T. L. Yahr, and D. W. Frank. 1998. Identification and characterization of SpcU, a chaperone required for efficient secretion of the ExoU cytotoxin. J. Bacteriol. 180:6224-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleiszig, S. M., D. J. Evans, N. Do, V. Vallas, S. Shin, and K. E. Mostov. 1997. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65:2861-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 18.Goncz, K. K., L. Feeney, and D. C. Gruenert. 1999. Differential sensitivity of normal and cystic fibrosis airway epithelial cells to epinephrine. Br. J. Pharmacol. 128:227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassl, G. A., M. Kracht, A. Wiedemann, E. Hoffmann, M. Aepfelbacher, C. von Eichel-Streiber, E. Bohn, and I. B. Autenrieth. 2003. Activation of NF-kappaB and IL-8 by Yersinia enterocolitica invasin protein is conferred by engagement of Rac1 and MAP kinase cascades. Cell. Microbiol. 5:957-971. [DOI] [PubMed] [Google Scholar]
- 20.Hardt, W. D., and J. E. Galan. 1997. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc. Natl. Acad. Sci. USA 94:9887-9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertz, C. J., Q. Wu, E. M. Porter, Y. J. Zhang, K. H. Weismuller, P. J. Godowski, T. Ganz, S. H. Randell, and R. L. Modlin. 2003. Activation of Toll-like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin-2. J. Immunol. 171:6820-6826. [DOI] [PubMed] [Google Scholar]
- 23.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72:847-855. [PubMed] [Google Scholar]
- 25.Holtmann, H., R. Winzen, P. Holland, S. Eickemeier, E. Hoffmann, D. Wallach, N. L. Malinin, J. A. Cooper, K. Resch, and M. Kracht. 1999. Induction of interleukin-8 synthesis integrates effects on transcription and mRNA degradation from at least three different cytokine- or stress-activated signal transduction pathways. Mol. Cell. Biol. 19:6742-6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islam, D., B. Veress, P. K. Bardhan, A. A. Lindberg, and B. Christensson. 1997. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. Infect. Immun. 65:739-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabarowski, J. H., K. Zhu, L. Q. Le, O. N. Witte, and Y. Xu. 2001. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science 293:702-705. [DOI] [PubMed] [Google Scholar]
- 29.Kazmierczak, B. I., K. Mostov, and J. N. Engel. 2004. Epithelial cell polarity alters Rho-GTPase responses to Pseudomonas aeruginosa. Mol. Biol. Cell. 15:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazmierczak, B. I., K. Mostov, and J. N. Engel. 2001. Interaction of bacterial pathogens with polarized epithelium. Annu. Rev. Microbiol. 55:407-435. [DOI] [PubMed] [Google Scholar]
- 31.Kierbel, A., A. Gassama-Diagne, K. Mostov, and J. N. Engel. 2005. The phosphoinositol-3-kinase-protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell. 16:2577-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause, A., H. Holtmann, S. Eickemeier, R. Winzen, M. Szamel, K. Resch, J. Saklatvala, and M. Kracht. 1998. Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J. Biol. Chem. 273:23681-23689. [DOI] [PubMed] [Google Scholar]
- 33.Ktistakis, N. T., C. Delon, M. Manifava, E. Wood, I. Ganley, and J. M. Sugars. 2003. Phospholipase D1 and potential targets of its hydrolysis product, phosphatidic acid. Biochem. Soc. Trans. 31:94-97. [DOI] [PubMed] [Google Scholar]
- 34.Kube, D., U. Sontich, D. Fletcher, and P. B. Davis. 2001. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am. J. Physiol. Lung Cell Mol. Physiol. 280:L493-L502. [DOI] [PubMed] [Google Scholar]
- 35.Kurahashi, K., O. Kajikawa, T. Sawa, M. Ohara, M. A. Gropper, D. W. Frank, T. R. Martin, and J. P. Wiener-Kronish. 1999. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 104:743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laxalt, A. M., and T. Munnik. 2002. Phospholipid signalling in plant defence. Curr. Opin. Plant Biol. 5:332-338. [DOI] [PubMed] [Google Scholar]
- 37.Lee, C. A., M. Silva, A. M. Siber, A. J. Kelly, E. Galyov, and B. A. McCormick. 2000. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc. Natl. Acad. Sci. USA 97:12283-12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 39.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMorran, B., L. Town, E. Costelloe, J. Palmer, J. Engel, D. Hume, and B. Wainwright. 2003. Effector ExoU from the type III secretion system is an important modulator of gene expression in lung epithelial cells in response to Pseudomonas aeruginosa infection. Infect. Immun. 71:6035-6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monack, D. M., D. Hersh, N. Ghori, D. Bouley, A. Zychlinsky, and S. Falkow. 2000. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J. Exp. Med. 192:249-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muir, A., G. Soong, S. Sokol, B. Reddy, M. I. Gomez, A. Van Heeckeren, and A. Prince. 2004. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30:777-783. [DOI] [PubMed] [Google Scholar]
- 43.Orth, K. 2002. Function of the Yersinia effector YopJ. Curr. Opin. Microbiol. 5:38-43. [DOI] [PubMed] [Google Scholar]
- 44.Orth, K., Z. Xu, M. B. Mudgett, Z. Q. Bao, L. E. Palmer, J. B. Bliska, W. F. Mangel, B. Staskawicz, and J. E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 290:1594-1597. [DOI] [PubMed] [Google Scholar]
- 45.Perdomo, O. J., J. M. Cavaillon, M. Huerre, H. Ohayon, P. Gounon, and P. J. Sansonetti. 1994. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J. Exp. Med. 180:1307-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez, A., and P. B. Davis. 2004. Gene profile changes after Pseudomonas aeruginosa exposure in immortalized airway epithelial cells. J. Struct. Funct. Genomics 5:179-194. [DOI] [PubMed] [Google Scholar]
- 47.Plotkowski, M. C., S. de Bentzmann, S. H. Pereira, J. M. Zahm, O. Bajolet-Laudinat, P. Roger, and E. Puchelle. 1999. Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am. J. Respir. Cell Mol. Biol. 20:880-890. [DOI] [PubMed] [Google Scholar]
- 48.Ramos, H. C., M. Rumbo, and J. C. Sirard. 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12:509-517. [DOI] [PubMed] [Google Scholar]
- 49.Richards, M. J., J. R. Edwards, D. H. Culver, R. P. Gaynes, et al. 1999. Nosocomial infections in medical intensive care units in the United States. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 50.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 51.Saliba, A. M., D. O. Nascimento, M. C. Silva, M. C. Assis, C. R. Gayer, B. Raymond, M. G. Coelho, E. A. Marques, L. Touqui, R. M. Albano, U. G. Lopes, D. D. Paiva, P. T. Bozza, and M. C. Plotkowski. 2005. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell. Microbiol. 7:1811-1822. [DOI] [PubMed] [Google Scholar]
- 52.Sansonetti, P. J., J. Arondel, M. Huerre, A. Harada, and K. Matsushima. 1999. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect. Immun. 67:1471-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawa, T., M. Ohara, K. Kurahashi, S. S. Twining, D. W. Frank, D. B. Doroques, T. Long, M. A. Gropper, and J. P. Wiener-Kronish. 1998. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect. Immun. 66:3242-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 56.Schweizer, H. P., and T. T. Hoang. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15-22. [DOI] [PubMed] [Google Scholar]
- 57.Shaver, C. M., and A. R. Hauser. 2004. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect. Immun. 72:6969-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stirling, F. R., A. Cuzick, S. M. Kelly, D. Oxley, and T. J. Evans. 2006. Eukaryotic localization, activation and ubiquitinylation of a bacterial type III secreted toxin. Cell. Microbiol. [Online.] doi: 10.1111/j.1462-5822.2006.00710.x. [DOI] [PubMed]
- 59.Taylor, G. D., M. Buchanan-Chell, T. Kirkland, M. McKenzie, and R. Wiens. 1995. Bacteremic nosocomial pneumonia. A 7-year experience in one institution. Chest 108:786-788. [DOI] [PubMed] [Google Scholar]
- 60.Vallis, A. J., V. Finck-Barbancon, T. L. Yahr, and D. W. Frank. 1999. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect. Immun. 67:2040-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 62.Viboud, G. I., S. S. So, M. B. Ryndak, and J. B. Bliska. 2003. Proinflammatory signalling stimulated by the type III translocation factor YopB is counteracted by multiple effectors in epithelial cells infected with Yersinia pseudotuberculosis. Mol. Microbiol. 47:1305-1315. [DOI] [PubMed] [Google Scholar]
- 63.West, S. E., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]
- 64.Wilson, R., and R. B. Dowling. 1998. Lung infections. 3. Pseudomonas aeruginosa and other related species. Thorax 53:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]