Abstract
Enteroviral meningitis causes appreciable morbidity in adults, including hospitalization, decreased activity, and headache. Limited data define the natural history of disease. No antiviral therapeutic agent has demonstrated improved outcome in controlled clinical trials. Pleconaril, an inhibitor of enterovirus replication, was tested in two placebo-controlled clinical trials. Of 607 randomized patients in a multicenter, double-blind placebo-controlled study of pleconaril (200 mg three times daily versus an identical-appearing placebo), 240 patients were confirmed to have enterovirus infection. The time to headache resolution was evaluated by using Kaplan-Meier survival methodology. A Cox regression model evaluated multivariate factors associated with disease resolution. Resolution of headache in patients with concomitant moderate to severe nausea at baseline occurred at a median of 9.5 days in the absence of therapy and was reduced to 7.0 days for pleconaril recipients (P = 0.009). For a headache score of >5 alone, treated patients resolved headache significantly more rapidly (P = 0.005). Males resolved headache 50% faster than females. Regardless of randomization group, patients with a baseline headache score of 5 or greater resolved headache 50% more slowly than patients with a baseline headache score of 4. No differences in either clinical or laboratory adverse events were noted. Over 50% of untreated patients had a persistent headache that was greater than 1 week in duration. Pleconaril shortened the course of illness compared to placebo recipients, especially in the early disease course. However, the benefit was achieved only modestly in a subgroup analysis of patients with more severe disease after adjusting for confounding variables.
Enteroviruses are the most common cause of viral meningitis, resulting in an estimated 75,000 cases annually in the United States (4, 7). Diagnosis of enterovirus infection of the central nervous system has been improved significantly by PCR assessment of cerebrospinal fluid (CSF) for the detection of viral RNA (3). While the incidence is higher in children, disease is not uncommon in adults (8). Data on the natural history of enteroviral meningitis in adults are limited; however, significant morbidity has been reported, including hospitalization, protracted illness, and impairment of normal activities (8).
Antiviral therapy of enteroviral meningitis is limited (10). The only nonproven therapeutic options for enteroviral meningitis are immune serum globulin and pleconaril. Pleconaril is an orally administered antiviral agent that inhibits enterovirus replication by a capsid-binding mechanism (4). Pleconaril attains severalfold-higher concentrations within the central nervous system than in serum, a feature that is beneficial for patients with infection of the brain (6). Pleconaril was initially evaluated for the treatment of rhinovirus infections and failed to secure the approval of the Food and Drug Administration because of the finding of induction of CYP 3A enzyme activity and the potential for drug interactions, particularly the interference with oral contraceptives. As a consequence, the sponsoring pharmaceutical company elected not to pursue licensure of pleconaril for other indications, including enteroviral meningitis. Recently, it was licensed to another firm in consideration of topical (intranasal) therapy for the common cold. Regardless, the data presented here define the therapeutic outcome of the clinical trials performed for treatment of enteroviral meningitis and further elaborate the natural history of disease.
MATERIALS AND METHODS
Source of data and study objectives.
ViroPharma Incorporated (Exton, PA) provided the data from two multicenter, randomized, placebo-controlled clinical trials to one of the authors (R.J.W.) for independent statistical analyses. After approval of the University of Alabama at Birmingham Institutional Review Board, analyses were undertaken to assess the natural history of disease as well as efficacy of pleconaril; these later analyses were defined as exploratory. The primary end point for both studies was resolution of headache, albeit with slight differences. For study 843-009 (study A), the efficacy of pleconaril (200 or 400 mg three times daily [t.i.d.] per orum versus an identical placebo for 7 days) was defined as the time to reduction of mild headache (headache score of 2 or less) on two consecutive days after initiation of treatment. Study 843-018 (study B) was designed to evaluate the time to complete resolution of headache (score 0) for subjects treated with pleconaril (200 mg t.i.d. versus placebo for 7 days; a limited number of patients were randomized to 400 mg t.i.d. also per orum). Relapse was defined as a headache score of 3 or more accompanied by at least one other sign or symptom with a score of >0 for two consecutive days.
For these analyses, we defined the time to complete resolution of headache as the first day with a headache score of zero.
Study population. (i) Inclusion/exclusion criteria.
Both studies had identical entry criteria and enrolled subjects who were male or postmenopausal or were surgically sterile female subjects over 14 years of age. The volunteers were judged to have viral meningitis predicated upon clinical presentation. CSF examination was required for both laboratory evaluation and diagnostic assessment by PCR. The CSF required >10 white blood cells/mm3, with no restrictions on protein or glucose concentrations. Headache and at least one additional symptom/sign of meningitis (photophobia, nuchal rigidity, fever, myalgia, or nausea/vomiting) were required for eligibility. Hospitalization was not required. Subjects were required to have a total morbidity score (TMS) of 9 or greater with a headache score of 4 or greater. The headache had to be present less than 48 h prior to study drug administration. The TMS was calculated as the sum of the scores for the following individual symptoms: headache, nuchal rigidity, photophobia, myalgia, fever, and nausea/vomiting. Each symptom/sign, other than headache, was ranked by the subject on a four-point scale of 0 (absent) to 3 (severe). For instance, for nausea/vomiting, 0 = none, 1 = mild (nausea present, no emesis), 2 = moderate (two or fewer episodes of emesis in the preceding 24 h), and 3 = severe (more than two episodes of emesis in the preceding 24 h). Headache was ranked on a seven-point scale of 0 to 6: 0 = none, 1 = barely noticeable, 2 = mild (able to function), 3 = moderate (requires medication), 4 = moderately severe (able to function but prefers to stay in bed), 5 = severe (must stay in bed), and 6 = very severe (incapacitating). Therefore the maximum TMS is 21. The TMS was piloted in a preliminary trial (7).
Exclusion criteria included age of <14 years, pregnancy, and being an immunocompromised host (as defined by an underlying malignancy on or off chemotherapy within the last 3 years or human immunodeficiency virus infection).
(ii) Diagnosis of enteroviral meningitis.
Cerebrospinal fluid at study entry was evaluated for the presence of enteroviral RNA by PCR. All assays were performed at a Central Laboratory (H. Rotbart, Children's Hospital, University of Colorado, Denver, Colorado). Results of the assay were not available to investigators until after subjects had completed the trial.
(iii) Randomization.
Prior to randomization, all patients provided written informed consent. Local institutional review boards at participating institutions approved the study protocols. Both studies were conducted between 1999 and 2002. In both studies pleconaril was administered in an oral liquid formulation and treatment assignment was based on a computer-generated randomization schedule. Patients were randomized so that for every two patients who received pleconaril at 200 mg t.i.d., one patient received a matching volume (5 ml) of placebo; similarly, for every two patients who received pleconaril at 400 mg t.i.d., one patient received a matching volume (10 ml) of placebo. In study B, which recruited patients over a longer period of time than study A, enrollment at 400 mg t.i.d. was discontinued through a protocol amendment based on a preliminary analysis of data from another protocol (data not available to these investigators) that showed no clinical advantage at this dosage. Patients enrolled subsequently in study B were randomized so that for every one patient who received pleconaril at 200 mg t.i.d., one patient received a matching volume (5 ml) of placebo. A computer-generated card randomization in blocks of six was utilized.
For both studies, subjects meeting the inclusion criteria received their first dosage within 48 h after onset of headache. For study A, 198 patients were randomized: 62 received placebo, 68 and 68 received 200 mg t.i.d. and 400 mg t.i.d. of pleconaril, respectively. For study B, 409 patients were randomized: 180 received placebo, 181 received pleconaril at 200 mg t.i.d., and 48 received pleconaril at 400 mg t.i.d.. Because enrollment at 400 mg t.i.d. was discontinued, patients receiving this dosage were not included in the current analysis.
Data collection.
Subjects were either assessed in the hospital or contacted daily by a study coordinator who recorded symptoms, activity, and analgesic use until the volunteer resumed full activity and had a TMS score of 3 or less and a headache score of 2 or less. Thereafter, subjects were assessed every other day until discharged at day 28 or completely well or had a headache score of 0.
Statistical analyses.
As per protocol definition, efficacy analyses included intent-to-treat infected subjects: all enterovirus-confirmed subjects with a TMS score of >9, including a headache score of >4, who received at least one dose of study medication. Subjects found to be negative by PCR for enterovirus were excluded from these analyses but were included in all safety assessments. Assessment of efficacy differences in treatment groups was made using Kaplan-Meier estimates and rank statistic. Prognostic variables examined with a Cox regression model included age, sex, race, and baseline headache score. Demographic and baseline clinical characteristics were compared between the two protocols by the chi-square test for categorical variables and t test for continuous variables. Because both studies utilized virtually identical protocols, the populations were pooled for some of the statistical analyses of efficacy, such as headache resolution. In such models the study was entered as a covariate (0 or 1), and an interaction term was added to assess the relationship of treatment effect across the two studies.
Safety end points included the incidence of reported clinical adverse events and laboratory aberrations reflecting hematologic, hepatic, and renal function. These were compared by the chi-square statistic or Fisher's exact test.
RESULTS
Demographics and study population characteristics.
A total of 36 and 60 institutions participated in studies A and B, respectively, enrolling a total of 607 patients. Of the total, 240 patients were confirmed to have enteroviral infection by PCR (39%). The primary efficacy analysis for study A was performed on 79 enterovirus-confirmed patients: 37 randomized to placebo and 42 to pleconaril at 200 mg t.i.d.. For study B, 161 patients had confirmed enterovirus infection: 75 randomized to placebo and 86 to pleconaril at 200 mg t.i.d. There were no significant differences in the distribution of demographic factors by treatment group for study B, but there was a higher proportion of males randomized to pleconaril in study A (Table 1). Overall, approximately 75% of enterovirus-confirmed patients were between the ages of 20 and 40. No demographic differences were detected between clinical trial sites for either study. Furthermore, when the demographic characteristics for those with enteroviral proven disease were compared with those who were PCR negative, no differences were demonstrated (data not shown).
TABLE 1.
Summary of baseline demographics: all enterovirus-confirmed patients for protocols 843-009 and 843-018
| Characteristic | No. (%) of patients in treatment group
|
P value | |
|---|---|---|---|
| Pleconaril, 200 mg t.i.d. | Placebo | ||
| Study Aa | |||
| Age groups | |||
| <20 | 14 (33.3) | 3 (8.1) | 0.03 |
| 20-29 | 11 (26.2) | 18 (48.7) | |
| 20-39 | 8 (19.1) | 10 (27.0) | |
| ≥40 | 9 (21.4) | 6 (16.2) | |
| Gender | |||
| Male | 29 (69) | 13 (35) | 0.003 |
| Female | 13 (31) | 34 (65) | |
| Race | |||
| White | 27 (64) | 28 (76) | 0.27 |
| Other | 15 (36) | 9 (24) | |
| Study Bb | |||
| Age groups | |||
| <20 | 24 (27.9) | 16 (21.3) | 0.43 |
| 20-29 | 32 (37.2) | 24 (32.0) | |
| 20-39 | 24 (27.9) | 30 (40.0) | |
| ≥40 | 6 (7.0) | 65 (6.7) | |
| Gender | |||
| Male | 49 (57) | 36 (48) | 0.26 |
| Female | 37 (43) | 39 (52) | |
| Race | |||
| White | 70 (81) | 63 (84) | 0.66 |
| Other | 16 (19) | 12 (16) | |
For the pleconaril group, n = 42; for the placebo group, n = 37.
For the pleconaril group, n = 86; for the placebo group, n = 75.
There were no significant differences in the median baseline symptoms (headache, fever, stiff neck, light sensitivity, myalgia, nausea/vomiting) by treatment group in either protocol (Table 2). Median baseline symptom scores showed that the baseline headache score was more severe in study B (P < 0.0001) than in study A. The majority of enterovirus-confirmed patients had moderate or severe nausea at baseline (58%).
TABLE 2.
Summary of median baseline sign and symptom scores for all enterovirus-confirmed patients in study A and study Ba
| Parameter | Value for treatment group
|
|
|---|---|---|
| Pleconaril, 200 mg | Placebo | |
| Study A | ||
| No. of patients | 42 | 37 |
| Baseline symptom score for: | ||
| Headache | 5 | 5 |
| Fever | 1 | 1 |
| Stiff neck | 2 | 2 |
| Light sensitivity | 1 | 2 |
| Myalgia | 1 | 1 |
| Nausea/vomiting | 1 | 2 |
| Total symptom score | 12 | 12 |
| Study B | ||
| No. of patients | 86 | 75 |
| Baseline symptom score for: | ||
| Headache | 6 | 6 |
| Fever | 1 | 1 |
| Stiff neck | 2 | 2 |
| Light sensitivity | 2 | 2 |
| Myalgia | 1 | 1 |
| Nausea/vomiting | 2 | 2 |
| Total symptom score | 13 | 13 |
The range of all symptoms is 0 to 3 except the range for headache, which is 0 to 6. Therefore, the maximum TMS score is 21.
Relapse events.
For both studies, only two patients had a relapse of headache. For these patients, the time to headache resolution was adjusted to reflect the time to subsequent headache score of zero after the first relapse with no further relapse. Both patients were randomized to pleconaril at 200 mg t.i.d.
Headache resolution.
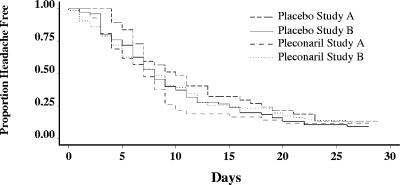
Utilizing the data from both clinical trials, no statistically significant difference was observed between enterovirus-confirmed patients in the pleconaril (200 mg t.i.d.) and placebo groups for the median time to complete resolution of headache (P = 0.15) (Fig. 1). No patient had evidence of a persistent headache after 28 days.
FIG. 1.
Time to resolution of headache, stratified by treatment and study protocol.
Subgroup analyses of patients with risk factors at baseline.
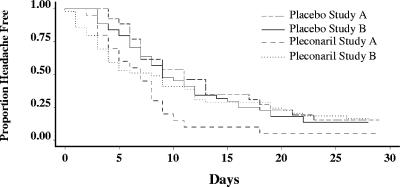
Among patients presenting with moderate or severe nausea, the median time to headache resolution (score of zero) was significantly shorter among the pleconaril recipients (7 days) than among the placebo recipients (11 days) in study A (Fig. 2) (P = 0.009). For patients presenting with moderate or severe nausea at baseline in study B, the pleconaril patients experienced a shorter time to headache resolution (8 days) than the placebo group (9 days) but not significantly so (P = 0.15). For the combined groups, the pleconaril recipients had a shorter time to complete resolution of headache (7 days) than the placebo recipients (9.5 days) (P = 0.009) (Fig. 2). Of note, severity of nausea did not influence drug compliance.
FIG. 2.
Time to resolution of headache in patients with moderate to severe nausea at baseline, stratified by treatment and study protocol.
Among patients with severe or very severe headache at baseline (headache score of 5 or 6), the median time to headache resolution was shorter for patients treated with pleconaril (8 days) than for those treated with placebo (9 days) (P = 0.05 for the combined studies).
Multivariate analysis of headache resolution.
Table 3, illustrates the Cox multivariate model of risk factors for headache resolution in patients with moderate to severe nausea at baseline. Patients treated with pleconaril experienced nearly a twofold-faster time to headache reduction (P = 0.035), controlling for study protocol (analysis I, below). Adjusting for other confounders, male patients experienced a 50% faster headache resolution than females (analysis II). Patients with a baseline headache score of 5 or 6 were about 50% less likely to resolve than patients with a baseline headache score of four. Treatment with pleconaril was not independently associated with reduction in headache morbidity overall (P = 0.11) after controlling for confounders (analysis II, below).
TABLE 3.
Multivariate analysis of factors associated with headache resolution in patients with moderate to severe nausea at baselinea
| Factor | HRb (95% CI)
|
|
|---|---|---|
| Analysis I | Analysis II | |
| Treatment | ||
| Pleconaril (200 mg) | 1.95 (1.05, 3.63) | 1.67 (0.89, 3.13) |
| Placebo | 1.0 | 1.0 |
| Study | ||
| 84-009 | 1.0 | 1.0 |
| 84-018 | 1.16 (0.69, 1.95) | 1.10 (0.60, 2.01) |
| Treatment-study interaction | 0.55 (0.26, 1.19) | 0.62 (0.29, 1.33) |
| Gender | ||
| Female | 1.0 | |
| Male | 1.51 (1.04, 2.20) | |
| Baseline headache score | ||
| 4 | 1.0 | |
| 5 vs 4 | 0.48 (0.27, 0.85) | |
| 6 vs 4 | 0.55 (0.29, 1.02) | |
Analysis I includes treatment effect adjusting for study protocol and study-treatment interaction. Analysis II includes treatment effect adjusting for gender and baseline headache score in addition to study and study-treatment interaction.
HR, hazard ratio.
Total morbidity score resolution.
The maximum daily severity of the TMS, as defined above, was assessed at study entry and daily until the end of the study. The analysis of the time to first symptom score of zero among all enterovirus-confirmed patients with moderate or severe headache and nausea/vomiting at baseline did not show any statistically significant differences between the pleconaril and placebo randomization groups. However, in both studies patients with more-severe disease at baseline resolved the TMS symptoms about 3 days sooner among the treatment group than in the placebo group.
Safety.
For assessment of safety, all patients entered into the clinical trial, whether enterovirus proven or not, were assessed. Overall, treatment-emergent adverse events were reported by similar proportions of study A placebo (74%) and pleconaril (68%) recipients (P = 0.41) (Table 4). However, a lower proportion of pleconaril recipients (26%) than placebo recipients (39%) reported “treatment-related” emergent adverse events (P = 0.14). The majority of the events were mild or moderate in intensity.
TABLE 4.
Summary of adverse events for all treated patients
| Category | No. (%) of patients in treatment group
|
P value | |
|---|---|---|---|
| Pleconaril | Placebo | ||
| Study A | |||
| Treated patients | 68 | 62 | |
| Patients with ≥1 treatment-emergent event | |||
| All adverse events | 46 (68.0) | 46 (74.0) | 0.41 |
| Related adverse events | 18 (26.0) | 24 (39.0) | 0.14 |
| Patients with serious adverse events | |||
| All adverse events | 3 (4.0) | 1 (2.0) | 0.62 |
| Related adverse events | 0 (0.0) | 0 (0.0) | |
| Study B | |||
| Treated patients | 181 | 180 | |
| Patients with ≥1 treatment-emergent event | |||
| All adverse events | 116 (64.0) | 112 (62.0) | 0.71 |
| Related adverse events | 59 (33.0) | 51 (28.0) | 0.38 |
| Patients with serious adverse events | |||
| All adverse events | 8 (4.0) | 10 (6.0) | 0.47 |
| Related adverse events | 0 (0.0) | 2 (1.0) | 0.23 |
The findings were virtually identical in study B. Treatment-emergent adverse events were reported by 62% of placebo and 64% of pleconaril recipients (P = 0.71). Treatment-related adverse events were 28% and 33%, respectively, for the placebo and pleconaril recipients (P = 0.38). The most commonly reported treatment-emergent adverse events were headache, back pain, nausea, vomiting, and diarrhea (Table 5). There were no notable differences between groups. No patients in any randomization group experienced elevations in clinical chemistries that were due to the study drug or disease state.
TABLE 5.
Summary of the most common treatment-emergent adverse events (occurring in >2% of patients in any treatment group)
| Event
|
No. (%) of patients reporting event
|
|
|---|---|---|
| Pleconaril (200 mg t.i.d.) | Placebo | |
| Study Aa | ||
| Headacheb | 15 (22) | 12 (19) |
| Nausea | 7 (10) | 7 (11) |
| Diarrhea | 7 (10) | 1 (11) |
| Back pain | 6 (9) | 5 (8) |
| Vomiting | 4 (6) | 6 (10) |
| Dyspepsia | 2 (3) | 8 (13) |
| Constipation | 2 (3) | 1 (2) |
| Study Bc | ||
| Nausea | 27 (15) | 10 (6) |
| Back pain | 24 (13) | 17 (9) |
| Diarrhea | 14 (8) | 20 (11) |
| Vomiting | 14 (8) | 13 (7) |
| Constipation | 14 (8) | 8 (5) |
| Headache | 12 (7) | 14 (8) |
| Dizziness | 12 (7) | 9 (5) |
| Insomnia | 9 (5) | 9 (5) |
For pleconaril group, n = 68; for placebo group, n = 62.
No patient reported baseline chronic headache or migraines.
For pleconaril group, n = 181; for placebo group, n = 180.
DISCUSSION
Limited clinical studies of pleconaril have reported a reduction in duration of symptoms associated with enteroviral meningitis. One report of 16 adult and pediatric patients with enterovirus meningoencephalitis reported clinical benefit in 12 patients (75.0%) (9). Benefit was defined as improved neurologic status, growth/weight gain, diminished myositis/fasciitis, and improved vision. The study was uncontrolled and included patients of all ages, including young children. A second report on two immunodeficient patients suggested a successful response to pleconaril therapy by single photon emission tomography scans (11). A third report was of a double-blind placebo controlled trial of pleconaril with 12 infants with enterovirus meningitis (4 pleconaril; 8 placebo), which showed that pleconaril was well tolerated, although efficacy was not demonstrated (1) because of small numbers of volunteers enrolled.
Initial analyses of these efficacy studies of pleconaril for the treatment of enteroviral meningitis failed to define clinical benefit, according to the end points provided to the Food and Drug Administration for a registrational trial. However, this post hoc subgroup analysis suggests the beneficial contribution of pleconaril to the acceleration of headache resolution in patients with moderate to severe nausea at baseline compared to the placebo recipients, albeit a modest 1 to 2 days. In the population examined, over 50% of the patients experienced these symptoms, making the results generalizable to enteroviral meningitis patients seen routinely in emergency departments. Thus, the initial analyses were compromised by the lack of disease severity in half of the patients entered into the controlled clinical trial.
Two important risk factors impacted headache resolution. First, male patients resolved their symptoms 50% faster than women, as reported in other settings. Women report systematically greater pain and duration of headache than men (2, 5). Second, severity of headache at baseline influenced resolution. All patients in this study presented with a headache score of 4 (moderately severe) or greater at baseline. For those patients with severe or very severe headaches (score of >5), resolution was significantly slower than patients with scores of 4, especially during the first week. While the current status of licensure of pleconaril would suggest that it will not be available for the treatment of disease, should the drug be studied for this entity again, it might be considered for selective use in patients presenting with severe and incapacitating headaches.
Some caution should be exercised in interpretation of the results. First, the analyses are exploratory in nature and cannot be considered appropriate for licensure. Second, the actual numbers of enterovirus-confirmed patients, while large in comparison to those in all other reported studies, still remains limited, a point readdressed below. Third, pleconaril requires oral administration. In patients with severe nausea, compliance may be a factor that influences outcome; however, this did not appear to compromise the current trial.
Importantly, these data elaborate the natural history of enteroviral meningitis in adults and, hopefully, will provide insight into the design of future registrational trials of other antiviral medications. Several points warrant iteration. First, only approximately 40% of patients who presented with findings compatible with aseptic meningitis had enteroviral proven disease. The remaining patients had pleocytosis and clinical findings of unknown etiology. This figure is lower than would have been anticipated. In that all assays were performed in a central laboratory by experienced staff, this figure is likely correct. Second, the median time to complete resolution of headache was over a week, namely, 8 days (95% confidence interval [CI], 7 to 9 days) for both randomization groups. Third, 15% of patients had a headache that persisted for 3 weeks. For those individuals with a moderate to severe headache at baseline, pleconaril was beneficial but only after subgroup analysis. Under these circumstances, the mean duration of headache for placebo recipients was nearly 12 days, and 18% had a persistent headache at 3 weeks. Fourth, as demonstrated by multivariate analysis, the more severe the headache the longer the time to resolution. Fifth, the study defines populations for which clinical benefit is most likely with therapy, namely, those with more severe headaches at presentation with or without moderate nausea. This group could be the focus of future clinical trials.
Taken together, the outcome for the placebo recipients alone defines an illness that is not benign and one that remains a target for antiviral drug development. Hopefully, future efforts will be directed toward this important disease entity.
Acknowledgments
This work was funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract (NO1-AI-30025, NO1-AI-65306, NO1-AI-15113, and NO1-AI-62554), the General Clinical Research Unit (RR-032), and the State of Alabama.
Participants in Belgium were as follows: L. Ceulemans, St. Jozefklinlek, Bornem; J. De Bleecker, AZ St. Lucas, campus H. Familie, Gent; J. De Bleecker, UZ Gent, Gent; F. Deceuninck, Kliniek OLV Van Lourdes, Waregem; J. Dobbelaere, Regional ZH Heilig Hart, Leuven; M. Marchau, OLV Ziekenhuis, Geraardsbergen; C. Monte, AZ St. Elisabeth, Zottegem; J. Mulliez, Stedelijik Ziekenhuis Roeselare, Roselare; E. Tack, Stadtskliniek Sint-Niklaas, Sint-Niklaas; P. Tack, St-Adries ZH, Tielt; P. Vanooteghem, AZ Diest Campus St-Jozef, Diest; M. Vantomme, Polikliniek/Volkskliniek Gent, Gent; and E. Verjans, Regional Ziekenhuis Sint-Trudo Campus St. Jozef, Sint Truiden.
Participants in Canada were as follows: F. Aoki, Health Sciences Center, Winnepeg, Manitoba; G. Dow, The Moncton Hospital, Moncton, New Brunswick; R. Duperval, CUSE site Fleurimont, Fleurimont, Quebec; G. Evans, Kingston General Hospital, Kingston, Ontario; G. Garber, Ottawa General Hospital, Ottawa, Ontario; R. Lalonde, Royal Victoria Hospital, Montreal Chest Institute, Montreal, Quebec; J. Langley, IWK-Grace Health Center, Halifax, Nova Scotia; M. Miller, SMBD-Jewish General Hospital, Montreal, Quebec; A. Paradis, Centre Hospitalier Afillie Universitaire de Quebec, Quebec City, Quebec; N. Rau, Credit Valley Hospital, Mississauga, Ontario; C. Rotstein, Hamilton Health Sciences Corp., Hamilton, Ontario; R. Saginur, Ottawa Civic Hospital, Ottawa, ON; S. Shafran, University of Alberta Hospital, Edmonton Alberta; M. Silverman, Ajax, Ontario; and S. Trottier, CHUQ-Pavillon CHUL, Ste-Foy, Quebec.
Participants in Denmark were as follows: J. Gerstoft, Righospitalet, Copenhagen; J. Lundgren, Hvidovre University Hospital, Hvidovre; and C. Pederson, Odense University Hospital, Odense C.
Participants in France were as follows: J. Beytout, Hotel-Dieu, Clermont Ferrand Cedex; D. Christmann, CHU Hospital Civil, Strausbourg Cedex; F. Janon, Centre Hospitalier Universitaire Gui de Chauliac, Montpellier Cedex; H. Portier, CHRU Hosptial du Bocage, DIJON Cedex; and R. Verdon, CHU de Caen, CAEN Cedex.
Participants in Germany were as follows: D. Claus, Klinikum Darmstadt, Darmstadt, and H. Langohr, Stadtisches Klinikum, Fulda.
Participants in Israel were as follows: R. Lang, Meir Hospital, Kfar Sava, and R. Raz, Ha'emek Medical Center, Afula.
In Panama, X. Saez-Llorens, Hospital del Nino, Ciudad de Panama, participated.
Participants in Poland were as follows: W. Halota, University School of Medicine, Bydgiszcz, and H. Trocha, Provincial Hospital of Infectious Disease, Gdansk.
Participants in South Africa were as follows: G. Ellis, Medi Clinic Vergelegen, Somerset West; L. Hervst, Vaalmed, Vanderbijlpark; J. Mynhardt, Kimberley; and J. Smuts, Muelmed Hospital, Arcadia.
Participants in the United Kingdom were as follows: C. Leen, Western General Hospital, Edinburgh; B. Mandal, North Manchester General Hospital, Manchester; and M. Wansbrough-Jones, St. Georges Hospital, London.
Participants in the United States were as follows: M. Abzug, The Children's Hospital, Denver, Colo.; J. Bernstein, Wright State School of Medicine/VA Campus, Dayton, Ohio; D. Boenning, Children's National Medical Center, Washington, D.C.; A. Brayer, URMC, Rochester, N.Y.; K. Burkhart, The Milton S. Hershey Medical Center, Hershey, Pa.; D. Carrison, University Medical Center of Southern Nevada, Las Vegas, Nev.; T. Chonmaitree, University of Texas Medical Branch, The Children's Hospital, Galveston, Tex.; B. Congeni, Children's Hospital Medical Center of Akron, Akron, Ohio; C. D'Addezio, Bay Research Associates, Bay City, Mich.; D. Delaportas, Hagerstown, Md.; K. Denninghoff, UAB School of Medicine, Birmingham, Ala.; R. Dick, Rochester General Hospital, Rochester, N.Y.; R. Dubinsky, University of Kansas Medical Center, Kansas City, Kans.; L. Dunbar, LSU Medical Center, New Orleans, La.; M. Eberst, University of North Carolina at Chapel Hill, Chapel Hill, N.C.; K. Fife, Outpatient Clinical Research Facility, Indiana Cancer Pavilion, Indianapolis, Ind.; G. Fort, Landmark Medical Center, Woonsocket, R.I.; D. Gilbert, Providence Portland Medical Center, Portland, Ore.; P. Giordano, Orlando Regional Medical Center, Orlando, Fla.; L. Graff, New Britain General Hospital, New Britain, Conn.; M. Greer, University of Florida, Gainesville, Fla.; L. Haglund, University of Cincinnati School of Medicine, Cincinnati, Ohio; F. Harchelroad, Allegheny General Hospital, Pittsburgh, Pa.; C. Harris, Regions Hospital, St. Paul, Minn.; R. Holman, Infectious Diseases and Medical Associations, Arlington, Va.; R. Jackson, William Beaumont Hospital, Royal Oak, Mich.; R. Jacobs, Arkansas Children's Hospital, Little Rock, Ark.; F. Kahn, Montana Health Research Institute, Billings, Mont.; D. Lang, Children's Hospital of Orange County, Orange, Calif.; A. Lentnek, Promina Northwest Physicians Group, Marietta, Ga.; M. Levitt, Highland General Hospital, Oakland, Calif.; R. Lichenstein, Western Health Center, Baltimore, Md.; C. Lucasti, South Jersey Infectious Diseases, Somers Point, N.J.; J. Lutz, Central California Medical Research, Fresno, Calif.; J. Maisel, Bridgeport Hospital, Bridgeport, Conn.; D. Martin, Ohio State University, Columbus, Ohio; H. Meislin, University of Arizona, Tucson, Ariz.; D. Mildvan, Beth Israel Medical Center, New York, N.Y.; G. Moran, Olive View—UCLA Medical Center, Sylmar, Calif.; J. O'Brien, Orlando Regional Medical Center, Orlando, Fla.; B. O'Neil (Wayne State University, Detroit, MI), Richmond, Va.; S. Opal, Memorial Hospital, Pawtucket, R.I.; S. Parillo, Albert Einstein Medical Center, Philadelphia, Pa.; J. Peacock, Wake Forest University, Winston-Salem, N.C.; C. Pollack, Maricopa Medical Center, Phoenix, Ariz.; C. Pollack, Phoenix, Ariz.; J. Pressman, San Diego Digestive Disease Consultants, Inc., San Diego, Calif.; O. Ramilo, Southwest Medical Center, Dallas, Tex.; M. Reiss, Beta Research, Inc., Westmont, Ill.; M. Reiss, Hinsdale, Ill.; J. Romero, Creighton University, Omaha, Neb.; M. Rush, Truman Medical Center West, Kansas City, Mo.; R. Salata, University Hospitals of Cleveland, Cleveland, Ohio; W. Salzer, University of Missouri—Columbia, Columbia, Mo.; R. Schafermeyer, Carolinas Medical Center, Charlotte, N.C.; M. Schmidt, INOVA Institute of Research and Education, Falls Church, Va.; R. Silverman, Long Island Jewish Medical Center, New Hyde Park, N.Y.; A. Singer, University Hospital & Medical Center, Stony Brook, N.Y.; J. Smith, University of Texas HSC San Antonio, San Antonio, Tex.; S. Sperber, Hackensack University Medical Center, Hackensack, N.J.; C. Terregino, Cooper Hospital/University Medical Center, Camden, N.J.; B. Tiffany, Tampa General Hospital, Tampa, Fla.; E. Tobin, Albany Medical College, Albany, N.Y.; T. Vats, Texas Tech University Health Sciences Center, Amarillo, Tex.; M. Wallace, Naval Medical Center of San Diego, San Diego, Calif.; S. Wambsgans, Eisenhower Army Medical Center, Fort Gordon, Ga.; L. Weiner, SUNY Health Sciences Center, Syracuse, N.Y.; R. Winn, Scott & White Clinic/Scott & White Memorial Hospital, Temple, Tex.; S. Wright, Vanderbilt University Medical Center, Nashville, Tenn.; K. Young, Harbor-UCLA Medical Center, Torrance, Calif.; and J. Zimmerman, Ben Taub General Hospital, Houston, Tex.
REFERENCES
- 1.Abzug, M. J., G. Cloud, J. Bradley, P. J. Sanchez, J. Romero, D. Powell, M. Lepow, C. Mani, E. V. Capparelli, S. Blount, F. Lakeman, R. J. Whitley, D. W. Kimberlin, and National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. 2003. Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr. Infect. Dis. J. 22:335-341. [DOI] [PubMed] [Google Scholar]
- 2.Celentano, D. D., M. S. Linet, and W. F. Stewart. 1990. Gender differences in the experience of headache. Soc. Sci. Med. 30:1289-1295. [DOI] [PubMed] [Google Scholar]
- 3.Chambon, M., C. Archimbaud, J. L. Bailly, C. Henquell, C. Regagnon, F. Charbonne, and H. Peigue-Lafeuille. 2001. Circulation of enteroviruses and persistence of meningitis cases in the winter of 1999-2000. J. Med. Virol. 65:340-347. [DOI] [PubMed] [Google Scholar]
- 4.Pevear, D. C., T. M. Tull, M. E. Seipel, and J. M. Groarke. 1999. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 43:2109-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raftery, K. A., R. Smith-Coggins, and A. H. Chen. 1995. Gender-associated differences in emergency department pain management. Ann. Emerg. Med. 26:414-421. [DOI] [PubMed] [Google Scholar]
- 6.Romero, J. R. 2001. Pleconaril: a novel antipicornaviral drug. Expert Opin. Investig. Drugs 10:369-379. [DOI] [PubMed] [Google Scholar]
- 7.Rotbart, H. A. 1995. Enteroviral infections of the central nervous system. Clin. Infect. Dis. 20:971-981. [DOI] [PubMed] [Google Scholar]
- 8.Rotbart, H. A., P. J. Brennan, K. H. Fife, J. R. Romero, J. A. Griffin, M. A. McKinlay, and F. G. Hayden. 1998. Enterovirus meningitis in adults. Clin. Infect. Dis. 27:896-898. [DOI] [PubMed] [Google Scholar]
- 9.Rotbart, H. A., A. D. Webster, and Pleconaril Treatment Registry Group. 2001. Treatment of potentially life-threatening enterovirus infections with pleconaril. Clin. Infect. Dis. 32:228-235. [DOI] [PubMed] [Google Scholar]
- 10.Stalkup, J. R., and S. Chilukuri. 2002. Enterovirus infections: a review of clinical presentation, diagnosis, and treatment. Dermatol. Clin. 20:217-223. [DOI] [PubMed] [Google Scholar]
- 11.Tormey, V. J., J. R. Buscombe, M. A. Johnson, A. P. Thomson, and A. D. Webster. 2003. SPECT scans for monitoring response to pleconaril therapy in chronic enteroviral meningoencephalitis. J. Infect. 46:138-140. [DOI] [PubMed] [Google Scholar]