Abstract
Toll-like receptors (TLRs) are key components of the innate immune system that trigger antimicrobial host defense responses. The aim of the present study was to analyze the effects of probiotic Escherichia coli Nissle strain 1917 in experimental colitis induced in TLR-2 and TLR-4 knockout mice. Colitis was induced in wild-type (wt), TLR-2 knockout, and TLR-4 knockout mice via administration of 5% dextran sodium sulfate (DSS). Mice were treated with either 0.9% NaCl or 107 E. coli Nissle 1917 twice daily, followed by the determination of disease activity, mucosal damage, and cytokine secretion. wt and TLR-2 knockout mice exposed to DSS developed acute colitis, whereas TLR-4 knockout mice developed significantly less inflammation. In wt mice, but not TLR-2 or TLR-4 knockout mice, E. coli Nissle 1917 ameliorated colitis and decreased proinflammatory cytokine secretion. In TLR-2 knockout mice a selective reduction of gamma interferon secretion was observed after E. coli Nissle 1917 treatment. In TLR-4 knockout mice, cytokine secretion was almost undetectable and not modulated by E. coli Nissle 1917, indicating that TLR-4 knockout mice do not develop colitis similar to the wt mice. Coculture of E. coli Nissle 1917 and human T cells increased TLR-2 and TLR-4 protein expression in T cells and increased NF-κB activity via TLR-2 and TLR-4. In conclusion, our data provide evidence that E. coli Nissle 1917 ameliorates experimental induced colitis in mice via TLR-2- and TLR-4-dependent pathways.
Although the etiopathology of inflammatory bowel disease (IBD) is not completely understood, it is evident that intestinal inflammation is a result of an uncontrolled cell-mediated immune response of the host to ubiquitous luminal antigens (12, 15, 55). This assumption is supported by several lines of evidence. First, the incidence of inflammation is greatest in that part of the intestine harboring the highest concentrations of luminal bacteria (66). Second, surgical reduction of the bacterial load ameliorates intestinal inflammation. Furthermore, mucosal inflammation can be experimentally induced by instillation of fecal contents from an inflamed gut into a noninflamed gut of susceptible individuals (14). Third, oral tolerance toward the commensal flora seems to be lost in IBD (30). In different models of IBD using genetically manipulated animals, such as T-cell receptor knockout mice, spontaneous colitis does not develop if the animals are raised under germfree conditions (23, 45). Furthermore, antibiotic as well as probiotic therapy attenuates both experimental colitis and human IBD (19, 22, 31, 54, 55, 58). Taken together, these results emphasize the importance of the commensal microflora in the induction, perpetuation, and maintenance of chronic intestinal inflammation and imply the existence of an imbalance between aggressive (detrimental) and protective (beneficial) bacteria which may play an essential role in the pathogenesis of IBD (55, 69).
Probiotic bacteria are live nonpathogenic microorganisms, which confer a health benefit to the host when administered in adequate amounts (52). Probiotics limit the influence of aggressive bacteria by several mechanisms. They suppress the growth of pathogens through release of antimicrobial factors (28, 29) and are thought to compete with microbial pathogens for the limited number of receptors on the epithelial cells (51) and thus may decrease the adherence of pathogens to the mucosal surface. They modulate the function of the gut-associated lymphoid tissue and enhance the mucosal barrier function (25, 35, 46, 54, 59). Further mechanisms of action may include the reduction of bacterial endotoxin concentrations in blood serum due to decreased translocation of bacterial cell components from the gut into the systemic circulation, competition with pathogenic bacteria for nutrients, and lowering of intraluminal pH by the production of short-chain fatty acids, which fosters the growth of nonpathogenic commensal bacteria and at the same time hinders bacterial overgrowth by pathogens (31, 43, 54, 62). Hence, probiotics have been evaluated in the last few years as an alternative and safe treatment modality for IBD. Several randomized, placebo-controlled studies have clearly demonstrated the beneficial effects of probiotics in the treatment of ulcerative colitis and pouchitis (17, 18, 31-33, 35, 53), and a pilot study in patients with Crohn's disease has shown promising results (36). Despite the demonstrated clinical benefit, the underlying modes of probiotic action in intestinal inflammation have yet to be elucidated at the cellular and molecular level.
Escherichia coli Nissle 1917 is a nonpathogenic E. coli strain that has been characterized extensively at the phenotypic level as well as at the molecular genetic level (7, 8, 20, 21, 65). Exploring the modes of action of this bacterium, we have recently demonstrated that E. coli Nissle 1917 distinctively inhibited the expansion of peripheral (but not mucosal) T cells via Toll-like receptor 2 (TLR-2) signaling (64). This leads to interference with the establishment of newly recruited T cells in the gut and limit intestinal inflammation.
TLRs have been identified as signaling receptors of the innate immune system recognizing a wide variety of molecular patterns typical for microorganisms (37). TLRs have evolved as essential elements of an alarm system suited for alerting the host to the presence of microbes and for initiating anti-infective inflammatory responses (67). Different TLRs are selectively activated by different microbial ligands (10). TLR-2 recognizes lipoteichoic acid and bacterial lipoproteins abundant in the cell wall of gram-positive and gram-negative bacteria (27), whereas TLR-4 is the major receptor for lipopolysaccharides of gram-negative bacteria (5, 67). In addition, several experimental studies with TLR knockout mice have demonstrated that TLRs are involved in the production and release of immunomodulatory cytokines (24, 37, 56, 71). With respect to this, the cytokine pattern after TLR signaling differs depending on the bacterial stimulus used and the cell type examined (24, 44). However, little is known about the role and interaction of TLRs with the adaptive immune system during intestinal inflammation.
An array of different experimental models now exist for studying the etiopathogenesis of ulcerative colitis and Crohn's disease and for testing new treatment options (13, 23). In the present study, we mainly investigated the in vivo effects of the probiotic E. coli strain Nissle 1917 in the well-established model of dextran sodium sulfate (DSS)-induced experimental colitis in mice. This animal model is characterized by a typical Th1 as well as Th2 cytokine profile (13). Furthermore, since TLRs are critically involved in intestinal inflammation, we administered E. coli Nissle 1917 to TLR-2 knockout and TLR-4 knockout animals in order to explore the importance of TLRs for the biological action of this probiotic.
MATERIALS AND METHODS
Reagents and antibodies.
CD3 monoclonal antibody (MAb; clone OKT3; Janssen-Cilag, Neuss, Germany) and CD28 (ANC28.1/5D10; Ancell Corp., Bayport, MN) were used for human T-cell activation. Anti-CD3 MAb (clone 145-2C11; eBioscience, San Diego, CA), CD28, and phorbol myristate acetate and ionomycin (both Sigma-Aldrich, Taufkirchen, Germany) were used for mouse T-cell activation. For flow cytometry, fluorescein isothiocyanate (FITC)-labeled anti-human TLR-2 (clone 6C2) and anti-human TLR-4 (clone HTA125) MAb were purchased from eBioscience. FITC-labeled anti-mouse interleukin-1 (IL-4; clone 11B11), anti-mouse gamma interferon (IFN-γ; clone XMG1.2), anti-mouse tumor necrosis factor alpha (TNF-α; clone MP6-XT22), and anti-mouse phycoerythrin (PE)-labeled anti-CD3 (clone 145-2C11) MAb were bought from BD Pharmingen (Heidelberg, Germany). FITC- and PE-labeled isotype controls were from Dako (Hamburg, Germany). Brefeldin A and saponin were purchased from Sigma-Aldrich, and paraformaldehyde was obtained from Roth (Karlsruhe, Germany). The cytofluorometric bead array kit was purchased from BD Pharmingen. Triacylated lipopeptides [Pam3CSK4; lipopeptide (LP)3] and diacylated lipopeptides (Pam2CSK4; LP2), first described by Mühlradt et al. (39), were purchased from EMC microcollections (Tübingen, Germany). Lipopolysaccharide (LPS) from Salmonella enterica serovar Minnesota Re595 was purchased from Sigma-Aldrich and found to be devoid of any lipoprotein contaminations in previous studies. Plasmids encoding human TLR-4 (hTLR-4), human CD14 (hCD14), and human TLR-2 (hTLR-2), as well as β-galactosidase and the ELAM NFκ B reporter plasmid (luciferase), were kindly provided by C. J. Kirschning (University of Munich, Munich, Germany). The plasmid encoding human MD2 (hMD2) was kindly provided by K. Miyake (University of Tokyo, Tokyo, Japan) and O. Takeuchi (University of Osaka, Osaka, Japan).
E. coli culture and generation of conditioned media.
E. coli Nissle 1917 conditioned medium was generated as described by Yan and Polk (72). Briefly, E. coli Nissle 1917-layered beads (provided by Ardeypharm, Herdecke, Germany) were incubated for 16 h at 37°C in Luria-Bertani broth. The bacterial culture was then harvested by centrifugation at 1,000 × g for 15 min. The supernatant was discarded, and the bacterial pellet was washed twice in phosphate-buffered saline and then resuspended in T-cell medium (RPMI, 10% fetal calf serum, 1.5% HEPES) without antibiotics. After 2 h at 37°C and 5% CO2, the culture was centrifuged at 1,000 × g, and the supernatant was recovered and sterile filtered through a 0.22-μm-pore-size syringe-driven filter. As determined by using a Limulus amebocyte lysate, the E. coli Nissle 1917-conditioned medium contained 1,300 × 109 endotoxin units/ml.
Transient transfection of HEK 293-cells and measurement of luciferase activity.
Transient transfection of human embryonic kidney cells (HEK) 293 cells was performed by using FuGENE 6 Transfection Reagent (Roche Diagnostics, Mannheim, Germany) as previously described (41, 57). Briefly, cells were plated out in 12-well plates with 105 cells per well and then incubated overnight with plasmids containing NF-κB reporter luciferase (120 ng). In previous experiments we determined that TLR-4 is more sensitive in response to its ligand (LPS) than TLR-2 in response to lipopeptides (41, 57). To correct differences in transfection efficiency, cells were transfected with Rous sarcoma virus-β-galactosidase (40 ng) and cotransfected with either hTLR-4 (2 ng) or hTLR-2 (40 ng). Since the LPS receptor complex consists of TLR-4 and the accessory molecule MD-2 (60), hTLR-4 transfected cells were also cotransfected with hMD2. At 24 h after transfection cells were stimulated with various potential ligands or controls in Dulbecco modified Eagle medium. At 20 h after stimulation, cell extracts were prepared for determination of the luciferase and β-galactosidase activity using chemiluminescence assays (Luciferase and β-Gal Reporter Gene Assay; Roche Diagnostics). The results are expressed as the ratio of luciferase to β-galactosidase and are presented as mean values ± the standard error of the mean (SEM) of triplicate experiments.
Animals.
C57BL/6 mice were bred under specific-pathogen-free conditions at the Bundesinstitut für Risikobewertung (Berlin, Germany). Mice were housed in filter-top cages and provided with sterile water and food ad libitum. After transfer to our animal facility, mice maintained for 3 weeks under conventional housing, ensuring similar colonization of the mucosa. TLR-2 knockout and TLR-4 knockout mice were described previously (68, 71). The use of the animals was approved by the animal care committee of the local government.
Treatment protocol.
Wild-type (wt), TLR-2 knockout, and TLR-4 knockout C57BL/6 mice (body weight, approximately 22 g) were each divided into four groups. All groups received 5% DSS (ICN Biomedicals, Inc., Meckenheim, Germany) to induce colitis. The DSS-treated control groups received either 100 μl of isotonic sterile saline by gastric gavage (n = 10) or by rectal installation (n = 10) twice daily. The treatment groups received 100 μl of E. coli Nissle 1917 (108 CFU per ml) (DSM 6601, Mutaflor; Ardeypharm) twice daily either by gastric gavage (n = 10) or by rectal installation (n = 10). Normal, non-DSS-treated wt control C57BL/6 mice received either 100 μl of isotonic sterile saline or 100 μl of 107 E. coli Nissle 1917 by gastric gavage. All treatments were performed from day 0 until day 8 when the animals were evaluated after sacrifice.
Determination of disease activity.
The well-established Rachmilewitz disease activity index (DAI) was determined by an investigator blinded to the protocol according to a standard scoring system (11, 48). The combined score of the extent of body weight loss and detection of blood in the stool is defined as follows. Loss in body weight was scored as: 0, no loss; 1, a 5 to 10% loss of body weight; 2, a 10 to 15% loss; 3, a 15 to 20% loss; and 4, a >20% loss. A fecal occult blood test (hemoCARE; Care Diagnostica, Moellersdorf, Austria) was used to screen occult blood in the stool and was scored as follows: 0, no blood; 2, positive; and 3, gross blood.
Histological grading of colitis.
Colonic tissues were removed for histological analysis and used to determine myeloperoxidase (MPO). For histological grading, the tissues were fixed in buffered 4% formalin, embedded in paraffin, cut into 5-μm slices (colon and rectum), and stained with hematoxylin and eosin. The sections were scored blindly by one investigator (A. Dankof) for histological evidence of inflammation with a scoring system described in detail previously (13). Briefly, the tissue samples were evaluated for the amount and depth of inflammation with a range of 0 to 3 and the amount of crypt damage or regeneration with a rage of 0 to 4 as indicated in Table 1.
TABLE 1.
Histological grading of colitis according to Dieleman et al. (13)
| Feature graded | Grade | Description |
|---|---|---|
| Inflammation | 0 | None |
| 1 | Slight | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Regeneration | 4 | No tissue repair |
| 3 | Surface epithelium not intact | |
| 2 | Regeneration with crypt depletion | |
| 1 | Almost complete regeneration | |
| 0 | Complete regeneration or normal tissue | |
| Crypt damage | 0 | None |
| 1 | Basal one-third damaged | |
| 2 | Basal two-thirds damaged | |
| 3 | Only surface epithelium intact | |
| 4 | Entire crypt and epithelium lost | |
| % Involvement | 1 | 1-25 |
| 2 | 26-50 | |
| 3 | 51-75 | |
| 4 | 76-100 |
Determination of MPO activity.
Colonic samples, including tissue from the middle to distal colon, were obtained from all animals. Samples were rinsed with cold phosphate-buffered saline, blotted dry, and immediately snap-frozen in liquid nitrogen. The samples were stored at −80°C, thawed for analysis, and weighed, and 50 mg of tissue was suspended in 50 mM potassium phosphate buffer (Kpi [pH 6.0], containing 0.5% hexadecyltrimethylammonium bromide [0.1 g/20 ml Kpi]) (Sigma-Aldrich) and homogenized. A 1-ml sample of the homogenate was sonicated by using a homogenizator (Sonoplus HD2070; Bandelin Electronic, Berlin, Germany) for 30 s, with continuous operation. The sample was then centrifuged at 200 × g for 10 min at 4°C. The reaction was started by mixing and incubating the supernatant (100 μl) at 20°C for 10 min with a solution composed of 2,810 μl of 50 mM Kpi, 30 μl of 20 mg/ml O-dianisidine dihydrochloride (Sigma-Aldrich), and 30 μl of 20 mM hydrogen peroxide (Roth). After 10 min the reaction was terminated by the addition of 30 μl of 2% sodium azide, and the absorbance was read at 460 nm in a spectrophotometer (ELx800 Universal Microplate Reader; Bio-Tek Instruments, Inc., Winooski, VT). The MPO activity is expressed as the amount of enzyme necessary to produce a change in absorbance of 1.0 per min per g of tissue (wet weight).
Flow cytometric analysis.
For isolation of peripheral blood human or murine T lymphocytes (PBT), peripheral blood mononuclear cells (PBMC) from healthy volunteers or mice were isolated from heparinized venous blood by using Ficoll-Hypaque density gradients. PBMC were then incubated for 30 min at 4°C with magnetically labeled CD19, CD14, and CD16 antibodies directed against B lymphocytes, monocytes, and neutrophils, respectively (Miltenyi Biotec, Inc., Bergisch-Gladbach, Germany). T cells were then collected by using a magnetic cell sorting system (MACS; Miltenyi Biotec). For surface staining (TLR expression), human PBT were stimulated with anti-human CD3 (OKT3; 10 μg/ml) and CD28 MAb (5 μg/ml) in the presence or absence of 40% (vol/vol) E. coli Nissle 1917-conditioned medium. After 48 h, the cells were collected, washed, and stained with FITC-labeled TLR-2 and TLR-4 MAbs or with isotype-matched nonspecific mouse MAb (Dako) for 30 min at 4°C. Cells were then washed twice with flow buffer, fixed in 1% paraformaldehyde, and analyzed by using a single laser flow cytometer (FACSCalibur; Becton Dickinson, California) using CellQuest software (BD Pharmingen). Each analysis was performed on at least 10,000 events. To measure cytokine secretion, 105 murine T cells were isolated and cultured in complete medium in the presence of stimulating anti-murine CD3 (clone 145-2C11; 10 μg/ml) and CD28 (5 μg/ml) MAbs at 37°C and 5% CO2. After 72 h, the supernatants were collected, and cytokine secretion was determined by a cytometric bead array performed according to the manufacturer's instructions (BD Pharmingen). Briefly, bead populations with distinct fluorescence intensities, coated with capture antibody proteins, were mixed with PE-conjugated detection antibodies, and recombinant standards or test samples were incubated to form sandwich complexes. After acquisition of sample data using flow cytometry, the cytokine concentrations were calculated by using the BD CBA analysis software.
Statistical analysis.
Data are expressed as means ± the SEM. Statistical analysis for significant differences was performed by using analysis of variance, the Student t test for parametric samples, and the Mann-Whitney rank sum test for nonparametric samples (SigmaStat, version 2.03; Sigma, Chicago, IL).
RESULTS
E. coli Nissle 1917 increases TLR-2 and TLR-4 expression.
We have previously demonstrated that E. coli Nissle 1917 increases the number of γ/δ- positive T cells (64). γ/δ T cells have a protective role in various animal models of chronic inflammation (34), and γ/δ T cells are known to express TLR-2 mRNA. Furthermore, TLR-2 knockout mice show an impaired increase of γ/δ T cells after exposure to native lipid A (38, 51). We therefore assessed the expression of TLR-2 and TLR-4 in PBT in response to E. coli Nissle 1917. As determined by flow cytometric analysis, the mean fluorescence intensity of TLR-2 expression on PBT before coculture with E. coli Nissle 1917 was lower (52.6 ± 6.3) than in the group treated with 40% (vol/vol) E. coli Nissle 1917-conditioned medium (165.3 ± 18.1) (P < 0.01). To a lesser extent, but also statistically significant, the mean fluorescence intensity of TLR-4 expression on PBT before coculture with E. coli Nissle 1917 was lower (48.3 ± 5.9) than in the group treated with 40% (vol/vol) E. coli Nissle 1917-conditioned medium (91.1 ± 8.6) (P < 0.05).
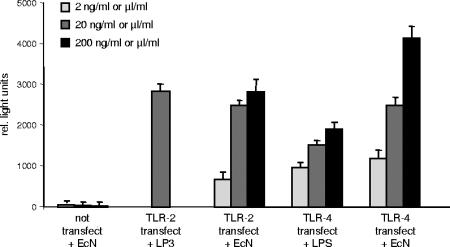
We next explored whether E. coli Nissle 1917-conditioned medium utilizes the TLR pathway. We therefore transfected TLR-2- and TLR-4-negative HEK 293 cells with plasmids encoding TLR-2 and TLR-4 and measured NF-κB activation after exposure to E. coli Nissle 1917-conditioned medium. As depicted in Fig. 1, E. coli Nissle 1917 supernatants induced a significant dose-dependent increased in NF-κB activity in TLR-2- and TLR-4-transfected cells, suggesting a TLR-2- and TLR-4-dependent activation of NF-κB by E. coli Nissle 1917. Lipoprotein (LP3), a known substrate for TLR-2 and LPS, a well-known stimulator of TLR-4, were used as controls.
FIG. 1.
E. coli Nissle 1917-conditioned medium increases NF-κB activity via TLR-2- and TLR-4-dependent pathways. After TLR-2 and TLR-4 transfection, E. coli Nissle 1917-conditioned medium increases NF-κB activity. HEK 293 cells were transiently transfected as described in Materials and Methods. At 24 h after transfection cells were stimulated with LP3 or LPS (concentration in ng/ml) or E. coli Nissle 1917 supernatants (EcN) (concentration in μl/ml) or controls in Dulbecco modified Eagle medium. At 20 h after stimulation, cell extracts were prepared for determination of luciferase activity by using a Luciferase Reporter Gene Assay. LP3 was used at 20 ng/ml only. Each bar represents mean ± the SEM value of three separate experiments.
E. coli Nissle 1917 ameliorates DSS-induced colitis only in wt mice and not in TLR-2 and TLR-4 knockout mice.
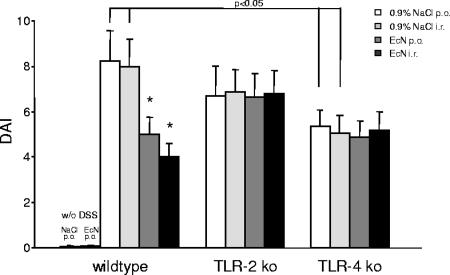
Acute colitis was induced in wt, TLR-2 knockout, and TLR-4 knockout mice via administration of 5% DSS in the drinking water. Orally treated animals received either sterile physiological saline as DSS-treated control or 100 μl of 107 E. coli Nissle 1917 twice daily by gastric gavage from day 0 until they were sacrificed on day 8. The rectally treated animals received either sterile saline as DSS-treated control group or 100 μl of 107 E. coli Nissle 1917 twice daily by rectal instillation from day 0 until they were sacrificed by day 8. As evaluated with the well-known Rachmilewitz DAI (11, 47, 49), wt and TLR-2 mice exposed to 5% DSS developed symptoms of acute colitis of comparable degree, with diarrhea observed first, followed by rectal bleeding and substantial weight loss (Fig. 2). In contrast, in TLR-4 knockout mice the DSS application induced significantly fewer symptoms of colitis compared to wt and TLR-2 knockout mice (P < 0.05) (Fig. 2). In wt mice both the oral and rectal administration of 107 E. coli Nissle 1917 clearly attenuated the severity of DSS-induced colitis, as evaluated with the DAI, compared to saline-treated control mice (Fig. 2). In contrast, when colitis was induced by DSS in TLR-2 knockout or TLR-4 knockout mice, E. coli Nissle 1917 failed to improve the experimental colitis (Fig. 2). Accordingly, in wt mice, but not in TLR-2 or TLR-4 knockout mice, E. coli Nissle 1917 treatment resulted in a longer colon length compared to saline treatment (data not shown).
FIG. 2.
Distinct effect of DSS application and E. coli Nissle 1917 treatment in wt mice and in TLR-2 and TLR-4 knockout mice. In TLR-4 knockout mice, DSS application induced significantly fewer symptoms of colitis compared to wt or TLR-2 knockout mice. wt mice, but not TLR-2 knockout or TLR-4 knockout mice, treated with E. coli Nissle 1917 showed a significant clinical improvement in disease activity, as evaluated with the DAI, compared to mice treated with 0.9% NaCl. Colitis was induced by DSS application in wt mice, TLR-2 knockout mice, and TLR-4 knockout mice. Mice were subsequently treated with an oral or rectal application of 107 E. coli Nissle 1917 (100 μl) or 0.9% NaCl (100 μl) twice daily for 8 days. After sacrifice, the DAI was determined as described in Materials and Methods. Each bar represents mean ± the SEM value of 10 animals. *, P < 0.05 for differences in E. coli Nissle 1917-treated groups versus saline-treated animals.
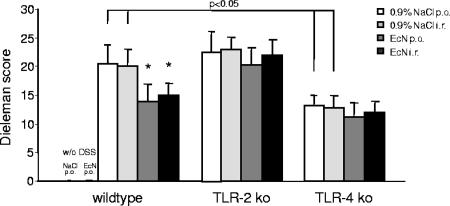
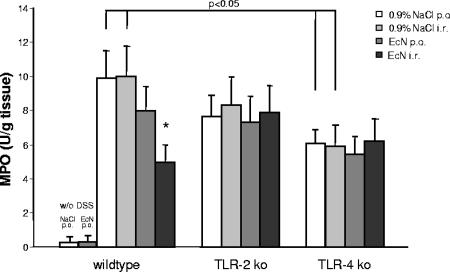
Consistent with the results of body weight, DAI scores, and colon length, histological assessment of colonic tissues using the well-established Dieleman score (13), previously described by Cooper et al. (11), revealed that the colons from DSS-induced wt and TLR-2 knockout mice contained more severe ulceration and inflammatory cell infiltrate than the colons from TLR-4 knockout mice (Fig. 3 and 4). In wt animals, oral or rectal treatment with 107 E. coli Nissle 1917 significantly reduced mucosal damage and facilitated mucosal repair compared to control treated wt mice (Fig. 3 and 4). Again, in contrast to wt mice, the degree of extensive superficial ulcerations and mucosal inflammatory reaction induced by DSS was not reduced by E. coli Nissle 1917 treatment in TLR-2 or TLR-4 knockout animals (Fig. 3 and 4). The distinct modulation of the DAI and histological damage was confirmed by a significant reduction in MPO activity in the colonic mucosa of wt mice treated rectally with 107 E. coli Nissle 1917 compared to DSS-treated control animals but not with TLR-2 knockout or TLR-4 knockout mice (Fig. 5).
FIG. 3.
Differential effect of E. coli Nissle 1917 treatment on inflammation and regeneration in wt mice but not in TLR-2 or TLR-4 knockout mice. In TLR-4 knockout mice DSS application induced significantly less inflammation compared to wt or TLR-2 knockout mice. wt mice, but not TLR-2 knockout or TLR-4 knockout mice, treated with E. coli Nissle 1917 showed significantly less inflammation or crypt damage and more regeneration, as evaluated with the Dieleman index, compared to mice treated with 0.9% NaCl. Colitis was induced by DSS application in wt mice, TLR-2 knockout mice, and TLR-4 knockout mice. Mice were subsequently treated with an oral or rectal application of 107 E. coli Nissle 1917 (100 μl) or 0.9% NaCl (100 μl) twice daily for 8 days. After sacrifice, tissue samples were obtained from the distal 5 cm of the colon and prepared for histological evaluation as described in Materials and Methods. Each bar represents mean ± the SEM value of 10 animals. *, P < 0.05 for differences in E. coli Nissle 1917-treated groups versus saline-treated animals.
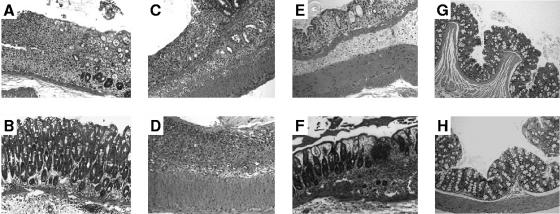
FIG. 4.
Differential effect of E. coli Nissle 1917 treatment on mucosal damage in wt mice but not in TLR-2 or TLR-4 knockout mice. wt (A) and TLR-2 knockout (C) mice exposed to 5% DSS developed symptoms of acute colitis to a comparable degree, with a patchy pattern of severe mucosal damage characterized by a loss of crypts, necrosis, and the local influx of inflammatory cells into the lamina propria. (E) In contrast, in TLR-4 knockout mice, DSS application induced significantly fewer symptoms of colitis compared to wt and TLR-2 knockout mice. E. coli Nissle 1917 treatment ameliorated the severity of DSS-induced colitis in wt mice (B) but not in TLR-2 knockout (D) or TLR-4 knockout (F) mice. DSS colitis was induced as described in Materials and Methods, and mice were treated orally with 100 μl of 0.9% NaCl (A, C, and E) or 100 μl of 107 E. coli Nissle 1917 (B, D, and F). Control, non-DSS-treated wt C57BL/6 mice received, by gastric gavage, either 100 μl of isotonic sterile saline (G) or 100 μl of 107 E. coli Nissle 1917 (H). After sacrifice at day 8, tissue samples were obtained from the distal 5 cm of the colon and prepared for histological evaluation as described in Materials and Methods (hematoxylin and eosin stain; original magnifications, ×100).
FIG. 5.
Distinct effect of DSS application and E. coli Nissle 1917 treatment on MPO activity in wt, TLR-2 knockout, and TLR-4 knockout mice. In TLR-4 knockout mice, DSS application induced significantly less MPO activity in colonic tissue compared to wt or TLR-2 knockout mice. wt mice, but not TLR-2 knockout or TLR-4 knockout mice, treated rectally with 107 E. coli Nissle 1917 showed a significant decrease in MPO activity compared to mice treated with 0.9% NaCl. Colitis was induced by DSS application in wt, TLR-2 knockout, and TLR-4 knockout mice. Mice were subsequently treated with an oral or rectal application of 107 E. coli Nissle 1917 (100 μl) or 0.9% NaCl (100 μl) for 8 days. After sacrifice, the MPO activity was determined as described in Materials and Methods. Each bar represents mean ± the SEM value of 10 animals. *, P < 0.05 for differences in E. coli Nissle 1917-treated groups versus saline-treated animals.
Modulation of cytokine secretion by E. coli Nissle 1917 in wt, TLR-2 knockout, and TLR-4 knockout mice.
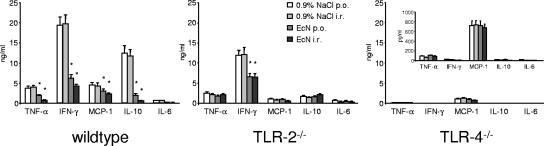
Cytokines are crucial for the proliferation and differentiation of T cells. Having demonstrated that E. coli Nissle 1917 substantially ameliorated DSS-induced colitis in wt mice, we isolated T cells from the peripheral blood of wt, TLR-2 knockout, and TLR-4 knockout animals treated with physiological saline or 107 E. coli Nissle 1917 twice daily and stimulated the cells for 3 days with anti-CD3 MAb. Then, the levels of TNF-α, IFN-γ, MCP-1, and IL-6 secretion were determined by cytometric bead array analysis. In wt animals, E. coli Nissle 1917 treatment significantly decreased the secretion of TNF-α, IFN-γ, MCP-1, and IL-10 (P < 0.01) (Fig. 6). In contrast, in TLR-2 knockout mice, the secretion of TNF-α, MCP-1, and IL-10 was remarkably reduced compared to wt animals and was not modulated by E. coli Nissle 1917 treatment. In these mice, only IFN-γ secretion was still elevated in saline-treated controls and could be reduced by both oral and rectal administration of E. coli Nissle 1917 (Fig. 6). In TLR-4 knockout mice, TNF-α, IFN-γ, IL-6, and IL-10 secretion was nearly completely abolished, and MCP-1 secretion was dramatically reduced compared to wt animals. In TLR-4 knockout mice, no differences in cytokine secretion between saline controls and E. coli Nissle 1917-treated animals could be detected (Fig. 6).
FIG. 6.
Differential cytokine secretion in wt, TLR-2 knockout, and TLR-4 knockout mice. In wt animals, E. coli Nissle 1917 treatment decreased the secretion of TNF-α, IFN-γ, MCP-1, and IL-10. In TLR-2 knockout mice, only IFN-γ secretion was still high in control animals and reduced by E. coli Nissle 1917, whereas the secretion of TNF-α, MCP-1, and IL-10 was reduced compared to wt controls and not altered by E. coli Nissle 1917 treatment. In TLR-4 knockout mice, TNF-α, IFN-γ, IL-6, and IL-10 secretion was nearly abolished, and MCP-1 secretion was dramatically reduced compared to wt animals. A total of 105 freshly isolated PBT from wt, TLR-2 knockout, and TLR-4 knockout mice, treated orally or rectally with 0.9% NaCl or 107 E. coli Nissle 1917, were cultured for 3 days in the presence of anti-CD3 and CD28 MAbs. Supernatants were then collected, and cytokine secretion was analyzed by using a cytometric bead array assay. Each bar represents the mean ± the SEM value of 10 animals. *, P < 0.05 for differences in E. coli Nissle 1917-treated groups versus saline-treated animals.
DISCUSSION
The innate immune system has evolved to perform recognition of conserved molecular structures of microorganism referred to as pathogen-associated molecular patterns. Pathogen-associated molecular patterns are recognized by corresponding receptors of the innate immune system, the so-called pattern recognition receptors (2). TLRs are members of the pattern recognition receptor family and are characterized by an extracellular domain with leucine-rich repeats and an intracellular domain homologous to the Toll/IL-1R. TLR-2 is the principal receptor for bacterial lipopeptides, and TLR-4 is the main receptor for LPS (67). In the murine gut, TLR-2 and TLR-4 are expressed in a compartmentalized manner and are upregulated during DSS-induced colitis (42, 70). TLR-4 polymorphisms are associated with Crohn's disease and ulcerative colitis (40), which makes it evident that alterations of the TLR expression and signaling are critically involved in the pathogenesis of IBD.
In IBD, T cells expand in an uncontrolled fashion, causing mucosal inflammation (63). We have previously demonstrated that E. coli Nissle 1917 supernatants inhibit T-cell cycling and expansion via the TLR-2 receptor pathway (64). Together with the recent identification of TLRs on T-cell populations, it became evident that TLRs not only recognize specific patterns of microbial compounds but also regulate both the innate and the adaptive immune systems (24).
In the present study, we demonstrated that the culture of activated T cells with E. coli Nissle 1917 supernatants increases TLR-2 and TLR-4 protein expression on the surface of PBMC, demonstrating a bacterial control of TLR-2 and TLR-4 expression in PBMC to increase its influence on cell function. Otherwise, E. coli Nissle 1917 would, by downregulating TLR expression, limit its own effects, which seems to be counterproductive. Recently, by demonstrating that mucosa-associated bacteria upregulate the expression of TLRs 1 to 4 in epithelial cells, the ability of bacteria to increase TLR expression was confirmed by other groups (16).
Activation of signaling through TLRs results in recruitment of the cytoplasmic adaptor molecule MyD88, activation of serine/threonine kinases of the IRAK family, and ultimately the degradation of IκB and translocation of NF-κB to the nucleus (24, 56). After transfection of HEK 293 cells with TLR-2 or with TLR-4 and MD2, we found that E. coli Nissle 1917 supernatants activated NF-κB translocation, demonstrating that NF-κB is activated by E. coli Nissle 1917 via TLR-2- and TLR-4-dependent pathways.
When acute colitis was induced by DSS application, wt and TLR-2 knockout mice developed significantly more symptoms of acute colitis than TLR-4 knockout mice, indicating that TLR-4 expression is required to fully develop mucosal damage in the DSS-induced model of acute colitis. Recently, it has been demonstrated that MyD88 knockout, TLR-2 knockout, and TLR-4 knockout mice exhibit increased susceptibility to DSS-induced colitis, as reflected by significantly higher lethality and higher clinical and histological scores compared to wt mice (1, 50). However, in contrast to our study design, no macroscopic or microscopic signs of intestinal inflammation were observed in their wt controls that were treated with low concentrations of 1.2 or 2% DSS in the drinking water (1, 50). Furthermore, the animals used in the study by Rakoff-Nahoum et al. (50) differed in genetic background (these authors used F2 generations from 129/SvJ × C57BL/6 crosses) and were bred and maintained under specific-pathogen-free conditions (1, 50). Since the presence of the intestinal microflora is required in most models of experimental colitis to obtain mucosal inflammation (6, 9), we maintained our mice in non-pathogen-free conventional housing conditions. Thus, although confirming the important link between the innate immune system and TLRs, the different study designs make it difficult to compare the two studies.
E. coli Nissle 1917 is used as therapy for ulcerative colitis and pouchitis; however, the underlying effects are still elusive. Schultz and coworkers demonstrated that orally administered E. coli Nissle 1917 reduces the secretion of IFN-γ and IL-6 in DSS-treated mice compared to DSS-treated controls but did not ameliorate mucosal inflammation (59). In contrast, in the CD4+ CD62L+ transfer model of chronic colitis, E. coli Nissle 1917 was found to reduce the secretion of proinflammatory cytokines and to ameliorate intestinal inflammation as well (59). More recently, Kamada et al. (26) reported that the oral application of E. coli Nissle 1917 to DSS-treated mice significantly ameliorated body weight loss, DAI scores, and macroscopic and microscopic damage of the gut mucosa. Our data are in accordance with those of Kamada et al., demonstrating that orally administrated E. coli Nissle 1917 ameliorates macroscopic and microscopic disease activity in C57BL/6 mice. In addition, we expanded this observation by showing that the rectal application of E. coli Nissle 1917 is even more efficient at ameliorating DSS-induced colitis. Modulation of disease activity by E. coli Nissle 1917 is not necessarily a specific effect of this bacterial strain. It may be speculated that other E. coli strains exert comparable effects in this model; however, E. coli Nissle 1917 is currently the only E. coli strain fulfilling safety requirements needed to treat humans. Therefore, from a therapeutic point of view, it does not seem relevant to evaluate the effect of a nonprobiotic E. coli strain in a therapeutic model of IBD. Interestingly, in contrast to wt mice, E. coli Nissle 1917 lost its protective function and did not ameliorate the macroscopic DAI score, the microscopic inflammation, or the recruitment of neutrophils into the intestinal mucosa in TLR-2 knockout or TLR-4 knockout mice.
Mucosal inflammation is initiated and perpetuated by the secretion of proinflammatory and chemotactic cytokines, and potent anti-inflammatory drugs are able to inhibit their release (3, 4). E. coli Nissle 1917 given orally or rectally to wt mice, significantly reduced the secretion of the proinflammatory antigen-specific T helper type 1 (Th1)-derived cytokines TNF-α and IFN-γ, as well as the chemokine MCP-1. There was a corresponding decrease in neutrophil infiltration and reduced MPO activity in the intestinal mucosa that confirmed the biological relevance of this finding. MyD88 knockout mice have a profound defect in the activation of the Th1 but not the Th2 immune response (56). However, the role of distinct TLRs is not completely explored yet, since it has been demonstrated by others that TLR-2 ligands are adjuvants for human Th1 responses (61). Using TLR-2 knockout mice, we demonstrated that, despite the lack of TLR-2, IFN-γ secretion still takes place and is modulated by E. coli Nissle 1917. In contrast, MCP-1 secretion was abolished in these mice, and TNF-α and IL-10 secretion was low and not modulated by E. coli Nissle 1917. Distinct from TLR-2 knockout mice, cytokine secretion was also altered in TLR-4 knockout mice in which TNF-α, IFN-γ, and IL-10 secretion was not detectable, and MCP-1 secretion five times lower than in wt animals. Our data thus confirm the important role of TLR signaling in the activation of Th1 immune response and support the notion that innate immune recognition is generally required for the initiation of adaptive immune responses. Furthermore, we provide evidence that the defects in cytokine secretion and responsiveness toward microbial patterns are distinct between TLR-2 and TLR-4 knockout mice.
In conclusion, our data provide clear evidence that E. coli Nissle 1917 ameliorates experimental colitis and that TLR-4 signaling is involved in the development of DSS-induced colitis in mice. E. coli Nissle 1917 modulates T-cell expansion and cytokine release by T cells through TLR-2 and TLR-4-dependent pathways, providing strong evidence that innate and adaptive immune responses are functionally linked.
Acknowledgments
This study was financially supported by the Deutsche Forschungsgemeinschaft (STU247/3-1 and 247/3-2 to A.S.); Ardeypharm GmbH, Herdecke, Germany; and the Charité Medical School, Berlin, Germany.
We thank Arturo Zychlinsky for helpful discussion and critical reading of the manuscript and Annett Rexin for technical assistance.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Araki, A., T. Kanai, T. Ishikura, S. Makita, K. Uraushihara, R. Iiyama, T. Totsuka, K. Takeda, S. Akira, and M. Watanabe. 2005. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterol. 40:16-23. [DOI] [PubMed] [Google Scholar]
- 2.Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 300:1524-1525. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart, D. C., and A. U. Dignass. 2004. Current biological therapies for inflammatory bowel disease. Curr. Pharm. Des. 10:4127-4147. [DOI] [PubMed] [Google Scholar]
- 4.Baumgart, D. C., B. Wiedenmann, and A. U. Dignass. 2003. Rescue therapy with tacrolimus is effective in patients with severe and refractory inflammatory bowel disease. Aliment. Pharmacol. Ther. 17:1273-1281. [DOI] [PubMed] [Google Scholar]
- 5.Beutler, B. 2002. TLR-4 as the mammalian endotoxin sensor. Curr. Top. Microbiol. Immunol. 270:109-120. [DOI] [PubMed] [Google Scholar]
- 6.Bhan, A. K., E. Mizoguchi, R. N. Smith, and A. Mizoguchi. 1999. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol. Rev. 169:195-207. [DOI] [PubMed] [Google Scholar]
- 7.Blum, G., R. Marre, and J. Hacker. 1995. Properties of Escherichia coli strains of serotype O6. Infection 23:234-236. [DOI] [PubMed] [Google Scholar]
- 8.Blum-Oehler, G., S. Oswald, K. Eiteljorge, U. Sonnenborn, J. Schulze, W. Kruis, and J. Hacker. 2003. Development of strain-specific PCR reactions for the detection of the probiotic Escherichia coli strain Nissle 1917 in fecal samples. Res. Microbiol. 154:59-66. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg, R. S., L. J. Saubermann, and W. Strober. 1999. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr. Opin. Immunol. 11:648-656. [DOI] [PubMed] [Google Scholar]
- 10.Cario, E. 2005. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut 54:1182-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper, H. S., S. N. Murthy, R. S. Shah, and D. J. Sedergran. 1993. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 69:238-249. [PubMed] [Google Scholar]
- 12.Danese, S., M. Sans, and C. Fiocchi. 2004. Inflammatory bowel disease: the role of environmental factors. Autoimmun. Rev. 3:394-400. [DOI] [PubMed] [Google Scholar]
- 13.Dieleman, L. A., M. J. Palmen, H. Akol, E. Bloemena, A. S. Pena, S. G. Meuwissen, and E. P. Van Rees. 1998. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114:385-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duchmann, R., I. Kaiser, E. Hermann, W. Mayet, K. Ewe, and K. H. Meyer zum Buschenfelde. 1995. Tolerance exists toward resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin. Exp. Immunol. 102:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiocchi, C. 1998. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115:182-205. [DOI] [PubMed] [Google Scholar]
- 16.Furrie, E., S. Macfarlane, G. Thomson, and G. T. Macfarlane. 2005. Toll-like receptors-2, -3, and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology 115:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gionchetti, P., C. Amadini, F. Rizzello, A. Venturi, and M. Campieri. 2002. Treatment of mild to moderate ulcerative colitis and pouchitis. Aliment. Pharmacol. Ther. 16(Suppl. 4):13-19. [DOI] [PubMed] [Google Scholar]
- 18.Gionchetti, P., F. Rizzello, A. Venturi, P. Brigidi, D. Matteuzzi, G. Bazzocchi, G. Poggioli, M. Miglioli, and M. Campieri. 2000. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119:305-309. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg, G. R. 2004. Antibiotics should be used as first-line therapy for Crohn's disease. Inflamm. Bowel. Dis. 10:318-320. [DOI] [PubMed] [Google Scholar]
- 20.Grozdanov, L., C. Raasch, J. Schulze, U. Sonnenborn, G. Gottschalk, J. Hacker, and U. Dobrindt. 2004. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 186:5432-5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grozdanov, L., U. Zahringer, G. Blum-Oehler, L. Brade, A. Henne, Y. A. Knirel, U. Schombel, J. Schulze, U. Sonnenborn, G. Gottschalk, J. Hacker, E. T. Rietschel, and U. Dobrindt. 2002. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J. Bacteriol. 184:5912-5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guslandi, M. 2005. Antibiotics for inflammatory bowel disease: do they work? Eur. J. Gastroenterol. Hepatol. 17:145-147. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann, J. C., N. N. Pawlowski, A. A. Kuhl, W. Hohne, and M. Zeitz. 2002. Animal models of inflammatory bowel disease: an overview. Pathobiology 70:121-130. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 25.Jain, P. K., C. E. McNaught, A. D. Anderson, J. MacFie, and C. J. Mitchell. 2004. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clin. Nutr. 23:467-475. [DOI] [PubMed] [Google Scholar]
- 26.Kamada, N., N. Inoue, T. Hisamatsu, S. Okamoto, K. Matsuoka, T. Sato, H. Chinen, K. S. Hong, T. Yamada, Y. Suzuki, T. Suzuki, N. Watanabe, K. Tsuchimoto, and T. Hibi. 2005. Nonpathogenic Escherichia coli strain Nissle1917 prevents murine acute and chronic colitis. Inflamm. Bowel. Dis. 11:455-463. [DOI] [PubMed] [Google Scholar]
- 27.Kirschning, C. J., and R. R. Schumann. 2002. TLR-2: cellular sensor for microbial and endogenous molecular patterns. Curr. Top. Microbiol. Immunol. 270:121-144. [DOI] [PubMed] [Google Scholar]
- 28.Klaenhammer, T. R. 1988. Bacteriocins of lactic acid bacteria. Biochimie 70:337-349. [DOI] [PubMed] [Google Scholar]
- 29.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 30.Kraus, T. A., L. Toy, L. Chan, J. Childs, and L. Mayer. 2004. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology 126:1771-1778. [DOI] [PubMed] [Google Scholar]
- 31.Kruis, W. 2004. Review article: antibiotics and probiotics in inflammatory bowel disease. Aliment. Pharmacol. Ther. 20(Suppl. 4):75-78. [DOI] [PubMed] [Google Scholar]
- 32.Kruis, W., P. Fric, J. Pokrotnieks, M. Lukas, B. Fixa, M. Kascak, M. A. Kamm, J. Weismueller, C. Beglinger, M. Stolte, C. Wolff, and J. Schulze. 2004. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 53:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruis, W., E. Schutz, P. Fric, B. Fixa, G. Judmaier, and M. Stolte. 1997. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 11:853-858. [DOI] [PubMed] [Google Scholar]
- 34.Kuhl, A. A., C. Loddenkemper, J. Westermann, and J. C. Hoffmann. 2002. Role of gamma delta T cells in inflammatory bowel disease. Pathobiology 70:150-155. [DOI] [PubMed] [Google Scholar]
- 35.Lammers, K. M., A. Vergopoulos, N. Babel, P. Gionchetti, F. Rizzello, C. Morselli, E. Caramelli, M. Fiorentino, A. d'Errico, H. D. Volk, and M. Campieri. 2005. Probiotic therapy in the prevention of pouchitis onset: decreased interleukin-1β, interleukin-8, and interferon-gamma gene expression. Inflamm. Bowel. Dis. 11:447-454. [DOI] [PubMed] [Google Scholar]
- 36.Malchow, H. A. 1997. Crohn's disease and Escherichia coli: a new approach in therapy to maintain remission of colonic Crohn's disease? J. Clin. Gastroenterol. 25:653-658. [DOI] [PubMed] [Google Scholar]
- 37.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 38.Mokuno, Y., T. Matsuguchi, M. Takano, H. Nishimura, J. Washizu, T. Ogawa, O. Takeuchi, S. Akira, Y. Nimura, and Y. Yoshikai. 2000. Expression of Toll-like receptor 2 on gamma delta T cells bearing invariant V gamma 6/V delta 1 induced by Escherichia coli infection in mice. J. Immunol. 165:931-940. [DOI] [PubMed] [Google Scholar]
- 39.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1998. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect. Immun. 66:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oostenbrug, L. E., J. P. Drenth, D. J. de Jong, I. M. Nolte, E. Oosterom, H. M. van Dullemen, L. K. van der, G. J. Te Meerman, S. G. van der, J. H. Kleibeuker, and P. L. Jansen. 2005. Association between Toll-like receptor 4 and inflammatory bowel disease. Inflamm. Bowel Dis. 11:567-575. [DOI] [PubMed] [Google Scholar]
- 41.Opitz, B., N. W. Schroder, I. Spreitzer, K. S. Michelsen, C. J. Kirschning, W. Hallatschek, U. Zahringer, T. Hartung, U. B. Gobel, and R. R. Schumann. 2001. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-κB translocation. J. Biol. Chem. 276:22041-22047. [DOI] [PubMed] [Google Scholar]
- 42.Ortega-Cava, C. F., S. Ishihara, M. A. Rumi, K. Kawashima, N. Ishimura, H. Kazumori, J. Udagawa, Y. Kadowaki, and Y. Kinoshita. 2003. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J. Immunol. 170:3977-3985. [DOI] [PubMed] [Google Scholar]
- 43.O'Sullivan, G. C., P. Kelly, S. O'Halloran, C. Collins, J. K. Collins, C. Dunne, and F. Shanahan. 2005. Probiotics: an emerging therapy. Curr. Pharm. Des. 11:3-10. [DOI] [PubMed] [Google Scholar]
- 44.Pasare, C., and R. Medzhitov. 2005. Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 560:11-18:11-18. [DOI] [PubMed] [Google Scholar]
- 45.Powrie, F., and M. W. Leach. 1995. Genetic and spontaneous models of inflammatory bowel disease in rodents: evidence for abnormalities in mucosal immune regulation. Ther. Immunol. 2:115-123. [PubMed] [Google Scholar]
- 46.Qin, H. L., T. Y. Shen, Z. G. Gao, X. B. Fan, X. M. Hang, Y. Q. Jiang, and H. Z. Zhang. 2005. Effect of lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. World J. Gastroenterol. 11:2591-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rachmilewitz, D., F. Karmeli, E. Okon, and M. Bursztyn. 1995. Experimental colitis is ameliorated by inhibition of nitric oxide synthesis. Gastroenterology 108:A897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rachmilewitz, D., F. Karmeli, K. Takabayashi, T. Hayashi, L. Leider-Trejo, J. Lee, L. M. Leoni, and E. Raz. 2002. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 122:1428-1441. [DOI] [PubMed] [Google Scholar]
- 49.Rachmilewitz, D., K. Katakura, F. Karmeli, T. Hayashi, C. Reinus, B. Rudensky, S. Akira, K. Takeda, J. Lee, K. Takabayashi, and E. Raz. 2004. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126:520-528. [DOI] [PubMed] [Google Scholar]
- 50.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118:229-241. [DOI] [PubMed] [Google Scholar]
- 51.Reid, G., J. Howard, and B. S. Gan. 2001. Can. bacterial interference prevent infection? Trends Microbiol. 9:424-428. [DOI] [PubMed] [Google Scholar]
- 52.Reid, G., J. Jass, M. T. Sebulsky, and J. K. McCormick. 2003. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 16:658-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rembacken, B. J., A. M. Snelling, P. M. Hawkey, D. M. Chalmers, and A. T. Axon. 1999. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635-639. [DOI] [PubMed] [Google Scholar]
- 54.Rosenfeldt, V., E. Benfeldt, N. H. Valerius, A. Paerregaard, and K. F. Michaelsen. 2004. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 145:612-616. [DOI] [PubMed] [Google Scholar]
- 55.Sartor, R. B. 2004. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126:1620-1633. [DOI] [PubMed] [Google Scholar]
- 56.Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 57.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 58.Schultz, M., J. Scholmerich, and H. C. Rath. 2003. Rationale for probiotic and antibiotic treatment strategies in inflammatory bowel diseases. Dig. Dis. 21:105-128. [DOI] [PubMed] [Google Scholar]
- 59.Schultz, M., U. G. Strauch, H. J. Linde, S. Watzl, F. Obermeier, C. Gottl, N. Dunger, N. Grunwald, J. Scholmerich, and H. C. Rath. 2004. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin. Diagn. Lab. Immunol. 11:372-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sieling, P. A., W. Chung, B. T. Duong, P. J. Godowski, and R. L. Modlin. 2003. Toll-like receptor 2 ligands as adjuvants for human Th1 responses. J. Immunol. 170:194-200. [DOI] [PubMed] [Google Scholar]
- 62.Sturm, A. 2002. Probiotika bei chronisch entzündlichen Darmerkrankungen, p. 121-126. In A. Dignass and J. Stein (ed.), Chronisch entzündliche Darmerkrankungen. Springer, Berlin, Germany.
- 63.Sturm, A., A. Z. Leite, S. Danese, K. A. Krivacic, G. A. West, S. Mohr, J. W. Jacobberger, and C. Fiocchi. 2004. Divergent cell cycle kinetics underlie the distinct functional capacity of mucosal T cells in Crohn's disease and ulcerative colitis. Gut 53:1624-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sturm, A., K. Rilling, D. C. Baumgart, K. Gargas, T. Abou-Ghazale, B. Raupach, J. Eckert, R. R. Schumann, C. Enders, U. Sonnenborn, B. Wiedenmann, and A. U. Dignass. 2005. Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via Toll-like receptor 2 signaling. Infect. Immun. 73:1452-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun, J., F. Gunzer, A. M. Westendorf, J. Buer, M. Scharfe, M. Jarek, F. Gossling, H. Blocker, and A. P. Zeng. 2005. Genomic peculiarity of coding sequences and metabolic potential of probiotic Escherichia coli strain Nissle 1917 inferred from raw genome data. J. Biotechnol. 117:147-161. [DOI] [PubMed] [Google Scholar]
- 66.Swidsinski, A., A. Ladhoff, A. Pernthaler, S. Swidsinski, V. Loening-Baucke, M. Ortner, J. Weber, U. Hoffmann, S. Schreiber, M. Dietel, and H. Lochs. 2002. Mucosal flora in inflammatory bowel disease. Gastroenterology 122:44-54. [DOI] [PubMed] [Google Scholar]
- 67.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 68.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR-2 and TLR-4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 69.Tamboli, C. P., C. Neut, P. Desreumaux, and J. F. Colombel. 2004. Dysbiosis in inflammatory bowel disease. Gut 53:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Underhill, D. M., and A. Ozinsky. 2002. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 14:103-110. [DOI] [PubMed] [Google Scholar]
- 71.Weiss, D. S., B. Raupach, K. Takeda, S. Akira, and A. Zychlinsky. 2004. Toll-like receptors are temporally involved in host defense. J. Immunol. 172:4463-4469. [DOI] [PubMed] [Google Scholar]
- 72.Yan, F., and D. B. Polk. 2002. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J. Biol. Chem. 277:50959-50965. [DOI] [PMC free article] [PubMed] [Google Scholar]