Abstract
Lyme disease, a global health concern, is caused by infection with Borrelia burgdorferi, B. afzelii, or B. garinii. The spirochete responsible for the disease in the United States is B. burgdorferi and is spread by the bite of an infected Ixodes tick. We utilized multiple two-dimensional gel techniques combined with proteomics to reveal the full humoral immune response of mice and Lyme patients to membrane-associated proteins isolated from Borrelia burgdorferi. Our studies indicated that a subset of immunogenic membrane-associated proteins (some new and some previously identified) was recognized by mice experimentally infected with Borrelia burgdorferi either by low-dose needle inoculation or by tick infestation. Moreover, the majority of these immunogenic membrane-associated proteins were recognized by sera from patients diagnosed with early-disseminated Lyme disease. These included RevA, ErpA, ErpP, DbpA, BmpA, FtsZ, ErpB, LA7, OppA I, OppA II, OppA IV, FlhF, BBA64, BBA66, and BB0323. Some immunogens (i.e., BBI36/38) were more reactive with sera from mice than Lyme patients, while additional membrane proteins (i.e., FlaB, P66, LA7, and Hsp90) were recognized more strongly with sera from patients diagnosed with early-localized, early-disseminated, or late (chronic)-stage Lyme disease. We were able to examine the humoral response in Lyme patients in a temporal fashion and to identify the majority of immunoreactive proteins as the disease progresses from early to late stages. This serologic proteome analysis enabled the identification of novel membrane-associated proteins that may serve as new diagnostic markers and, more importantly, as second-generation vaccine candidates for protection against Lyme disease.
Borrelia burgdorferi is the causative agent of Lyme disease in North America and is the most commonly reported vector-borne infectious agent in the United States (14, 15, 59). The spirochete infectious cycle is complex and involves the colonization and infection of Ixodes ticks, as well as a range of mammalian hosts, including humans (6, 33). The bacterium is thus exposed to variation in its environment as it moves between arthropod vector and mammal. During transmission, bacteria encounter significant changes in pH, temperature, and other aspects of the host milieu. B. burgdorferi responds with differential gene expression and changes in the composition of its membrane proteins (7, 9, 54). The ability of B. burgdorferi to alter its surface in response to these signals is a critical step in the pathogenesis of Lyme disease. This adaptation may aid the efficient transmission of the organism, as well as to predispose the mammalian host to chronic infection (51).
Translation of the sequenced B. burgdorferi B31 genome predicts an unusually basic proteome, with an average pI greater than 8.0 (43). Another distinct aspect is its high percentage of predicted lipoproteins, greater than 10% of the predicted proteome (20). Many lipoproteins have been found to undergo differential expression in the tick and mammalian hosts (35). The response of many previously characterized lipoproteins to simple environmental cues is well documented, such as the increased production of OspC at 35°C and pH 7.0 relative to 23°C and pH 8.0 (45, 56, 57). The alterations in a number of other membrane components, such as the Erp family of complement-binding proteins, the adhesins DbpA and -B, VlsE, and other complement-binding proteins such as CRASP-1, are thought to contribute to the successful transmission to the mammalian host during the blood meal of the Ixodes tick (18, 25, 26, 31, 60). Serologic data from human and murine hosts demonstrate a humoral response to a number of these membrane-associated lipoproteins (19, 30, 55, 60). However, the clinical course of infection in the two mammalian hosts differs: humans manifest the erythema chronicum migrans rash in early localized disease and arthritis later, whereas the infection of natural murine host species produces no skin findings and arthritis in a limited number of laboratory-derived strains (15, 69). These divergent clinical manifestations may have their foundation in the innate and adaptive immune response in each host to distinct membrane components.
Using two-dimensional electrophoresis with nonequilibrium pH gradient electrophoresis (2DE-NEPHGE) and with immobilized pH gradients (2DE-IPG), immunoblotting with murine and human sera, as well as matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis, we have identified a set of common immunogens among three distinct mouse strains and patients diagnosed with Lyme disease. These include a number of acidic, high-molecular-mass proteins, as well as basic lipoproteins of 30,000 to 45,000 Da. We correlate these data with immunoreactivity from human sera pools reflecting the temporal course of Borrelia infection in order to further our understanding of the evolving humoral response in Lyme disease. These studies reveal members of the paralogous gene family 54 (pgf 54) as consistently immunogenic across human and murine sera and identify novel acidic proteins that may be involved in the pathogenesis of infection in mammalian hosts.
MATERIALS AND METHODS
Bacterial strains and growth.
A nonclonal isolate of B. burgdorferi strain B31 (passaged five times in vitro after isolation from an experimentally infected mouse) was grown at 35°C to 5 × 107 cells per ml (mid-log phase) in 1 liter of BSK-H medium (Sigma, St. Louis, MO), adjusted to pH 7.0, and harvested by centrifugation (8,000 × g, 10 min, 4°C). The cell pellet was rinsed once with 200 ml of HN buffer (50 mM NaCl [Sigma] in 10 mM HEPES [Fisher, Pittsburgh, PA], pH 8.0) and centrifuged a second time. Cell pellets were suspended in 3 ml of 2.0 mM dithiothreitol (DTT; Calbiochem, San Diego, CA), 1.0 mM EDTA (pH 8.0; Calbiochem), and Complete EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN) in HN buffer and lysed by using a French press (Thermo IEC, Marietta, OH) (two passes at 18,000 lb/in2) as described previously (43). Total cell lysates generated in this manner were clarified of nonlysed cells and insoluble material by centrifugation (10,000 × g, 15 min, 4°C) and then subjected to subcellular fractionation to yield membrane-associated proteins.
Subcellular fractionation and isolation of membrane proteins.
Total cell lysate was subjected to ultracentrifugation (435,700 × g, 1 h, 4°C) in a Sorvall Discovery M150 SE Ultracentrifuge to pellet membrane vesicles. Membrane pellets were rinsed in 1.0 ml of HN buffer with the addition of protease inhibitors, reisolated by ultracentrifugation, and suspended in 200 to 400 μl of HN buffer plus complete protease inhibitor cocktail with the aid of a small Teflon coated homogenizer (Kontes, Vineland, NJ). Suspended membrane-associated proteins were stored at −80°C until use. Membrane protein quantities were determined by using a modified Lowry protein assay with bovine serum albumin as a standard (38).
2DE.
Prior to separation by using a 2D gel, membrane proteins were precipitated by the addition of 8 volumes acetone (Fisher), 1 volume of a saturated trichloroacetic acid solution (Fisher), and incubated at −20°C for 45 min. Precipitated protein samples were then subjected to centrifugation (16,000 × g, 20 min, 4°C), rinsed in 80% cold acetone in distilled water, air dried, and solubilized for isoelectric focusing by either 2DE-NEPHGE or IPGPhor pH 4-7 2DE-IPG strips (Amersham, Piscataway, NJ). For separation in the first dimension by 2DE-NEPHGE, proteins were suspended in C4TT NEPHGE buffer consisting of 7 M urea (Promega, Madison, WI), 2 M thiourea (Fisher), 4.0% (wt/vol) CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate; Calbiochem}, 1.0% (vol/vol) Triton X-100 (Bio-Rad, Hercules, CA), 65 mM DTT, and 2.0% (vol/vol) preblended ampholytes (pH 3 to 9.5; Amersham) and then incubated overnight at 23°C with gentle agitation. Samples were clarified by ultracentrifugation (435,700 × g, 30 min, 23°C), and 25 μg (immunoblots) or 150 μg (MALDI-TOF analysis) was loaded onto tube gels (2.0 mm in diameter by 12 cm in length) consisting of 8 M urea, 4.4% Duracryl acrylamide (Genomic Solutions, Ann Arbor, MI), 4.0% CHAPS (wt/vol), 1.0% (vol/vol) Triton X-100, and 2.0% (vol/vol) preblended ampholytes (pH 3 to 9.5). The first dimension was focused at 200 V for 1 h and then increased to 600 V for 4 h (total of 2,600 V · h). The tube gels were extruded and stored at −80°C until separated in the second dimension by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
For separation in the first dimension by IPG strips, membrane proteins were suspended in C4TT IPG pH 4 to 7 buffer consisting of 7 M urea, 2 M thiourea, 4.0% (wt/vol) CHAPS, 1.0% (vol/vol) Triton X-100, 100 mM DTT, and 0.5% IPGphor buffer 4-7 (Amersham) (vol/vol). Samples were clarified by ultracentrifugation (435,700 × g, 30 min, 23°C) as described above, and 50 μg (immunoblots) or 400 μg (MALDI-TOF analysis) was loaded onto 13 cm, pH 4-7 IPG strips and focused for 82,000 V · h using the IPGphor system (Amersham) (running conditions: 500 V for 1 h, 1,500 V for 1 h, and 8,000 V for 80,000 V · h). Once the strips had focused, they were stored at −80°C until separated in the second dimension by SDS-PAGE.
Prior to the separation of proteins in the second dimension, focused NEPHGE tube gels and IPG strips were equilibrated twice (10 ml, 10 to 30 min, 23°C) with SDS equilibration buffer composed of 3 M urea, 2.0% (wt/vol) SDS (Fisher), 1.0% DTT, and 10% (vol/vol) glycerol in 125 mM Tris (pH 8.8). Standard SDS-PAGE was performed by laying the tube gel or strip onto a 12.5% acrylamide gel (13.5 cm long by 1.5 mm wide by 14 cm high) on a Hoefer SE600 gel apparatus at 35 mA per gel. Broad-range protein standards or Precision Plus prestained protein standard (Bio-Rad) were used to estimate the relative molecular masses. Proteins separated for MALDI-TOF identification were stained by using the Silver Stain Plus Kit (Bio-Rad). Gel images were created by acquiring digitized images (400 dpi) using a Canon LiDE 30 flatbed scanner. Once scanned, gels were stored in 1.0% acetic acid (Fisher) at 4°C until the spots of interest were excised for mass spectrometry (MS).
MALDI-TOF MS analysis.
Protein spots of interest were manually picked (1.0 to 3.0 mm in diameter) and rinsed in 400 μl of distilled water. Silver ions were removed by adding 50 μl of fresh destaining solution (15 mM potassium ferricyanide and 50 mM thiosulfate in distilled water; Invitrogen, Carlsbad, CA) to each spot, followed by incubation for 20 min. Samples were then rinsed twice with 500 μl of distilled water and equilibrated with 200 mM ammonium bicarbonate (Fisher) for 20 min. Samples were rinsed three times in 400 μl of 50% acetonitrile (Fisher) in 25 mM ammonium bicarbonate (23°C for 15 min) and dehydrated in 400 μl of 100% acetonitrile (23°C for 10 min). Dehydrated gel punches were treated with trypsin manually. Samples were digested in situ with 200 to 300 ng of trypsin (Sigma) for 14 h at 37°C, and peptides were extracted twice with 50 μl of 50% acetonitrile-2.5% trifluoroacetic acid (Fisher) in distilled water and dried using a CentriVap Speed Vacuum (Labconco, Kansas City, MO). Extracted, dried peptides were mixed with 5 μl of α-cyano-4-hydroxycinnamic acid (CHCA), and 0.5 μl was spotted onto the target for MALDI-TOF analysis. MALDI-TOF was performed using the 4700 Proteomics Analyzer (ABI, Foster City, CA). Mass spectra were individually calibrated by using internal trypsin peaks with Data Explorer software available from ABI. Proteins were identified by using ProteinProspector (University of California, San Francisco; http://prospector.ucsf.edu/) set to a mass accuracy of ±50 ppm to compare unknown mass fingerprints to those of known proteins in the NCBI database using a species filter for the B. burgdorferi B31 genome.
Sera used for immunoblots.
Needle-inoculated pooled mouse sera (a gift from P. Rosa) was generated by the injection of RML white mice with 103 B. burgdorferi intradermally, with subsequent bleeds on 21 to 28 days postinfection (dpi) and was used at a 1:1,000 dilution. Tick bite sera were obtained from the white-footed mouse Peromyscus leucopus (the natural reservoir for Borrelia in the United States) at ∼30 dpi or ICR mice (∼18 dpi) as previously reported (9, 22) and used at a dilution of 1:1,000. Rabbit hyperimmune antiserum raised against live, low-passage B. burgdorferi B31 was produced as previously described and used at a dilution of 1:10,000 (9, 10). Human sera were obtained from the CDC Lyme patient serum panel and grouped into categories of negative, early localized, early disseminated, and late disseminated based on CDC guidelines and used at a dilution of 1:1,000. The individual CDC sera used and their clinical histories are found in Table 1.
TABLE 1.
Clinical descriptions of CDC Lyme disease patient seraa
| Pool | Patient identification | ELISA | Symptomology |
|---|---|---|---|
| Early localized | CDC 91-1222 | IgM+/IgG− | Single ECM, culture positive, 1-mo history |
| CDC 91-1347 | IgM+/IgG− | Single ECM, culture positive, 1-mo history | |
| CDC 91-1349 | IgM+/IgG− | Single ECM, culture positive, 1-mo history | |
| CDC 91-1350 | IgM+/IgG− | Single ECM, culture positive, 1-mo history | |
| CDC 91-1351 | IgM+/IgG− | Single ECM, culture positive, 1-mo history | |
| Early disseminated | CDC 90-2631 | IgM+/IgG+ | Single ECM, systemic illness, culture positive, 3-mo history |
| CDC 90-2668 | IgM+/IgG+ | Bell's palsy, systemic illness, Ab+, 2-mo history | |
| CDC 91-0865 | IgM+/IgG+ | Bell's palsy, ECM, Ab+, 6-wk history | |
| CDC 91-1104 | IgM+/IgG− | Multiple ECM, Ab+, 2-mo history | |
| CDC 91-1348 | IgM+/IgG+ | Single ECM, culture +, 3-mo history | |
| Late disseminated | CDC 91-0521 | IgM−/IgG+ | ECM, arthritis, Ab+, 5-mo history |
| CDC 91-0532 | IgM−/IgG+ | ECM, arthritis, CNS symptoms, Ab+, 13-yr history | |
| CDC 91-0533 | IgM+/IgG+ | Bell's palsy, arthritis, Ab+, 5-yr history | |
| CDC 91-0544 | IgM+/IgG+ | Arthritis, CNS symptoms, Ab+, 3 yr history | |
| CDC 92-0057 | IgM+/IgG+ | Severe CNS disease, culture positive, 4-yr history |
ELISA, enzyme-linked immunosorbent assay; ECM, erythema chronicum migrans; Ab+, B. burgdorferi Western blot positive; CNS, central nervous system.
Immunoblot analysis.
Proteins separated by either 2DE technique were prepared for immunoblotting by electrophoretic transfer to nitrocellulose (0.45-mm-pore-size Trans-Blot Transfer Medium; Bio-Rad) as described by Towbin et al. with a Bio-Rad Trans-Blot Cell (30 V, 12 h, 4°C) (65). After transfer, the proteins were visualized with Amido Blue Black (0.1% Amido Blue Black in 1.0% acetic acid), and standards were marked. The nitrocellulose membranes were blocked with 5% nonfat dry milk in 10 mM Tris-HCl-150 mM NaCl-0.1% Tween 20 at pH 8.0 (TBS-T20) for 3 h at 23°C, and immune serum diluted 1:1,000 in blocking solution was applied to the blot (1 h; 23°C). The blot was washed twice in 100 to 200 ml of TBS-T20 for 10 min. Secondary antibody (horseradish peroxidase-conjugated goat anti-human immunoglobulin G [IgG], anti-rabbit, or anti-mouse antibody; Sigma) was diluted 1:5,000 in TBS-T20 (with 1% nonfat dry milk for human sera) and applied to the blot (1 h, 23°C), followed by three washes with 100 to 200 ml of TBS-T20. Reactive bands were visualized with the enhanced chemiluminescence kit (Amersham) in accordance with the manufacturer's specifications.
RESULTS
Comparison of 2DE membrane immunoblots with mouse sera against needle-infected and tick-infected B. burgdorferi.
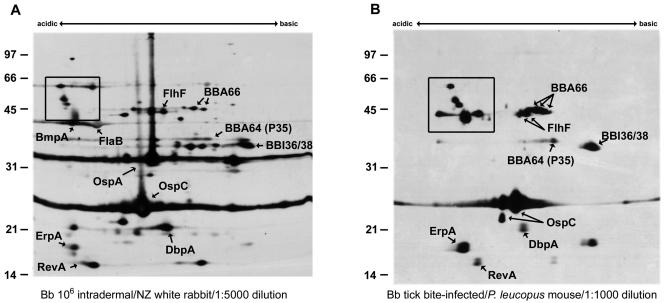
Figure 1 compares immunoblots of membrane proteins from B. burgdorferi grown at 35°C and pH 7.0 and separated with 2DE-NEPHGE. Immunoblots were probed with hyperimmune rabbit serum derived from needle-inoculated B. burgdorferi (Fig. 1A) as a reference immunoblot, or P. leucopus mouse serum (the reservoir host) infected via tick bite (Fig. 1B). MALDI-TOF identifications made in the present study and previous proteomic studies of the membrane fraction are listed in Table 2 (7, 43). Of the proteins identified previously and in the present study, ErpA/I/N (simply referred to herein as ErpA) is an OspE paralog (37, 63) with three identical loci identified in B. burgdorferi B31 designated ErpA, ErpI, and ErpN (12, 13, 41) and has formerly been shown to be temperature regulated and immunogenic in the murine and human host (41, 60). Furthermore, ErpA has also demonstrated an ability to bind factor H and is also termed BbCRASP-5 (complement regulator-acquiring surface protein) (3, 30, 66). Likewise, OspC and RevA are lipoproteins that are coordinately regulated by alterations in pH or temperature (8, 9, 53, 57, 70) and have been determined to be immunogenic in mammals (21, 24, 49, 50, 58, 68). The tick bite-infected sera (Fig. 1B) highlighted the immunogenicity of pgf 54 members BBA64, BBA66, and BBI36/38 (this protein pair is 98.6% identical in their primary sequences and thus are indistinguishable in our studies). All three proteins are reactive with both sera despite being present in low abundance by silver staining (data not shown). We also identified FlhF, a flagellum-associated protein, as an immunogenic membrane-associated protein in close proximity to BBA66. The acidic pole contained a group of high-molecular-mass membrane proteins (box) that were not identified in previous studies and yet are highly immunoreactive with the P. leucopus tick bite serum. Because of the low abundance of these proteins when they were visualized with silver staining, we were unable to identify them by MALDI-TOF from samples separated by 2DE-NEPHGE.
FIG. 1.
Comparison of B. burgdorferi membrane-associated proteins separated with 2DE-NEPHGE and immunoblotted with mammalian sera. Membrane-associated proteins (MAP) from B. burgdorferi were separated by 2DE-NEPHGE (100 μg of MAP/gel) and immunoblotted with needle-infected rabbit serum (9, 10) (A) or tick-infected P. leucopus mouse serum (B). Acidic and basic ends are noted. Relative molecular mass markers (in kilodaltons) are indicated to the left of each gel. Proteins identified by MALDI-TOF analysis of identical silver-stained spots are annotated and listed in Table 2. Boxed areas indicate unidentified acidic proteins.
TABLE 2.
Proteins identified by MALDI-TOFa
| Protein identification (additional designations) | % Coverage | Isoelectric point (pI) | Predicted molecular mass (Da) | Source or reference |
|---|---|---|---|---|
| RevA (BBM27, BBP27) | 36.0 | 5.6* | 15,628* | 7, 43; this study |
| ErpA/I/N (BBL39, BBP38, OspE paralog, BbCRASP-5) | 31.0 | 5.2* | 18,200* | 7, 43; this study |
| BBA03 | 65 | 5.3 | 19,222 | 43 |
| ErpP (BBN38, OspE paralog, BbCRASP-3) | 52 | 8.4* | 20,689* | This study |
| lipoprotein LA 7 (BB0365) | 72 | 5.5* | 21,866* | 43; this study |
| BB0323 | 25 | 9.4# | 29,000# | 43 |
| BBA64 (P35) | 36.0 | 9.3* | 31,878* | 7, 43 |
| BBI36/38 | 35.0 | 9.6* | 33,101* | 43; this study |
| BmpA (BB0383, P39) | 89.0 | 5.1* | 35,113* | 43; this study |
| FlaB (BB0147) | 60 | 5.5 | 35,765 | 43; this study |
| FtsZ (BB0299) | 55 | 4.9 | 42,398 | This study |
| ErpB/J/O (BBL40, BBP39, ElpB1) | 21.0 | 5.0* | 43,038* | This study |
| FlhF (BB0270) | 29.0 | 8.4 | 44.369 | This study |
| BBA66 | 18.0 | 9.4* | 43,787* | 7, 43 |
| OppA I, II, and IV (BB0328, BB0329, BBB16) | 50, 54, and 54 | 5.4*, 5.4*, and 5.5* | 57,937*; 58,549*; and 58,330* | 43; this study |
| OppA III (BB0330) | 40.0 | 9.1 | 62,355 | 43 |
| P66 (BB0603) | 68.0 | 6.0 | 68,173 | 43; this study |
| Hsp90 (BB0560) | 11.0 | 5.6 | 71,219 | 43; this study |
| P83/100 (BB0744) | 46.0 | 5.1 | 77,503 | 43; this study |
The asterisk denotes the predicted molecular mass and pI of lipoprotein after cleavage of the signal peptide. The “#” symbol denotes the predicted molecular mass and pI after CtpA processing.
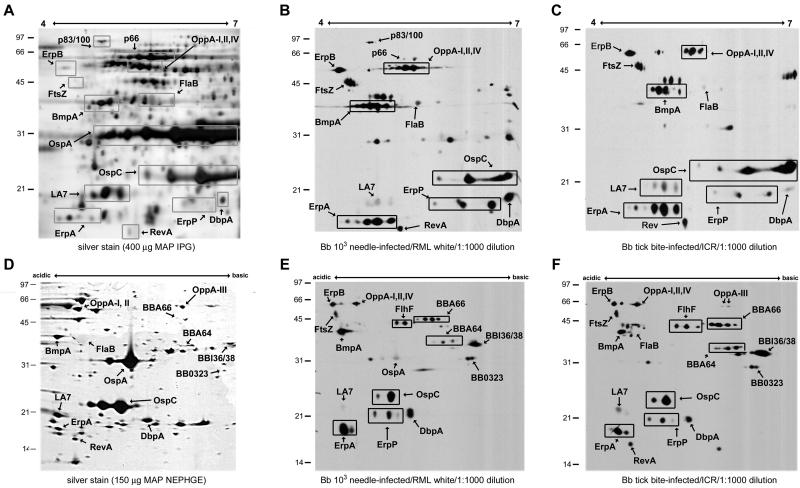
While high-density inoculation of rabbits with B. burgdorferi produces sera which react with a wide variety of membrane proteins as indicated in the reference immunoblot in Fig. 1A, tick-bite-infected hosts produce sera with a more limited humoral response to membrane proteins that are more likely to be present or expressed during transmission. We explored the possibility that low-density needle infection of mice might produce an attenuated immune response similar to natural tick bite infection. Figure 2 demonstrates that low-density (i.e., 103 B. burgdorferi) needle infection of RML white mice (Fig. 2B and E) produced sera very similar in profile to tick-infected ICR (Fig. 2C and F) or P. leucopus (Fig. 1B) mice. As expected, there was a reduction in the reactivity to OspA in sera from mice infected by tick bite or low-dose needle inoculation, and several other minor basic proteins are less immunoreactive with serum samples from low-dose needle inoculated mice compared to that from the high-dose inoculated rabbit reference serum.
FIG. 2.
Immunoblotting of B. burgdorferi membrane fraction proteins separated by 2DE with murine sera. B. burgdorferi MAP were separated with pH 4-7 2DE-IPG (A-C) or 2DE-NEPHGE (D-F) and visualized with silver staining (A and D) or immunoblotting (B, C, E, and F). Immunoblotting was performed with a 1:1,000 dilution of sera from low-density needle-infected RML white mice (B and E) or from tick-infected ICR mice (C and F). The pH range of each gel is indicated. Relative molecular mass markers (in kilodaltons) are indicated to the left of each gel. Proteins identified by MALDI-TOF analysis of identical silver-stained spots are annotated and listed in Table 2. Boxed areas designate protein isoforms with a single identification. For pH 4-7 2DE-IPG, 400 μg of MAP was used for silver staining and 50 μg of MAP was used for immunoblotting. 2DE-NEPHGE utilized 150 μg of MAP for silver staining and 25 μg of MAP for immunoblotting.
MALDI-TOF identification of acidic membrane proteins separated by pH 4-7 2DE-IPG.
Although we have previously reported the identification of pH-regulated membrane proteins in B. burgdorferi (7, 9), the immunoblots in Fig. 2 show reactivity with several acidic, high-molecular-mass proteins that are immunogenic in tick bite infection of mice. The identification of these proteins in previous studies was hampered by their relative low density. We attempted to improve our identification of these proteins by using IPG separation of membrane proteins with a more focused pH range (4.0 to 7.0). Expansion of the acidic membrane proteome with this technique also allowed for increased sample loading, more than twice the amount used in 2DE-NEPHGE. Figure 2 shows silver staining (Fig. 2A) and immunoblotting of pH 4-7 2DE-IPG-separated membrane proteins with needle-inoculated mouse serum (Fig. 2B). The immunoreactivity pattern of high-molecular-mass acidic proteins is comparable to that seen with rabbit sera in Fig. 1 and was used to pick individual proteins visualized with silver stain for analysis. Immunoreactive proteins identified with MALDI-TOF are labeled in Fig. 2A, and the corresponding identifications are listed in Table 2. We confirmed a number of previously recognized membrane proteins that are known to be immunogenic in mammalian infection, including RevA, ErpA, BmpA (P39), and several members of the OppA family (OppA I, II, and IV) (5, 8, 16, 60). ErpB/J/O (three identical loci in B. burgdorferi B31 [12, 13, 41] that we refer to simply as ErpB) was identified as an immunogenic protein present in low amounts in cell lysates (data not shown) or membrane-associated proteins, which we were previously unable to identify by MALDI-TOF analysis of 2DE-NEPHGE-separated proteins. ErpB, an Elp (OspE/F-like leader peptide) paralog and also referred to as ElpB1 in strain 297 (2, 61), is immunogenic in infected mice, baboons, and humans (28, 41, 60). In addition, FtsZ, a cell division protein with homology to tubulin, was immunogenic with this serum. Our identification of these membrane-associated proteins present in low amounts by MALDI-TOF has expanded the known proteomic map of the membrane fraction and allowed us to examine the immune response to B. burgdorferi membrane-associated proteins in a number of other mouse strains.
Immunoblotting of pH 4-7 2DE-IPG- and 2DE-NEPHGE-separated B. burgdorferi membrane proteins with murine sera.
The identification of low-abundance acidic proteins via 2DE-IPG, which show high degrees of immunogenicity with mouse sera, allowed us to more completely map the similarities in the humoral immune response to B. burgdorferi infection among outbred murine strains. Figure 2 shows pH 4-7 2DE-IPG-separated B. burgdorferi membrane proteins immunoblotted with sera from pooled low-density needle-inoculated RML white mice (Fig. 2B) and from tick-infected ICR mice (Fig. 2C). The map of the reactivity with these sera demonstrates a number of similarities between strains. Previously identified membrane proteins such as OspC, DbpA, BmpA, and ErpA are reactive in all pooled sera tested in our study, including other pooled RML white and ICR mouse sera (data not shown). FtsZ, identified in Fig. 2A, was immunogenic across all strains tested, an unexpected finding for a cell division-associated protein. OppA I, II, and IV, oligopeptide permease homologs, were reactive with all sera, as were ErpB and ErpP. Similar to ErpA, ErpP is an OspE paralog that demonstrates the ability to bind Factor H and is also called BbCRASP-3 (3, 30, 32, 39). Lipoprotein LA7 (p22) was recognized by all sera tested, although with various intensities. FlaB induced only faint responses in all murine sera examined. The reactivity of several of these proteins agrees with prior 2DE-IPG work performed with non-temperature-adapted or non-pH-adapted B. burgdorferi (16, 17) and adds the identification of a number of immunoreactive membrane-associated proteins present in lower quantity. These immunoblots also confirm the utility of the low-density needle infection (103) as a surrogate for tick bite infection, since the reactivity patterns of all four panels are very comparable.
Although we used the pH 4-7 2DE-IPG to identify acidic proteins present in low amounts in the membrane fraction, this pH range does not demonstrate the full humoral response to B. burgdorferi. We therefore examined the reactivity of RML white and ICR mouse sera with B. burgdorferi membrane proteins separated with 2DE-NEPHGE and correlated these blots with our previously established proteomic map (43). 2DE-NEPHGE offers a broader pH range resolution and highlights the predominantly basic membrane proteome of B. burgdorferi. Figure 2D demonstrates the pattern of 2DE-NEPHGE separation of B. burgdorferi strain B31 membrane-associated proteins, with corresponding identifications made by MALDI-TOF analysis. Figure 2E and F shows immunoblots of similarly separated proteins, with sera from pooled low-density needle-inoculated (103) RML white mice (Fig. 2E) and from tick-infected ICR mice (Fig. 2F). All sera showed comparable patterns of reactivity between pH 4-7 2DE-IPG and 2DE-NEPHGE, and the pattern of reactivity of acidic proteins with 2DE-NEPHGE allowed comparison with proteins identified previously using pH 4-7 2DE-IPG. All sera recognized BBA66 and BBI36/38, the basic pgf 54 lipoproteins recognized by P. leucopus serum, whereas BBA64 was recognized by the majority of tested sera. FlhF and BB0323 were immunogenic in all tested mouse sera. Higher-molecular-mass acidic proteins, including ErpB, OppA I, OppA II, OppA IV, BmpA, and FtsZ, were reactive across the panel, reproducing the results of the 2DE-IPG samples. The previously established immunogens—OspC, DbpA, BmpA, and ErpA—were recognized by each murine serum pool. We also observed immunoreactivity to RevA in all mouse sera when 2D-IPG or 2D-NEPHGE immunoblots were probed. The observed variability in RevA reactivity in some murine sera (compare Fig. 2B and E) was due to the equilibration steps of the NEPHGE tube gels prior to SDS-PAGE separation, which can cause the loss of low-molecular-mass proteins.
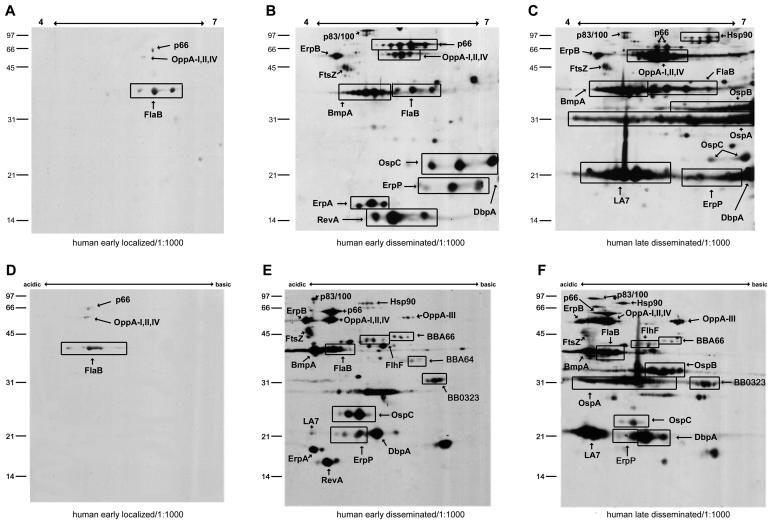
Temporal course of pooled CDC Lyme patient serum panel reactivity with 2DE-IPG- and 2DE-NEPHGE-separated B. burgdorferi membrane proteins.
The characterization of the murine immune response to B. burgdorferi membrane-associated proteins allowed us to compare this with the humoral response to B. burgdorferi infection in humans. There have been few previous reports using 2DE in the examination of human antibody reactivity from specific populations (such as cerebrospinal fluid profiles during neuroborreliosis and synovial fluid profiles during arthritis) with B. burgdorferi cell lysates (27, 40). These previous studies were performed with pH ranges that limited basic protein resolution or did not enrich for membrane proteins. Our work is differentiated by the increased range of basic protein resolution, as well as the analysis of temporal changes in the humoral immune response to B. burgdorferi membrane proteins. We obtained the CDC Lyme patient serum panel in order to compare and contrast the serologic response to infection in the early-localized (<1 month), early-disseminated (1 to 6 months), and late-disseminated (>6 months phases). Figure 3 shows immunoblotting of pH 4-7 2DE-IPG B. burgdorferi membrane proteins with pooled sera from early-localized (Fig. 3A), early-disseminated (Fig. 3B), and late-disseminated human infection (Fig. 3C). FlaB, the only antigen recognized strongly by early sera, was reactive throughout human exposure. P66 and OppA I, II, and IV are weakly reactive with the early localized pool. Pooled early-disseminated sera recognized OspC, DbpA, BmpA, and ErpA, which was similar to the murine response (refer to Fig. 2B and C). However, reactivity with P66 was much more prominent, as well as P83/100. Interestingly, FtsZ and ErpB were also immunogenic in the human, both in early- and late-disseminated disease. Although RevA and ErpA decreased in reactivity in late-disseminated sera at the dilution of 1:1,000, there was strong recognition of several new antigens, including LA7, OspB, and OspA. A number of antigens which the human sera pools react with (P66 and P83/100) have previously been described as immunogens in the human host (34, 44). The immunoblotting of 2DE-NEPHGE membrane fractions (Fig. 3D and E) with the same temporal groups detected a similar pattern of reactivity compared to the narrower pH range data in Fig. 3A to C. In addition, the reactivity of BBA66, FlhF, and BB0323 in early-disseminated sera was revealed with the improved pH range of the NEPHGE technique. Little reactivity with BBA64 or BBI36/38 was observed with either the early-disseminated or late Lyme sera pools at a dilution of 1:1,000, in contrast to the murine humoral response. These blots also confirm the reactivity of LA7 in late sera and the diminished recognition of OspC by these sera (comparing Fig. 3E and F).
FIG. 3.
Immunoblotting of B. burgdorferi membrane-associated proteins separated by 2DE with Lyme patient sera. MAP were separated with pH 4-7 2DE-IPG (A to C) or 2DE-NEPHGE (D to F) and immunoblotted with a 1:1,000 dilution of pooled CDC Lyme patient sera representing early-localized (A and D), early-disseminated (B and E), and late-disseminated (C and F) stages of Lyme disease. The pH range of each gel is indicated. Relative molecular mass markers (in kilodaltons) are indicated to the left of each gel. Proteins identified by MALDI-TOF analysis of identical silver-stained spots are annotated. Boxed areas designate protein isoforms with a single identification. For pH 4-7 2DE-IPG, 400 μg of MAP was used for silver staining and 50 μg of MAP was used for immunoblotting. 2DE-NEPHGE utilized 150 μg of MAP for silver staining and 25 μg of MAP for immunoblotting.
DISCUSSION
We describe here the serologic proteome analysis of murine and human humoral responses to B. burgdorferi membrane-associated proteins, using 2DE and MALDI-TOF identification of individual antigens. This study represents a comprehensive analysis of serologic reactivity in two dimensions and confirms a number of past observations regarding the humoral response in Lyme disease. We have identified a number of novel immunogens as well, including several members of the pgf 54 family of lipoproteins. The identified proteins and their reactivity with murine and human sera are listed in Table 3. The use of alternative methods of protein separation—both narrow, acidic range 2DE-IPG and broad pH range 2DE-NEPHGE—allowed us to identify abundant proteins with moderate reactivity (OspC), as well as very minor species that manifest significant immunogenicity in all hosts (FtsZ, FlhF, BB0323, and BBA66). The temporal map of the humoral response in humans reveals the shifting reaction to B. burgdorferi membrane components, highlighting differences in the antibody response over time. In total, these data represent a map of the humoral response to B. burgdorferi infection in both human and murine hosts and provide a number of areas of new investigation in the pathogenesis of Lyme disease.
TABLE 3.
Specific reactivity of serum poolsa
| Protein | Mouse sera
|
Human serab
|
||||
|---|---|---|---|---|---|---|
| P. leucopus | RML white | ICR | EL | ED | LD | |
| RevA (BBM27, BBP27) | + | + | + | + | ||
| ErpA/I/N (BBL39, BBP38, OspE paralog, BbCRASP-5) | + | + | + | + | ||
| Lipoprotein LA 7 (BB0365) | + | + | + | + | ||
| ErpP (BBN38, OspE paralog, BbCRASP-3) | + | + | + | + | + | |
| DbpA (BBA24) | + | + | + | + | + | |
| OspC (BBB19) | + | + | + | + | + | |
| BB0323 | + | + | + | + | + | |
| OspA (BBA15) | + | |||||
| BBI36/38 | + | + | + | |||
| BBA64 (P35) | + | + | + | + | ||
| BmpA (BB0383, P39) | + | + | + | + | + | |
| FlaB (BB0147) | + | + | + | |||
| FtsZ (BB0299) | + | + | + | + | + | |
| ErpB/J/O (BBL40, BBP39, ElpB1) | + | + | + | + | + | |
| FlhF (BB0270) | + | + | + | + | + | |
| BBA66 | + | + | + | + | + | |
| OppA I, II, and IV (BB0328, BB0329, BBB16) | + | + | + | −/+ | + | + |
| OppA III (BB0330) | + | + | ||||
| P66 (BB0603) | −/+ | + | + | |||
| Hsp90 (BB0560) | + | + | ||||
| P83/100 (BB0744) | + | + | ||||
−/+, weakly immunoreactive; +, strongly immunoreactive.
EL, early localized; ED, early disseminated; LD, late disseminated.
Investigation of humoral responses in humans and mice in past studies has focused on single clinical stages of Lyme disease (36) or on selected sites, including a 2DE-NEPHGE study of synovial fluid immunoglobulin reactivity with B. burgdorferi (40). Other work has focused on 2DE-IPG-separated B. burgdorferi antigens extracted with Triton X-114 from infected tissue in SCID mice or rabbits and then probed with immune rabbit or monospecific protein sera (16, 17). Our data correlate many of the identifications made in these studies, particularly in the more recent work with skin samples from infected mouse and rabbit hosts. The use of 2DE-IPG, as demonstrated by Crother et al. in recent studies, does reduce the separation of highly basic proteins, a recognized limitation of this electrophoretic technique (16, 17). The highly basic nature of the B. burgdorferi proteome (average pI of >8.0), however, adds importance to the improved resolution of these alkaline proteins. We have previously documented the efficiency of our CHAPS-based buffer system in combination with 2DE-NEPHGE for the identification of proteins with a pI as basic as 10.0 (7, 43). The increased resolution, as well as the comparison not only between species but also with the temporal course of human infection, allows us to better understand the humoral immune response to B. burgdorferi.
A key finding in the present study is the consistent immunogenicity of several members of the highly basic pgf 54 lipoproteins, including BBA64, BBA66, and BBI36/38. Our group has previously identified BBA64 and BBA66 as pH-regulated membrane proteins whose expression is increased at pH 7.0 relative to pH 8.0 (7). Ojaimi et al., in a gene array study, noted an increase in the transcription of BBA64 and BBA66 at 35°C relative to 23°C (46), again suggesting the increased presence of these proteins in the membrane during the transmission of B. burgdorferi into mammalian hosts. The identification of BBI36/38 in the pH 7.0 membrane proteome in a recent study confirmed another lipoprotein from pgf 54 as expressed at pH 7.0 and 35°C in culture. The reactivity of many sera with BBA64 (P35) has previously been established (1, 23), but the recognition of BBA66 and BBI36/38 as immunogens has not. The use of 2DE is critical to this recognition, since BBA66 (∼43 kDa) migrates at a molecular mass similar to that of FlaB (∼42 kDa) and BmpA (∼39-40 kDa), and BBI36/38 (∼33 kDa) is obscured in medium-derived B. burgdorferi by the expression of OspA and OspB. In previous studies examining 1DE of membrane proteins, it is likely that any specific reactivity with BBA66 would have been masked by the humoral response to these more abundant proteins. Other studies have noted the increased expression of pgf 54 member mRNA in response to temperature and exposure to blood, including BBA64 and BBA66 (46, 64). The function of this paralogous family remains unknown, but these data suggest a role for their expression under conditions that would simulate transmission to a mammalian host.
The immunogenicity of the pgf 54 members also highlights the contrast between abundant, moderately immunogenic proteins in the membrane and minor components which appear more highly immunogenic. BBA66 in particular is a minor protein difficult to visualize with silver staining and yet is immunogenic across mammalian hosts. FtsZ, a cell division-associated protein (20), was not identified in our previous study mapping the proteome of B. burgdorferi and, due to its presence in such limiting quantities, it required overloading with 2DE-IPG for identification in the present study. Similarly to BBA66, it is recognized across all of the sera we have examined in the present study. Interestingly, the 75-kDa Bartonella bacilliformis FtsZ homolog has been determined as a major immunogen in humans diagnosed with Carrion's disease (48). We would postulate that B. burgdorferi FtsZ, produced during spirochetal cell division in the persistently infected host, is recognized by the humoral response and may be a useful diagnostic target. What role FtsZ may play in Lyme borreliosis besides the putative role it serves in cell division remains to be determined. BB0323 is a membrane-associated protein of unknown function that is a substrate for the carboxyl-terminal protease (CtpA) of B. burgdorferi (47). Moreover, insertion of the mariner transposon into this gene leads to disruption of the outer membrane and the formation of large blebs in noninfectious B. burgdorferi clones (62). Interestingly, BB0323 contains a conserved LysM domain at the C terminus. LysM domains are found in prokaryotic and eukaryotic proteins and are thought to act as peptidoglycan-binding domains. These domains are found within cell wall-degrading enzymes, staphylococcal autolysins, and the invasion-associated protein P60 of Listeria monocytogenes (4, 11, 29). Lastly, the identification of FlhF, a putative flagellar GTP-binding protein, as immunogenic in all sera tested is intriguing given the evidence for a role as a sensor molecule in other bacterial models (52).
One limitation of this and any study seeking to analyze serologic samples is the use of culture-derived B. burgdorferi. While the ability to propagate B. burgdorferi in vitro is crucial to the progress made thus far in understanding the pathogenesis of Lyme disease, evidence that BSK-grown spirochetes may present a much different membrane protein composition is undeniable (42, 67). For example, in our experience OspA expression in vitro is persistent despite alterations in medium temperature and pH. Moreover, our current serologic proteome analysis technique is limited to proteins that we can visualize with dilute mammalian sera. We hypothesize that the expression of some low-abundance proteins such as pgf 54 members is greatly augmented during and after transmission by the tick, and we are currently involved in the study of this gene family in order to describe what its true in vivo expression and function may be during infection. Variations in medium composition, as well as interstrain differences in protein profiles, also make serologic results more challenging to interpret. We feel, however, that the present study clearly establishes the utility of a serologic proteome analysis in identifying novel antigens involved in the pathogenesis of B. burgdorferi infection. This tool will obviously be applicable to analyses in a wide variety of microbiologic systems where exploration of humoral immune response is critical. These data will direct further investigation into the antigenicity and possible protective roles of a number of B. burgdorferi proteins, and we hope will provide insight into the critical role of these membrane proteins in the development of Lyme disease in the human host.
Acknowledgments
This research was supported in part by NIH grant number AI055178, CDC grant number CI000181, and startup funds provided by the University of Pittsburgh School of Medicine. A.J.N. was supported by NIH T32 training grant HD042987.
We thank the University of Pittsburgh Genomics and Proteomics Core Laboratory for assistance with the MALDI-TOF MS.
Editor: A. D. O'Brien
REFERENCES
- 1.Aguero-Rosenfeld, M. E., J. Nowakowski, S. Bittker, D. Cooper, R. B. Nadelman, and G. P. Wormser. 1996. Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J. Clin. Microbiol. 34:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, D. R., M. J. Caimano, X. Yang, F. Cerna, M. V. Norgard, and J. D. Radolf. 1999. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect. Immun. 67:1526-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alitalo, A., T. Meri, H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 4.Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from Escherichia coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113-1119. [DOI] [PubMed] [Google Scholar]
- 5.Bryksin, A. V., H. P. Godfrey, C. A. Carbonaro, G. P. Wormser, M. E. Aguero-Rosenfeld, and F. C. Cabello. 2005. Borrelia burgdorferi BmpA, BmpB, and BmpD proteins are expressed in human infection and contribute to P39 immunoblot reactivity in patients with Lyme disease. Clin. Diagn. Lab. Immunol. 12:935-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease: a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 7.Carroll, J. A., R. M. Cordova, and C. F. Garon. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 68:6677-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll, J. A., N. El-Hage, J. C. Miller, K. Babb, and B. Stevenson. 2001. Borrelia burgdorferi RevA antigen is a surface-exposed outer membrane protein whose expression is regulated in response to environmental temperature and pH. Infect. Immun. 69:5286-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll, J. A., and F. C. Gherardini. 1996. Membrane protein variations associated with in vitro passage of Borrelia burgdorferi. Infect. Immun. 64:392-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll, S. A., T. Hain, U. Technow, A. Darji, P. Pashalidis, S. W. Joseph, and T. Chakraborty. 2003. Identification and characterization of a peptidoglycan hydrolase, MurA, of Listeria monocytogenes, a muramidase needed for cell separation. J. Bacteriol. 185:6801-6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 13.Casjens, S., R. van Vugt, K. Tilly, P. A. Rosa, and B. Stevenson. 1997. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J. Bacteriol. 179:217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. 2004. Lyme disease-United States, 2001-2002. Morb. Mortal. Wkly. Rep. 53:365-369. [PubMed] [Google Scholar]
- 15.Cerroni, L. 2004. Lyme borreliosis. Lancet 363:249. [DOI] [PubMed] [Google Scholar]
- 16.Crother, T. R., C. I. Champion, J. P. Whitelegge, R. Aguilera, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect. Immun. 72:5063-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crother, T. R., C. I. Champion, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 71:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Hage, N., K. Babb, J. A. Carroll, N. Lindstrom, E. R. Fischer, J. C. Miller, R. D. Gilmore, Jr., M. L. Mbow, and B. Stevenson. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147:821-830. [DOI] [PubMed] [Google Scholar]
- 19.Feng, S., E. Hodzic, B. Stevenson, and S. W. Barthold. 1998. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect. Immun. 66:2827-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 21.Fung, B. P., G. L. McHugh, J. M. Leong, and A. C. Steere. 1994. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect. Immun. 62:3213-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilmore, R. D., Jr., R. M. Bacon, A. M. Carpio, J. Piesman, M. C. Dolan, and M. L. Mbow. 2003. Inability of outer-surface protein C (OspC)-primed mice to elicit a protective anamnestic immune response to a tick-transmitted challenge of Borrelia burgdorferi. J. Med. Microbiol. 52:551-556. [DOI] [PubMed] [Google Scholar]
- 23.Gilmore, R. D., Jr., K. J. Kappel, and B. J. Johnson. 1997. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J. Clin. Microbiol. 35:86-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilmore, R. D., Jr., and M. L. Mbow. 1998. A monoclonal antibody generated by antigen inoculation via tick bite is reactive to the Borrelia burgdorferi Rev. protein, a member of the 2.9 gene family locus. Infect. Immun. 66:980-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo, B. P., E. L. Brown, D. W. Dorward, L. C. Rosenberg, and M. Hook. 1998. Decorin-binding adhesins from Borrelia burgdorferi. Mol. Microbiol. 30:711-723. [DOI] [PubMed] [Google Scholar]
- 26.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen, K., M. Cruz, and H. Link. 1990. Oligoclonal Borrelia burgdorferi-specific IgG antibodies in cerebrospinal fluid in Lyme neuroborreliosis. J. Infect. Dis. 161:1194-1202. [DOI] [PubMed] [Google Scholar]
- 28.Hefty, P. S., C. S. Brooks, A. M. Jett, G. L. White, S. K. Wikel, R. C. Kennedy, and D. R. Akins. 2002. OspE-related, OspF-related, and Elp lipoproteins are immunogenic in baboons experimentally infected with Borrelia burgdorferi and in human Lyme disease patients. J. Clin. Microbiol. 40:4256-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heilmann, C., J. Hartleib, M. S. Hussain, and G. Peters. 2005. The multifunctional Staphylococcus aureus autolysin aaa mediates adherence to immobilized fibrinogen and fibronectin. Infect. Immun. 73:4793-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraiczy, P., K. Hartmann, J. Hellwage, C. Skerka, M. Kirschfink, V. Brade, P. F. Zipfel, R. Wallich, and B. Stevenson. 2004. Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int. J. Med. Microbiol. 293(Suppl. 37):152-157. [DOI] [PubMed] [Google Scholar]
- 31.Kraiczy, P., J. Hellwage, C. Skerka, H. Becker, M. Kirschfink, M. M. Simon, V. Brade, P. F. Zipfel, and R. Wallich. 2004. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 279:2421-2429. [DOI] [PubMed] [Google Scholar]
- 32.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 33.Lane, R. S., J. Piesman, and W. Burgdorfer. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587-609. [DOI] [PubMed] [Google Scholar]
- 34.Lefebvre, R. B., G. C. Perng, and R. C. Johnson. 1990. The 83-kilodalton antigen of Borrelia burgdorferi which stimulates immunoglobulin M (IgM) and IgG responses in infected hosts is expressed by a chromosomal gene. J. Clin. Microbiol. 28:1673-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. DNA microarray assessment of putative Borrelia burgdorferi lipoprotein genes. Infect. Immun. 70:3300-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luft, B. J., W. Jiang, P. Munoz, R. J. Dattwyler, and P. D. Gorevic. 1989. Biochemical and immunological characterization of the surface proteins of Borrelia burgdorferi. Infect. Immun. 57:3637-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marconi, R. T., S. Y. Sung, C. A. Hughes, and J. A. Carlyon. 1996. Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J. Bacteriol. 178:5615-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 39.McDowell, J. V., J. Wolfgang, E. Tran, M. S. Metts, D. Hamilton, and R. T. Marconi. 2003. Comprehensive analysis of the factor h binding capabilities of borrelia species associated with Lyme disease: delineation of two distinct classes of factor h binding proteins. Infect. Immun. 71:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mensi, N., D. R. Webb, C. W. Turck, and G. A. Peltz. 1990. Characterization of Borrelia burgdorferi proteins reactive with antibodies in synovial fluid of a patient with Lyme arthritis. Infect. Immun. 58:2404-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, J. C., N. El-Hage, K. Babb, and B. Stevenson. 2000. Borrelia burgdorferi B31 erp proteins that are dominant immunoblot antigens of animals infected with isolate B31 are recognized by only a subset of human Lyme disease patient sera. J. Clin. Microbiol. 38:1569-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norris, D. E., B. J. Johnson, J. Piesman, G. O. Maupin, J. L. Clark, and W. C. t. Black. 1997. Culturing selects for specific genotypes of Borrelia burgdorferi in an enzootic cycle in Colorado. J. Clin. Microbiol. 35:2359-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowalk, A. J., C. Nolder, D. R. Clifton, and J. A. Carroll. 2006. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 6:2121-2134. [DOI] [PubMed] [Google Scholar]
- 44.Ntchobo, H., H. Rothermel, W. Chege, A. C. Steere, and J. Coburn. 2001. Recognition of multiple antibody epitopes throughout Borrelia burgdorferi p66, a candidate adhesin, in patients with early or late manifestations of Lyme disease. Infect. Immun. 69:1953-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ostberg, Y., J. A. Carroll, M. Pinne, J. G. Krum, P. Rosa, and S. Bergstrom. 2004. Pleiotropic effects of inactivating a carboxyl-terminal protease, CtpA, in Borrelia burgdorferi. J. Bacteriol. 186:2074-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padmalayam, I., B. Anderson, M. Kron, T. Kelly, and B. Baumstark. 1997. The 75-kilodalton antigen of Bartonella bacilliformis is a structural homolog of the cell division protein FtsZ. J. Bacteriol. 179:4545-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padula, S. J., F. Dias, A. Sampieri, R. B. Craven, and R. W. Ryan. 1994. Use of recombinant OspC from Borrelia burgdorferi for serodiagnosis of early Lyme disease. J. Clin. Microbiol. 32:1733-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Padula, S. J., A. Sampieri, F. Dias, A. Szczepanski, and R. W. Ryan. 1993. Molecular characterization and expression of p23 (OspC) from a North American strain of Borrelia burgdorferi. Infect. Immun. 61:5097-5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pal, U., and E. Fikrig. 2003. Adaptation of Borrelia burgdorferi in the vector and vertebrate host. Microbes Infect. 5:659-666. [DOI] [PubMed] [Google Scholar]
- 52.Pandza, S., M. Baetens, C. H. Park, T. Au, M. Keyhan, and A. Matin. 2000. The G-protein FlhF has a role in polar flagellar placement and general stress response induction in Pseudomonas putida. Mol. Microbiol. 36:414-423. [DOI] [PubMed] [Google Scholar]
- 53.Ramamoorthy, R., and D. Scholl-Meeker. 2001. Borrelia burgdorferi proteins whose expression is similarly affected by culture temperature and pH. Infect. Immun. 69:2739-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwan, T. G., K. K. Kime, M. E. Schrumpf, J. E. Coe, and W. J. Simpson. 1989. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect. Immun. 57:3445-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwan, T. G., and J. Piesman. 2000. Temporal Changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skare, J. T., D. M. Foley, S. R. Hernandez, D. C. Moore, D. R. Blanco, J. N. Miller, and M. A. Lovett. 1999. Cloning and molecular characterization of plasmid-encoded antigens of Borrelia burgdorferi. Infect. Immun. 67:4407-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevenson, B., J. L. Bono, T. G. Schwan, and P. Rosa. 1998. Borrelia burgdorferi erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect. Immun. 66:2648-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevenson, B., and J. C. Miller. 2003. Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J. Mol. Evol. 57:309-324. [DOI] [PubMed] [Google Scholar]
- 62.Stewart, P. E., J. Hoff, E. Fischer, J. G. Krum, and P. A. Rosa. 2004. Genome-wide transposon mutagenesis of Borrelia burgdorferi for identification of phenotypic mutants. Appl. Environ. Microbiol. 70:5973-5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sung, S. Y., C. P. Lavoie, J. A. Carlyon, and R. T. Marconi. 1998. Genetic divergence and evolutionary instability in ospE-related members of the upstream homology box gene family in Borrelia burgdorferi sensu lato complex isolates. Infect. Immun. 66:4656-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wallich, R., J. Pattathu, V. Kitiratschky, C. Brenner, P. F. Zipfel, V. Brade, M. M. Simon, and P. Kraiczy. 2005. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect. Immun. 73:2351-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang, G., R. Iyer, S. Bittker, D. Cooper, J. Small, G. P. Wormser, and I. Schwartz. 2004. Variations in Barbour-Stoenner-Kelly culture medium modulate infectivity and pathogenicity of Borrelia burgdorferi clinical isolates. Infect. Immun. 72:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilske, B., V. Preac-Mursic, G. Schierz, R. Kuhbeck, A. G. Barbour, and M. Kramer. 1988. Antigenic variability of Borrelia burgdorferi. Ann. N. Y. Acad. Sci. 539:126-143. [DOI] [PubMed] [Google Scholar]
- 69.Wright, S. D., and S. W. Nielsen. 1990. Experimental infection of the white-footed mouse with Borrelia burgdorferi. Am. J. Vet. Res. 51:1980-1987. [PubMed] [Google Scholar]
- 70.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]