Abstract
Environmental stress affects plant growth and development. Several plant hormones, such as salicylic acid, abscisic acid (ABA), jasmonic acid (JA), and ethylene play a crucial role in altering plant morphology in response to stress. Developmental regulation often has the cell cycle machinery among its targets. We analyzed the effect of JA and ABA on cell cycle progression in synchronized tobacco (Nicotiana tabacum) BY-2 cells. Both compounds were found to prevent DNA replication, keeping the cells in the G1 stage, when applied just before the G1/S transition. However, ABA did not have any effect on subsequent phases of the cell cycle when applied at a later stage, whereas JA effectively prevented mitosis on application during DNA synthesis. This demonstrates that JA treatment can freeze synchronized BY-2 cells in both the G1 and G2 stages of the cell cycle. Jasmonate administered after the S-phase was less effective in decreasing the mitotic index, suggesting that cell sensitivity toward JA is dependent on the cell cycle phase. In cultures detained in the G2-phase, we observed a reduced histone H1 kinase activity of kinases associated with the p13sucl protein.
In nature, plants are constantly exposed to environmental changes that force them to adapt, and only the proper cooperation of all their organs ensures their survival and production of healthy progeny. It is frequently observed that stress signals not only induce plant resistance but also affect growth rate and cell division. For example, in wheat (Triticum aestivum) seedlings subjected to a mild water stress, leaf elongation is reduced in correlation with a rapid decrease of mitotic activity in mesophyll cells of the first leaf (Schuppler et al., 1998). Similar effects were found in water-stressed roots of maize (Zea mays; Sacks et al., 1997) and sunflower (Helianthus annuus; Robertson et al., 1990), where an involvement of abscisic acid (ABA) in the inhibition of the cell cycle was suggested. Salt stress, which can be mediated by ABA, disrupts growth in Arabidopsis. Plants have shortened petioles, roots, and hypocotyls and a drastic decrease in lateral root formation. At the molecular level, salt treatment strongly reduced the expression of the cyclinA2;1 and cyclinB1;1 (Burssens et al., 2000b). Cell cycle progression can be also disturbed by oxidative stress. When generated artificially by the application of menadion, a source of semiquinone radicals and hydroquinones, oxidative stress disrupted DNA replication and delayed mitotic entry in tobacco (Nicotiana tabacum) BY-2 cells and in the apical root and shoot meristems of whole tobacco plants (Reichheld et al., 1999). In cultured parsley (Petroselinum crispum) cells, UV light has been shown to decrease expression of the histone H2A, H2B, H3, and H4, CDKA, and cyclin B genes (Longemann et al., 1995). Certain elements of stress signaling spatially correlate with cell divisions in plant tissue. ANP1 and NPK1 are kinases of the mitogen-activated protein (MAP) kinase kinase kinase class, isolated from Arabidopsis and tobacco, respectively. They mediate response to H2O2, one of the most common reactive oxygen species released during defense responses, which also serves as a stress messenger (Nishihama et al., 1997; Hirt, 2000; Kovtun et al., 2000). NPK1 is essential for cell plate formation (Nishihama et al., 2001). In dividing cells, they are expressed in a cell cycle-dependent manner, suggesting their involvement in the interplay between cell cycle progression and oxidative stress (Nakashima et al., 1998; Hirt, 2000).
Most of the external signals, however, do not exert their effect directly on proteins that are involved in cell division, but rather trigger their response in differentiated tissue, and in some way this response is then coupled to the regulation of the cell cycle within the meristems.
Among the messengers that may pass this type of information are phytohormones. There is growing evidence for both positive and negative hormonal regulation of cell division. Exogenous application of cytokinins to a hormone-depleted cell culture of Arabidopsis caused accumulation of transcripts of cyclin A1 (Burssens et al., 2000a) and cyclin D3 (cycD3; Riou-Khamlichi et al., 2000). cycD3 transcripts also accumulated in whole plants treated with zeatin, and constitutive expression of cycD3 conferred callus formation in the absence of cytokinin (Riou-Khamlichi et al., 1999). Taken together, these data suggest that the induction of cell division by cytokinins involves increased expression of cycD3. However, the activity of phosphatase cdc25, which activates cyclin-dependent kinase (CDK) by the removal of a phosphate group from a Tyr moiety in the ATP-binding loop, has also been shown to be cytokinin dependent (Zhang et al., 1996). Redig et al. (1996) demonstrated that the zeatin level fluctuates in synchronized tobacco BY-2 cells, peaking at late S and before G2/M transition. When zeatin production was blocked, mitotic activity ceased and could only be restored by application of exogenous zeatin (Laureys et al., 1998), suggesting that cytokinins are essential not only for the initiation of the cell cycle but also for the progression through it.
The information on auxin effects in the cell cycle is much more modest. It has been shown that in a hormone-depleted Arabidopsis cell culture, naphthyl-acetic acid application caused an increase in cycA2 transcript (Burssens et al., 2000a), and hormone-depleted protoplasts of petunia (Petunia hybrida) resumed cell division only when subsequently treated with 2,4-dichlorophenoxyacetic acid (2,4-D) and benzylaminopurine but not after treatment with cytokinin alone (Trehin et al., 1998). Another class of plant hormones, gibberellins, increased the expression of histone H3 and a mitotic cyclin, cycOs1, in the intercalary meristem of deepwater rice (Oryza sativa). This may explain the positive effect of gibberellins on internodal elongation in monocotyledonous plants (Sauter, 1997).
Certain hormones might act as messengers of negative regulation of cell division. For example, exogenous ABA reduced bromodeoxyuridine incorporation and mitotic events in root meristems of Arabidopsis and sunflower (Robertson et al., 1990; Leung et al., 1994). One of the possible mechanisms underlying the ABA effect on the cell cycle is suggested in the study of Wang et al. (1998). They cloned an Arabidopsis protein named ICK1, which showed a homology to the cyclin-dependent kinase inhibitor p27Kip1. ICK1 interacted with both CDKA and cycD3 and inhibited the histone H1 kinase activity of the complex. When overexpressed in Arabidopsis, it caused dramatic growth inhibition and decrease in the total number of cells per plant (Wang et al., 2000). Exogenous application of ABA up-regulated ICK1 expression, which may lead to a block of G1/S transition (Wang et al., 1998).
In our research, we investigated the possible interactions between another stress signal messenger, jasmonic acid (JA), and cell cycle progression. JA is widely known to inhibit root growth in Arabidopsis. This feature has been used in isolating mutants defective in jasmonate signaling (Staswick et al., 1992; Feys et al., 1994; Berger et al., 1996). However, the exact mechanism of root growth inhibition is not known. It is not directly linked with the wound and pathogen responses generally accepted to be the main function of jasmonates. There are several reports suggesting a role of JA in cell wall synthesis (Koda, 1997), which might affect cell elongation. On the other hand, it has also been reported that exogenous JA, when applied to growing soybean (Glycine max) callus, counteracted the positive effect of cytokinins and inhibited growth, presumably due to the inhibition of cell division (Ueda and Kato, 1982). We investigated the effects of JA on cell cycle progression. Using a synchronized tobacco BY-2 cell line as a model culture (Nagata et al., 1992), we compared the effect of JA with that of ABA. We found that exogenous application of JA and ABA resulted in phase-specific disruption of the cell cycle progression in BY-2 cells. Both ABA and JA prevented G1/S transition, but only JA, when given during the S-phase, was capable of preventing and delaying the mitotic entry without direct effect on DNA synthesis. This, in turn, suggests that the jasmonate response is broader that that of ABA and in this specific case, JA is not acting downstream of ABA, unlike suggested elsewhere for wound response (Peña-Cortés and Willmitzer, 1995).
RESULTS
JA Prevents Mitosis in Tobacco BY-2
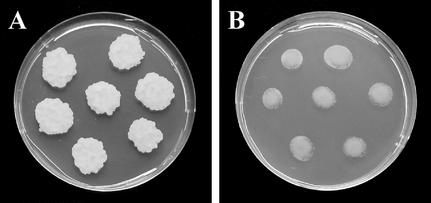
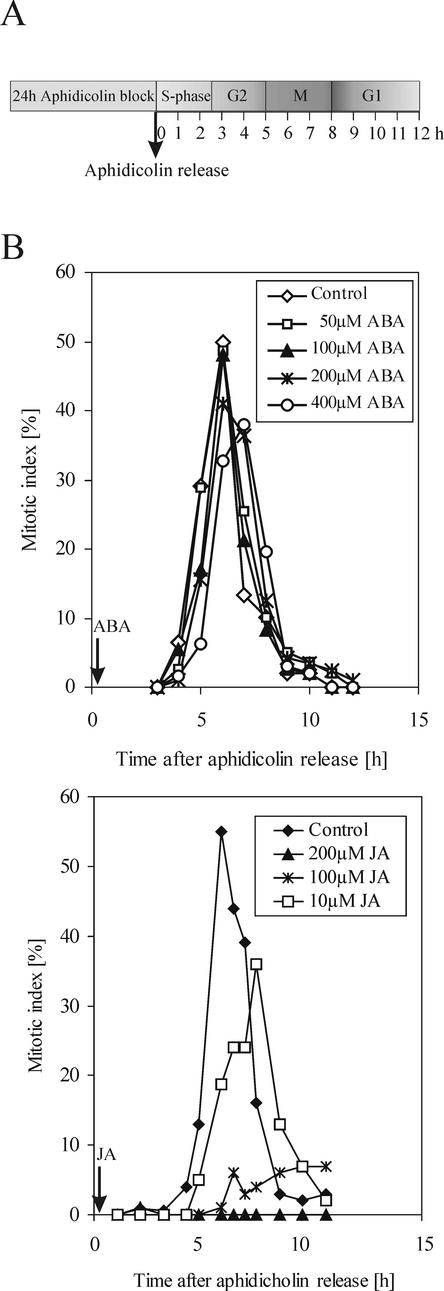
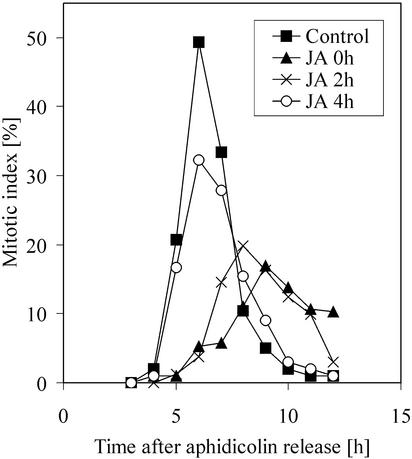
BY-2 callus grown in agar-hardened medium in the presence of 100 μm JA showed clear-cut reduced growth as compared with the untreated control (Fig. 1). Subsequently, we examined the effect of JA on the cell cycle progression. Duration of the specific phases of the cell cycle progression as shown in Figure 2A was estimated by means of flow cytometry, abundance of histone H4 mRNA, thymidine incorporation, and mitotic index (Nagata et al., 1992; Laureys et al., 1998; Ehsan et al., 1999; Reichheld et al., 1999). After aphidicolin release, at the beginning of the experiment, the main population of cells was at the early S-phase. Such cultures were divided into four subcultures, to which hormones were applied. We compared the effect of (±)JA and (±)ABA on the mitotic ratio. Although the ABA-treated culture showed little, if any, response (Fig. 2B), JA treatment decreased the mitotic index in a concentration-dependent manner (Fig. 2B). Besides a moderate reduction, 10 μm JA concentration caused a 1- to 2-h delay of the occurrence of the mitotic peak. Similar results were obtained when methyl jasmonate was used (data not shown). Fluorescein diacetate staining revealed that cells remained viable during the experiments and 24 h afterward. Data presented in Figure 2B were confirmed by multiple repetitions of this experiment.
Figure 1.
JA inhibits growth of BY-2 callus when applied to the agar medium: A, Control; B, 100 μm JA.
Figure 2.
A, Scheme of aphidicolin-based synchronization protocol. B, The effect of various concentrations of ABA and JA on mitotic activity in synchronized BY-2 cells. Both hormones were applied immediately after aphidicolin release.
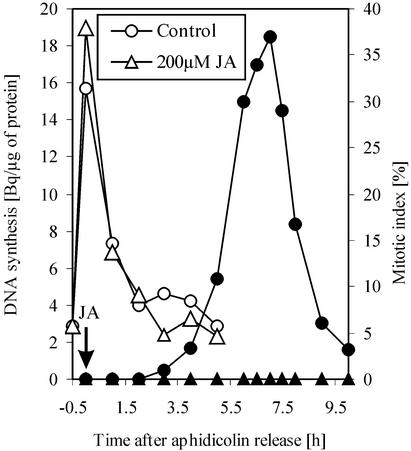
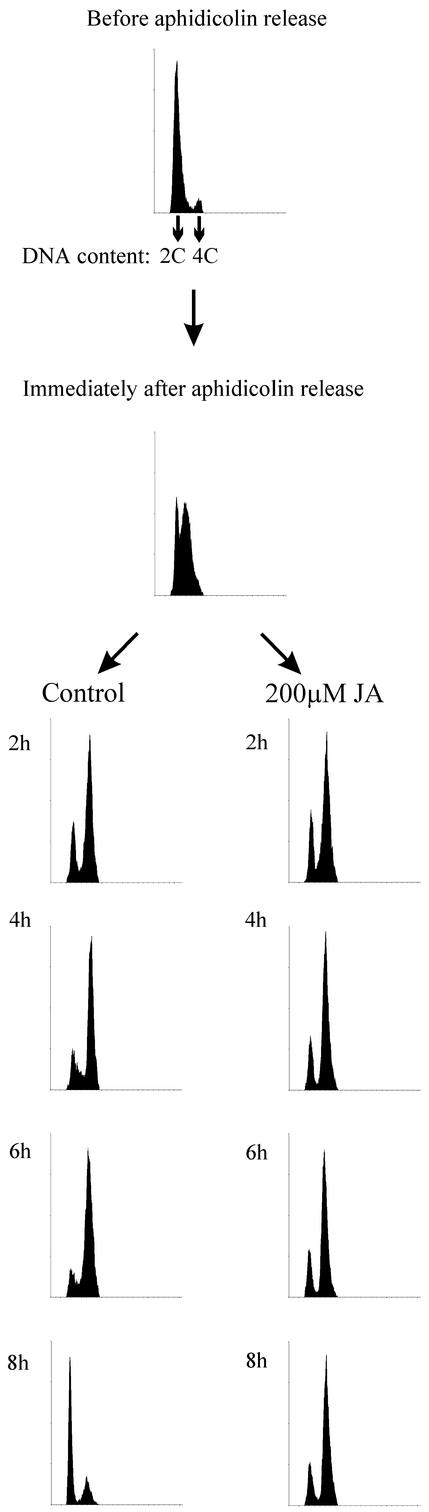
To examine whether the DNA synthesis was affected by JA treatment, the aphidicolin-released culture was divided in two subcultures: One of them was treated with 200 μm JA, and the other one was kept as control. DNA synthesis in both cultures was monitored by flow cytometry and thymidine incorporation (Figs. 3 and 4). The thymidine incorporation protocol used was a pulse-labeling method, which reflected the rate and frequency of replication. Thymidine incorporation did not differ between JA treated and control culture (Fig. 3). Flow cytometry results (Fig. 4) revealed that both cultures formed comparable G2 populations (about 70% of all cells). However, the transition from 4C to 2C DNA content occurred only in the control culture between the 6th and 8th hour after aphidicolin release.
Figure 3.
DNA replication during treatment with a JA concentration, which effectively prevents mitosis. Thymidine incorporation by the synchronized culture treated immediately after aphidicolin release compared with untreated control. White symbols correspond to thymidine incorporation and black symbols to mitotic index.
Figure 4.
Flow cytometric analysis of the synchronized culture treated in the same manner as in Figure 3. We took samples before, after the aphidicolin release and later, every 2 h from jasmonate-treated and reference cultures.
Mitotic Block Depends on the Application Time
As DNA synthesis appeared to be undisturbed by jasmonate application, we examined further the time requirement of JA application on the inhibition and delay of mitosis. The aphidicolin-synchronized culture was divided into five subcultures: control and four others to which 100 μm of JA was applied at 0, 1, 2, or 4 h after aphidicolin release. The control culture treated with a corresponding volume of methanol reached a mitotic peak of 50% at 6 h after release (Fig. 5). In the culture treated with JA at 0 h, a mitotic peak of only 16% was observed 9 h after aphidicolin release. JA treatment at late S-phase (2 h) was less effective, and application just before G2/M transition (4 h) resulted in a mitotic peak at the same time as in the control yet slightly reduced in height. This experiment revealed that the effect of JA depended on the stage of the cell cycle in which cells were treated.
Figure 5.
The effect of application time of 100 μm JA on the frequency of mitosis in synchronized BY-2 cells. After aphidicolin release, the culture was divided into four subcultures. JA was added immediately, 2 or 4 h after the release. One subculture was kept untreated as control.
Effect of Jasmonate and ABA on G1/S Transition
Using aphidicolin-released cultures limited our observation to S, G2, and mitotic stages of the cell cycle because by the time cells reached another division cycle, they lost synchrony to such an extent that obtained results became difficult to interpret.
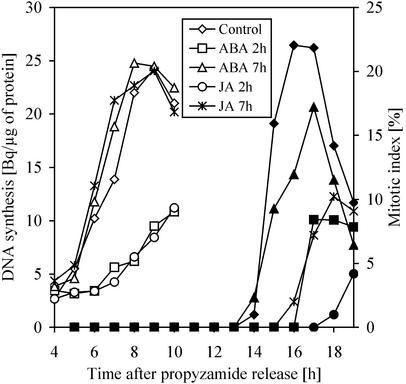
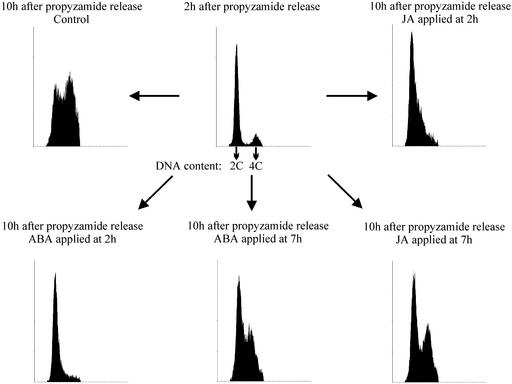
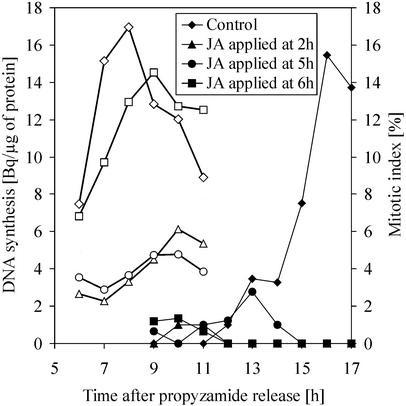
Prior knowledge that ABA acts negatively on the cell cycle progression at the level of the G1/S transition led us to compare the effect of ABA and JA at this point. To focus on G1/S transition, the aphidicolin-synchronized culture was subsequently treated with propyzamide, an agent that inhibits tubulin polymerization and therefore prevents spindle assembly and chromosome separation. Propyzamide was removed when most of the cells were arrested in metaphase. The scheme in Figure 6 represents the approximate timing of the cell cycle phases within the experimental setup. Using this method, a highly synchronized culture (>90% of the cells) at M/G1 transition can be obtained. In a first set of experiments, we compared the effect of ABA and JA on DNA synthesis and mitosis. The propyzamide-released culture was divided into five subcultures: One was treated with 200 μm ABA 2 h after the release, which corresponded to early G1-phase; a second one was supplied with ABA at 7 h, when cells were already involved in DNA synthesis; a third one was treated at 2 h with 100 μm JA; a fourth one was supplied with JA at 7 h; and the fifth one was treated with an equal volume of methanol and kept as a control. At appropriate time points, samples were taken for mitotic index, thymidine incorporation, and flow cytometric analysis (Figs. 7 and 8). The control culture reached S-phase between 6 and 11 h and its mitotic peak 16 h after propyzamide release. Both of the cultures that were treated with 200 μm ABA or 100 μm JA during S-phase (7 h after the release) showed similar DNA synthesis profiles as the control (Fig. 7). Flow cytometry showed the formation of a G2 population (4C DNA content) in both cultures (Fig. 8). The mitotic index was slightly reduced in the ABA-treated culture, with a mitotic peak at 17 h. A stronger reduction of mitotic index occurred in the JA-treated culture, consistent with previous results (Fig. 2B) showing that it was JA and not ABA that interfered with G2/M transition. In contrast, cultures supplied with either 100 μm JA or 200 μm ABA at the 2nd h, which was before G1/S transition, displayed similar strongly reduced thymidine incorporation. Corresponding flow cytometric data showed only a minor amount of JA- or ABA-treated cells with 4C DNA content at the 10th h of the experiment when compared with the control (Fig. 8). Taken together, these data strongly support the idea of a negative regulation of G1/S transition by both ABA and JA. There was also a difference in mitotic index between ABA- and JA-treated cultures. Although both hormones similarly decreased thymidine incorporation when applied before G1/S transition, JA treated-cultures had almost no mitotic events, whereas cell divisions were still observed after ABA treatment. As shown above, JA prevents DNA synthesis when applied at G1, 2 h after propyzamide release. To pinpoint more precisely the possible targets affected by the presence of jasmonate, we applied JA at various points between propyzamide release and initiation of DNA synthesis (Fig. 9). The propyzamide-released culture was divided into six subcultures to which 100 μm JA was applied 1, 2, 3, 4, 5, or 6 h after release, and one culture was left untreated as the control. As in previous experiments, JA applied before G1/S transition, i.e. before the 6th h after propyzamide release, caused a strong and similar decrease in thymidine incorporation. For sake of clarity, only data for the 2nd, 5th, and 6th h were presented in Figure 9. When applied at the 6th h, when most of the cells were at the beginning of S-phase, JA had only a moderate effect on DNA synthesis. These data pointed to a very specific target on which JA acts during G1/S transition.
Figure 6.
Scheme of synchronization protocol with subsequent block and release of aphidicolin and propyzamide.
Figure 7.
The effect of 200 μm ABA and 100 μm JA applied at different stages of the cell cycle on thymidine incorporation (represented by white symbols) and mitotic index (black symbols) in synchronized BY-2 cells. Hormone was applied at G1 (2 h after the release) or early S-phase (7 h after the release) and culture was sampled for thymidine incorporation, mitotic index, and flow cytometry (see Fig. 8). The results are compared with an untreated reference culture.
Figure 8.
The effect of 200 μm ABA and 100 μm JA applied at different stages of the cell cycle. Hormone was applied at G1 (2 h after the propyzamide release) or early S-phase (7 h after the release), and culture was sampled for thymidine incorporation, mitotic index (see Fig. 7), and flow cytometry. The central graph represents the G1 DNA content in the culture 2 h after the propyzamide was removed. Surrounding graphs represent DNA content in the treated samples isolated 8 h later, which corresponds to the late S-phase in the untreated culture (top left).
Figure 9.
The effect of application time of JA on the initiation of DNA synthesis. The culture, prepared by combined aphidicolin/propyzamide block, was divided into subcultures to which JA was added at a given time point. The subcultures were sampled for thymidine incorporation (white symbols) and mitotic index (black symbols).
Histone H1 Kinase Activity in Jasmonate-Treated Cells
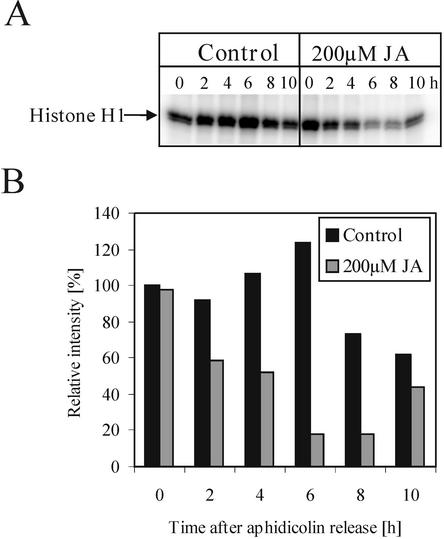
There was an intriguing discrepancy between the application time (0 up to 2 h after aphidicolin release, which corresponds to S-phase) when jasmonate treatment has its maximal effect on G2/M transition (Fig. 5) and the apparent undisturbed progression of replication (Figs. 3 and 4). This excluded the possibility of toxicity of the high concentrations of JA used. On the other hand, it raised the question of which of the elements of the cell cycle machinery were affected and how.
We measured the activities of CDKs, the core protein kinases of the cell cycle, upon separation from other kinases by affinity purification with p13-agarose. The histone H1 kinase activity in the cycling control culture remained high, with a transient slight increase in the activity at 6 h (Fig. 10), just before the mitotic peak, which occurred at 7 h (data not shown). Treatment with 200 μm JA resulted in a decrease of the H1 kinase activity detectable as soon as 2 h after aphidicolin release (Fig. 10), whereas the level of the CDKA protein remained unaffected, as detected by western blot using anti-PSTAIRE antibody (data not shown).
Figure 10.
Histone H1 kinase activity in jasmonates-treated synchronized BY-2 cells. The aphidicolin-released culture was divided into two subcultures, one immediately treated with 200 μm JA to suppress mitosis completely, the other left untreated. A, Histone H1 phosphorylation by p13 affinity purified protein from treated and untreated cultures, sampled as indicated. B, Quantification of the data presented in A.
DISCUSSION
In experiments reported by Ueda and Kato (1982) on the soybean callus, growth inhibition caused by jasmonate was attributed to the interference with action of the exogenous cytokinins, whose presence was essential for the survival of the culture. BY-2 cells synthesize sufficient endogenous cytokinins to sustain growth independently of exogenous supply (Nagata et al., 1992). In BY-2 callus, however, we also observed growth inhibition by JA. Detailed analysis of the cell cycle progression in synchronized suspension culture revealed that G1/S and G2/M transitions were blocked. JA has been reported to change the ratio between cytokinin ribosides and free bases and to decrease the level of zeatin in potato (Solanum tuberosum; Dermastia et al., 1994). In BY-2 cells, zeatin levels peak sharply before G2/M transition (Redig et al., 1996), and its exogenous supply can rescue G2 arrest caused by the inhibition of cytokinin production (Laureys et al., 1998). However, a block of G2/M transition caused by JA could not be recovered by zeatin treatment (data not shown). This means that the effect of JA was not directly linked with cytokinin action, and we have to seek its targets elsewhere.
We decided to compare the effects of JA on cell cycle progression with those of ABA, which were already documented in Arabidopsis (Leung et al., 1994; Wang et al., 1998). Moreover, ABA was proposed to be a primary factor in wound response, which triggers JA synthesis (Peña-Cortés and Willmitzer, 1995). ABA has been previously reported to diminish the number of mitotic events and thymidine incorporation in root meristems of sunflower (Robertson et al., 1990) and to reduce bromodeoxyuridine incorporation into Arabidopsis root meristems (Leung et al., 1994).
Our results demonstrate that exogenous ABA application inhibits G1/S transition in synchronized BY-2 cells, which is in agreement with previous literature data indicating ICK1 among targets of ABA action (Wang et al., 1998). This protein is a potent inhibitor of histone H1 kinase activity of the p13suc1-associated kinases. It can interact with both CDKA1 and cycD3, and its expression is induced by ABA (Wang et al., 1998).
We also found that ABA had no effect on further cell cycle progression when applied during S-phase. In contrast with ABA, JA was also able to hinder G2/M transitions. The effect of JA on G2/M transitions was remarkably more pronounced when applied during S-phase than when applied at the late G2-phase. This suggests that either the JA sensor mechanisms are most sensitive during S-phase or that there is delay between signal and response. The decrease in CDK activity detectable after 2 h of treatment suggests that the response is fast and occurs already in late S-phase. However, what is really triggered by JA in the cell remains an enigma.
There is evidence for the regulation of gene expression by jasmonates in BY-2 cells. For example, cathepsin D inhibitor from potato is positively regulated by JA in potato plants, and a cathepsin D promoter-β-glucuronidase fusion remains jasmonate inducible when expressed in BY-2 cells (Ishikawa et al., 1994). This suggests that the signaling and transcription machinery necessary to confer jasmonate responsiveness of a gene promoter is present in BY-2. JA treatment was reported to change the protein expression pattern in nonsynchronized BY-2, and several cDNAs were identified by differential screening (Imanishi et al., 1998). Another interesting feature of the effect of jasmonate on BY-2 cells is the intriguing sensitivity of the microtubular network of S-phase cells (Abe et al., 1990). Synchronized BY-2 cells respond with a total disruption of microtubular network when JA is applied but only when the cells are engaged in DNA synthesis. The microtubules seem to restore at the later stages of the cell cycle. Our data demonstrate undisturbed DNA synthesis despite the lack of organized microtubular structures. This can be easily understood because propyzamide or oryzaline treatment, which also disrupts the microtubular network completely, manifests its effect only at metaphase, when the lack of mitotic spindle stops the cycle, leaving the condensed chromosomes scattered in the cytoplasm. Thus, the lack of microtubular network during S-phase is not sufficient to prevent DNA synthesis or mitotic entry, and other mechanisms must be involved in the cell cycle arrest caused by jasmonates. León et al. (1998) demonstrated that JA-induced gene expression in Arabidopsis was inhibited by (2,5-di-tert-butyl)-1,4-hydroqinone, which mobilizes Ca2+ from internal stores. The phosphatase inhibitor okadaic acid had a similar effect, whereas a kinase inhibitor, staurosporine, positively regulated the expression of the genes normally induced by JA, thus indicating the need for protein phosphatase acting downstream of JA (Rojo et al., 1998). Because plant MAP kinases mediate various aspects of the stress responses, the hypothesis that jasmonate signaling might negatively cross-react with a MAP kinase pathway, which is positively controlled by phosphorylation, seems very tempting. However, two tobacco kinases (which are involved in wound response) related to mitotic alfalfa (Medicago sativa) kinase MMK3 (Bögre et al., 1999), salicylic acid-induced protein kinase, and wound-induced protein kinase are not influenced by JA (Kumar and Klessig, 2000). The only kinase in tobacco for which there was reported control by jasmonates is WAPK; expression of this kinase is induced by JA but its sequence is not related to MAP kinases (Lee et al., 1998).
It has been clear that JA inhibits plant growth, because plant extracted jasmonates were shown to inhibit sheath elongation in rice seedlings and hypocotyl and root elongation in lettuce (Lactuca sativa) seedlings (Yamane et al., 1981). Plant growth may be inhibited by the disruption of either the meristem activity or cell expansion in the elongation zone. Our results seem to favor the first possibility, because we observe the disturbance of both the G1/S and G2/M transitions after JA treatment. The down-regulation of CDK activity after jasmonate treatment of aphidicolin-released cells suggests the possibility that JA targets the cell cycle machinery as a part of a stress response.
Inhibition of root elongation in Arabidopsis was exploited to isolate jasmonate-insensitive mutants jar1 (Staswick et al., 1992), jin1 and jin 4 (Berger et al., 1996), and coi1 (Feys et al., 1994), all of them nonallelic. Only the COI1 gene has been identified (Xie et al., 1998), revealing a sequence with a F-box motif and a Leu-rich repeat that are characteristic for the component of Skp1/Cdc53(cullin)/F-box protein complex, which is involved in other organisms in the targeting for proteolysis cell cycle components, such as yeast (Saccharomyces cerevisiae) Cln1 and Cln2 cyclins, human cyclin E, and mouse (Mus musculus) p27KIP1 CDK inhibitor (Kipreos and Pagano, 2000).
Also, plant B-type cyclins, which accumulate before mitotic entry, undergo proteolysis in a ubiquitin-dependent manner to permit anaphase and exit from mitosis (Genschik et al., 1998).
The activity of the CDK decreased after JA treatment, whereas the level of the PSTAIRE protein remained unaffected. This suggests that decreased CDK activity could be related to cyclin availability. However, to our knowledge, there is no evidence of any involvement of Coi protein in cell cycle regulation, nor has its expression been demonstrated in BY-2 cells.
A second level of the control of CDKA activity involves the phosphorylation and subsequent removal of the phosphate group from the Tyr residue by cdc25. The Tyr phosphatase activity of the enzyme specific for the CDK is positively regulated by cytokinins (Zhang et al., 1996). In human cells, cdc25 is also involved in the response to UV-mediated DNA damage in a p53-independent pathway, where DNA damage induces S-phase arrest via activation of Chk1 kinase, which phosphorylates cdc25A and targets it for proteasome degradation (Mailand et al., 2000). In plant cells, there is growing evidence for the role of jasmonates in UV response (Conconi et al., 1996). Some of the genes induced by jasmonates are common for UV and pathogen response, such as chalcone synthase, Phe ammonia lyase in parsley and Arabidopsis (Longemann et al., 1995; Long and Jenkins, 1998), and polyphenol oxidase, Leu aminopeptidase, Thr deaminase, and proteinase inhibitors in tomato (Lycopersicon esculentum) plants (Conconi et al., 1996). UV exposure cannot induce mRNA expression of those genes in the tomato JL-5 mutant, defective in jasmonate synthesis, suggesting the requirement for octadecanoid compounds for a proper response (Conconi et al., 1996). On the other hand, the levels of neither 12-oxophytodienoic acid nor JA increase after UV exposure of tomato plants (Stratmann et al., 2000), which might imply that the actual cross-talk between jasmonate signaling and UV response is downstream of jasmonates.
UV and fungal elicitor exposure of a cell culture of parsley decreases the expression of many genes essential for the cell cycle, such as histones H2A, H2B, H3, H4, CDKA, and cyclin B1;1 (Longemann et al., 1995), leading to the inhibition of growth. If there is cross-talk between those two pathways, one might expect JA to exert a negative effect on cell proliferation by triggering similar check point mechanisms as UV light. We then can assume a physiological role for the cell cycle block caused by jasmonates as being a distress signal, which slows the vegetative growth during defense responses.
MATERIALS AND METHODS
Cell Culture and Synchronization
BY-2 cells were maintained as described by Nagata et al. (1992) with modifications: The culture was refreshed weekly by transfer of 0.5 mL of a 7-d-old culture into 50 mL of fresh Murashige and Skoog medium (Duchefa, Haarlem, The Netherlands) pH 5.8, containing 3% (w/v) Suc (Duchefa), 0.2 g L−1 KH2PO4 (Merck, Darmstadt, Germany), 10 mg L−1 myo-inositol (Sigma, Bornem, Belgium), 1 mg L−1 thiamin hydrochloride (Sigma), and 0.2 mg L−1 2,4-D (Serva, Hiedelberg, Germany), referred to hereafter as “medium.” The culture was kept at 27°C at constant darkness and 130 rpm. For maintenance on petri dishes, medium was additionally supplied with 1% (w/v) agar (Sigma). The synchronization protocol was based on the method of Nagata et al. (1992) as illustrated in Figure 2A. The stationary culture was transferred in a proportion 14:100 to the fresh medium, supplied with 5 mg L−1 aphidicolin (ICN Biomedicals, Asse, Belgium). After 24 h of incubation, cells were released by extensive washing with fresh medium without 2,4-D and thiamine, (4 L per 100 mL of blocked culture). Afterward, cells were transferred into fresh medium and divided equally into an appropriate number of subcultures, which were subsequently treated with methanol solutions of (±)JA, (±)methyl jasmonate (Apex Organics, Honiton, Devon, UK), or (±)ABA (Sigma) in various concentrations, prepared to obtain a 1:1000 dilution with the volume of experimental culture. An appropriate volume of methanol was added to the control culture. For a double block, an additional step was included. After aphidicolin release 1.54 mg L−1 propyzamide was added and after approximately 6 h, when at least 60% of the cells were either in prophase or in metaphase, the culture was released by washing and cells were transferred to the fresh medium.
Thymidine Incorporation
DNA synthesis was monitored in 1-mL samples by pulse labeling with 1 μCi of [methyl-3H]thymidine (Amersham Pharmacia Biotech Benelux, Roosendaal, The Netherlands) for 30 min at 28°C on a rotary shaker. Labeled cells were collected by centrifugation 5 min at 2,000 rpm and immediately frozen in liquid N2. Total DNA and protein was extracted by grinding and precipitated with 10% (w/v) trichloracetic acid, containing 10 mm thymidine (Sigma). The pellet was washed with 5% (w/v) trichloracetic acid, 70% (v/v) ethanol, and acetone, and suspended in 0.2 n NaOH. Protein content was measured with the Bradford reagent (Bio-Rad Laboratories, Hercules, CA), using bovine serum albumin as standard. The remaining sample was hydrolyzed overnight in 37°C, and incorporated radioactivity was measured by scintillation counting. Upon quench correction, total DNA synthesis was expressed as Bq per μg of protein in the sample.
Protein Extraction and Histone H1 Kinase Assays
Kinase activity was measured according to Reichheld et al. (1999). Protein extracts were prepared by grinding cells in liquid N2 with mortar and pestle to a fine powder. Frozen powder was suspended in extraction buffer, containing 60 mm β-glycerolophosphate, 15 mm p-nitrophenylphosphate, 25 mm Tris-HCl, pH 7.5, 15 mm ethyleneglycol-bis-(-aminoethyl ether)-N,N′-tetraacetic acid (EGTA), 15 mm MgCl2, 2 mm dithiothreitol, 1 mm NaVO3, 50 mm NaF, 20 mg L−1 antipain, 20 mg L−1 aproteine, 20 mg L−1 soybean (Glycine max) trypsine inhibitor, 100 μm benzamidine, 1 mm phenylmethylsulfonyl fluoride, and 0.1% (v/v) Nonidet P-40 and centrifuged at 4°C, 13,000 rpm for 5 min. An amount of supernatant, corresponding to 100 μg of protein, was adjusted with extraction buffer to equal volume where necessary and incubated at 4°C for 2 h with p13suc1-agarose beads (Oncogene, San Diego). After three times of washing with extraction buffer, beads were incubated for 20 min in the reaction buffer containing 50 mm Tris-HCl, pH 7.8, 15 mm MgCl2, 5 mm EDTA, 2 mm dithiothreitol, 2 mg L−1 of recombinant cAMP-dependent kinase inhibitor (Sigma), 0.6 g L−1 of histone H1 (Sigma), 10 μm ATP, and 15 mCi L−1 of [γ-32P]ATP (Amersham Pharmacia Biotech).
Adding 5× Laemmli loading buffer stopped reaction, and histone was separated from ATP by SDS-PAGE in 17.5% (w/v) acrylamide gel. Radioactivity of the bands was visualized and quantified with PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Microscopy and Flow Cytometry
Fluorescein diacetate 5 g L−1 (Serva) and 4′,6-diamino-phenylindole were used for viability staining and DNA visualization, respectively. For mitotic index analysis, cells were sampled during the experiment, 0.5 mL per sample, and fixed in solution ethanol/acetic acid 3:1 (v/v). Cells were washed in phosphate-buffered saline, stained with 4′,6-diamino-phenylindole, and counted (Nikon fluorescent microscope, Nikon Europe, Badhoevedorp, The Netherlands). Five hundred cells were counted per slide, and stages from early prophase until anaphase were considered as mitotic. Two-milliliter samples were taken for flow cytometry. Cells were washed with 0.66 m sorbitol in Murashige and Skoog medium, pH 5.8, and the cell wall was removed by treatment for 1 h at 37°C with 0.1% (w/v) pectolyase and 2% (w/v) cellulase (Sigma) dissolved in 0.66 m sorbitol in Murashige and Skoog medium. Protoplasts were washed twice with buffer containing 45 g L−1 mannitol and 18 g L−1 Glc in Murashige and Skoog medium, pH 5.8, and sedimented by centrifugation at 1,500g for 5 min. Nuclei were released from the pellet in 250 μL of Galbraith buffer (45 mm MgCl2, 30 mm sodium citrate, 20 mm 3-(N-morpholino)-propanesulfonic acid [MOPS], 1% [v/v] Triton X-100, pH 7.0) and fixed with 5 μL of 37% (v/v) formaldehyde (Sigma). DNA staining was performed according to the method of Vindelov et al. (1983). Fixed samples were washed with phosphate-buffered saline and filtered through a 20-μm nylon membrane. One-hundred microliters of filtrate was treated with 200 μL of solution A containing 3.4 mm trisodium citrate, 0.1% (v/v) Nonidet P-40, 0.5 mm Tris, pH 7.6, and 1.5 mm spermine tetrahydrochloride (Sigma; stock solution), to which 30 mg L−1 trypsine was added. After 10 min of incubation at room temperature, 150 μL of solution B containing the stock solution to which 0.5 g L−1 trypsine inhibitor and 100 mg L−1 of ribonuclease A were added and the sample was incubated for another 10 min. Finally, the nuclei were stained with solution C containing the stock solution to which 0.4 g L−1 propidium iodide (Sigma) and 1.2 g L−1 spermine tetrahydrochloride were added, and the sample was incubated for 1 h at 4°C and analyzed on FACScan (Becton-Dickinson, San Jose, CA) analytical flow cytometer.
ACKNOWLEDGMENTS
The authors thank Dr. Lieven de Veylder for kindly sharing his expertise in kinase assays and Prof. Herman Slegers and his coworkers for help with radioactivity work, especially Bert Grobben for PhosphorImager analysis.
Footnotes
This work was supported by Geconcenteerde Onderzoeks Actie and by the Belgian Program on Interuniversity Poles of Attraction (Prime Minister's Office, Science Programming, grant no. 15).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010592.
LITERATURE CITED
- Abe M, Shibaoka H, Yamane H, Takahashi N. Cell cycle-dependent disruption of microtubules by methyl jasmonate in tobacco BY-2 cells. Protoplasma. 1990;156:1–8. [Google Scholar]
- Benedetti CE, Xie D, Turner JG. COI1-dependent expression of an Arabidopsis vegetative storage protein in flowers and siliques and in response to coronatine or methyl jasmonate. Plant Physiol. 1995;109:567–572. doi: 10.1104/pp.109.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Bell E, Mullet JE. Two methyl jasmonate-insensitive mutants show altered expression of AtVsp in response to methyl jasmonate and wounding. Plant Physiol. 1996;111:525–531. doi: 10.1104/pp.111.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögre L, Calderini O, Binarova P, Mattauch M, Till S, Kiegerl S, Jonak C, Pollaschek C, Barker P, Huskisson NS. A MAP kinase is activated late in plant mitosis and becomes localized to the plane of cell division. Plant Cell. 1999;11:101–113. [PMC free article] [PubMed] [Google Scholar]
- Burssens S, de Almeida Engler J, Beeckman T, Richard C, Shaul O, Ferreira PCG, Van Montagu M, Inzé D. Developmental expression of the Arabidopsis thaliana CycA2;1 gene. Planta. 2000a;211:623–631. doi: 10.1007/s004250000333. [DOI] [PubMed] [Google Scholar]
- Burssens S, Himanen K, van de Cotte B, Beeckman T, Van Montagu M, Inzé D. Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana. Planta. 2000b;211:632–640. doi: 10.1007/s004250000334. [DOI] [PubMed] [Google Scholar]
- Conconi A, Smerdon MJ, Hove G, Ryan C. The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature. 1996;383:826–829. doi: 10.1038/383826a0. [DOI] [PubMed] [Google Scholar]
- Dermastia M, Ravnikar M, Vilhar B, Kovac M. Increased level of cytokinin ribosides in jasmonic acid-treated potato (Solanum tuberosum) stem node cultures. Physiol Plant. 1994;92:241–246. [Google Scholar]
- Ehsan H, Roef L, Witters E, Reichheld J-P, Van Bockstaele D, Inzé D, Van Onckelen Indomethacin-induced G1/S phase arrest of the plant cell cycle. FEBS Lett. 1999;458:349–353. doi: 10.1016/s0014-5793(99)01152-7. [DOI] [PubMed] [Google Scholar]
- Feys BJ, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschik P, Cirqui MC, Parmentier Y, Derevier A, Fleck J. Cell cycle-dependent proteolysis in plants: identification of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor MG132. Plant Cell. 1998;10:2063–2075. doi: 10.1105/tpc.10.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt H. Connecting oxidative stress, auxin, and cell cycle regulation through a plant mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2000;97:2405–2407. doi: 10.1073/pnas.97.6.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi S, Hashizume K, Kojima H, Ichihara A, Nakamura K. An mRNA of tobacco cell, which is rapidly inducible by methyl jasmonate in the presence of cycloheximide, codes for putative glycosyltransferase. Plant Cell Physiol. 1998;39:202–211. doi: 10.1093/oxfordjournals.pcp.a029358. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Yoshikara T, Nakamura K. Jasmonate-inducible expression of a potato cathepsin D inhibitor-GUS gene fusion in tobacco cells. Plant Mol Biol. 1994;26:403–414. doi: 10.1007/BF00039549. [DOI] [PubMed] [Google Scholar]
- Kipreos E, Pagano M. The-F box protein family. Genome Biol. 2000;1:1–7. doi: 10.1186/gb-2000-1-5-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda Y. Possible involvement of jasmonates in various morphogenic events. Physiol Plant. 1997;100:639–646. [Google Scholar]
- Kovtun Y, Chiu W-L, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Klessig DF. Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene and jasmonic acid. Mol Plant Microbe Interact. 2000;13:347–351. doi: 10.1094/MPMI.2000.13.3.347. [DOI] [PubMed] [Google Scholar]
- Laureys F, Dewitte W, Witters E, Van Montagu M, Inzé D, Van Onckelen H. Zeatin is indispensable for the G2-M transition in tobacco BY-2 cells. FEBS Lett. 1998;426:29–32. doi: 10.1016/s0014-5793(98)00297-x. [DOI] [PubMed] [Google Scholar]
- León J, Rojo E, Titarenko E, Sánchez-Serrano JJ. Jasmonic acid-dependent and -independent wound signal transduction pathways are differentially regulated by Ca2+/calmodulin in Arabidopsis thaliana. Mol Gen Genet. 1998;258:412–419. doi: 10.1007/s004380050749. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lee MH, Cung WI, Liu JR. WAPK, a Ser/Thr protein kinase gene of Nicotiana tabacum, is uniquely regulated by wounding, abscisic acid and methyl jasmonate. Mol Gen Genet. 1998;259:516–522. doi: 10.1007/s004380050843. [DOI] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris P-C, Guerrier D, Chefdor F, Giraudat J. Arabidopsis ABA Response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Long JC, Jenkins GI. Involvement of plasma membrane redox activity and calcium homeostasis in the UV-B and UV-A/blue light induction of gene expression in Arabidopsis. Plant Cell. 1998;10:2077–2086. doi: 10.1105/tpc.10.12.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longemann E, Wu SC, Schröder J, Schmelzer E, Somssich IE, Hahlbrock K. Gene activation by UV light, fungal elicitor or fungal infection in Petroselinum crispum is correlated with repression of cell cycle-related genes. Plant J. 1995;8:865–876. doi: 10.1046/j.1365-313x.1995.8060865.x. [DOI] [PubMed] [Google Scholar]
- Mailand N, Falck J, Lukas C, Syljuåsen RG, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol. 1992;132:1–30. [Google Scholar]
- Nakashima M, Hirano K, Nakashima S, Banno H, Nishihama R, Machida Y. The expression pattern of the gene for NPK1 protein kinase related to mitogen-activated protein kinase (MAPKKK) in a tobacco plant: correlation with cell proliferation. Plant Cell Physiol. 1998;39:690–700. doi: 10.1093/oxfordjournals.pcp.a029423. [DOI] [PubMed] [Google Scholar]
- Nishihama R, Banno H, Kawahara E, Irie K, Machida Y. Possible involvement of differential splicing in regulation of the activity of Arabidopsis ANP1 that is related to mitogen-activated protein kinase kinase kinases (MAPKKKs) Plant J. 1997;12:39–48. doi: 10.1046/j.1365-313x.1997.12010039.x. [DOI] [PubMed] [Google Scholar]
- Nishihama R, Ishikawa M, Araki S, Soyano T, Asada T, Machida Y. The NPK1 mitogen-activated protein kinase kinase kinase is a regulator of cell-plate formation in plant cytokinesis. Genes Dev. 2001;15:352–363. doi: 10.1101/gad.863701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Cortés H, Willmitzer L. The role of hormones in gene activation in response to wounding. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 395–414. [Google Scholar]
- Redig P, Shaul O, Inzé D, Van Montagu M, Van Onckelen H. Levels of endogenous cytokinins, indole-3-acetic acid and abscisic acid during the cell cycle of synchronized tobacco BY-2 cells. FEBS Lett. 1996;391:175–180. doi: 10.1016/0014-5793(96)00728-4. [DOI] [PubMed] [Google Scholar]
- Reichheld J-P, Vernoux T, Lardon F, Van Montagu M, Inzé D. Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J. 1999;17:647–656. [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH. Cytokinin activation of Arabidopsis cell division through a d-type cyclin. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH. Sugar control of the plant cell cycle: differential regulation of Arabidopsisd-type cyclin gene expression. Mol Cell Biol. 2000;20:4513–4521. doi: 10.1128/mcb.20.13.4513-4521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Hubick KT, Yeung EC, Reid DM. Developmental responses to drought and abscisic acid in sunflower roots.2. Mitotic activity. J Exp Bot. 1990;41:339–350. [Google Scholar]
- Rojo E, Titarenko E, Leon J, Berger S, Vancanneyt G, Sanchez-Serrano JJ. Reversible protein phosphorylation regulates jasmonic acid-dependent and -independent wound signal transduction pathways in Arabidopsis thaliana. Plant J. 1998;13:153–165. doi: 10.1046/j.1365-313x.1998.00020.x. [DOI] [PubMed] [Google Scholar]
- Sacks MM, Silk WK, Burman P. Effect of water stress on cortical cell division rates within the apical meristem of primary roots of maize. Plant Physiol. 1997;114:519–527. doi: 10.1104/pp.114.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter M. Differential expression of CAK (cdc2-activating kinase)-like protein kinase, cyclins and cdc2 genes from rice during the cell cycle and in response to gibberellin. Plant J. 1997;11:181–190. doi: 10.1046/j.1365-313x.1997.11020181.x. [DOI] [PubMed] [Google Scholar]
- Schuppler U, He P-H, John PCL, Munns R. Effect of water stress on cell division and cell-division-cycle 2-like cell-cycle kinase activity in wheat leaves. Plant Physiol. 1998;117:667–678. doi: 10.1104/pp.117.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P, Su W, Howell S. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA. 1992;89:6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JW, Stelmach B, Weiler E, Ryan C. UVB/UVA radiation activates a 48 kDa myelin basic protein kinase and potentiates wound signalling in tomato leaves. Photochem Photobiol. 2000;71:116–123. doi: 10.1562/0031-8655(2000)071<0116:sipuur>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Trehin C, Planchais S, Glab N, Perennes C, Tregear J, Bergounioux C. Cell cycle regulation by plant growth regulators: involvement of auxin and cytokinin in the re-entry of Petunia protoplasts into cell cycle. Planta. 1998;206:215–224. doi: 10.1007/s004250050393. [DOI] [PubMed] [Google Scholar]
- Ueda J, Kato J. Inhibition of cytokinin-induced plant growth by jasmonic acid and its methyl ester. Physiol Plant. 1982;54:249–253. [Google Scholar]
- Vindelov LL, Cristiensen IJ, Nissen NI. A detergent-Trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983;3:323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Wang H, Qi Q, Schorr P, Cutler A, Crosby WL, Fowke LC. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J. 1998;15:501–510. doi: 10.1046/j.1365-313x.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou Y, Gilmer S, Whitwil S, Fowke LC. Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J. 2000;24:613–623. doi: 10.1046/j.1365-313x.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Xie D, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene reacquired for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- Yamane H, Takagi H, Abe H, Yokota T, Takahashi N. Identification of jasmonic acid in three species of higher plants and its biological activities. Plant Cell Physiol. 1981;22:689–697. [Google Scholar]
- Zhang K, Letham DS, John PCL. Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta. 1996;200:12–20. doi: 10.1007/BF00196642. [DOI] [PubMed] [Google Scholar]