Abstract
In this paper we report on the susceptibilities of a range of Bacillus species to the human antimicrobial peptide LL-37. B. subtilis showed a low level of resistance to killing by LL-37 (50% growth-inhibitory concentration [GI50], 1 μg/ml). B. cereus and B. thuringiensis showed intermediate levels of resistance to killing (GI50s, 33 μg/ml and 37 μg/ml, respectively). B. anthracis showed the highest level of resistance (GI50s, 40 to 66 μg/ml). The degradation of LL-37 by B. anthracis culture supernatant was blocked by the metalloprotease inhibitors EDTA and 1,10-phenanthroline, and the gene encoding the protease responsible for LL-37 degradation was not plasmid borne. Our findings suggest that alongside the classical plasmid-based virulence determinants, extracellular metalloproteases of B. anthracis may play a role in survival in the host.
Bacillus anthracis, the etiological agent of anthrax, has been recognized as a human and animal pathogen for centuries. Although it is primarily a disease of animals, the potential for anthrax to be used as a biological weapon (21) has prompted renewed interest in identifying effective medical countermeasures to this disease. B. anthracis is a rod-shaped, gram-positive, aerobic/facultatively anaerobic, spore-forming bacterium. The spores can remain dormant in the environment for extended periods of time and are highly resistant to hostile environmental conditions. Anthrax predominantly affects herbivores, such as cattle, goats, and sheep, although all mammals, including humans, are susceptible. The bacterium is mainly transmitted via spores rather than vegetative cells, and infection is usually acquired through entry into the body of spores from soil or infected animal products via inhalation, ingestion, or cutaneous abrasions (46).
Classically, the virulence of B. anthracis is determined by two major virulence factors, the anthrax toxin complex and a poly-d-glutamic acid capsule, which are encoded by plasmids pXO1 and pXO2, respectively (13, 25, 47). The loss of either plasmid markedly reduces virulence. Indeed, the Sterne animal vaccine strain is attenuated as a consequence of the loss of pXO2. However, there is mounting evidence for the presence of chromosomally located virulence factors, since double-cured strains B. anthracis (pXO1− pXO2−) retain some virulence in mice (11). B. anthracis isolates are considered clonal, and apart from differences in plasmid content, only a few phenotypic variations between strains have been reported. Some isolates are resistant to penicillin, and a small group of isolates have an impaired ability to degrade a number of proteinaceous substrates (7, 10, 42, 43). Most significantly, and for reasons which are not clear, strains containing both virulence plasmids (pXO1+ pXO2+) may differ in virulence by up to 1,000-fold (42, 43).
Extracellular proteases have been implicated in the virulence of a large number of pathogenic bacterial species, as reviewed elsewhere (26). These proteases can perform a wide range of functions, including the acquisition of nutrients, deregulation of critical host processes (including interruption of cascade pathways, cytokine networks, and destruction of cell surface receptors), and inactivation of antimicrobial peptides (44).
Culture supernatant from B. anthracis Sterne has recently been shown to contain at least five distinct extracellular proteases (2). Subcutaneous inoculation of mice with concentrated filtered culture supernatant resulted in hemorrhage; however, the effect was abrogated when the culture supernatant was administered with immune sera raised in rabbits or chemical protease inhibitors (33). B. anthracis strains are considered clonal and possess an extremely high degree of homology at the whole-genome level. The inability to degrade extracellular proteins was previously identified in a small group of B. anthracis strains, including strain Vollum (10). Both the Ames and Vollum strains of B. anthracis are capable of causing disease in animals. However, the current U.S. vaccine is less effective at inducing a protective immune response in animals challenged with Ames, while complete protection was achieved against Vollum, thereby suggesting that this strain may be somewhat less virulent (22, 48). In this paper we hypothesize that the decreased virulence of B. anthracis Vollum may correspond to the reduced ability to degrade extracellular proteins.
Antimicrobial peptides are a ubiquitous component of the innate immune response and exhibit broad-range antimicrobial activities against gram-positive and gram-negative bacteria, fungi, parasites, and enveloped viruses (16, 17, 18, 19). Alongside their direct antimicrobial activities, these peptides play additional roles as mediators of inflammation and stimulators of the immune system (3, 6, 37). LL-37 is a unique human antimicrobial peptide that was initially isolated from human bone marrow (1, 24). LL-37 is constitutively expressed within neutrophils and is inducibly expressed on body surfaces, including the skin and respiratory epithelia, in response to infection, inflammation, or trauma (5, 12, 41).
It has previously been reported that a range of pathogens, including Enterococcus faecalis, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella enterica, Staphylococcus aureus, and Streptococcus pyogenes (15, 30, 35, 40), utilize proteases to confer resistance to the antimicrobial activities of LL-37. In this study we aimed to determine whether variations in the extracellular proteolytic activities of B. anthracis strains provide resistance to LL-37.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and media.
The Bacillus species used in this study included B. subtilis 168 and NCTC 3610; B. atrophaeus subsp. globigii (formerly B. subtilis var. globigii); B. brevis NCTC 2611; B. cereus 569, ATCC 10876 NCTC 10334, NCTC 9946, NCTC 9939, and NCTC 11143; B. thuringiensis subsp. israelensis and B. thuringiensis subsp. israelensis; and a range of B. anthracis strains, including wild-type strain Ames (pXO1+ pXO2+), Sterne (pXO1+ pXO2−), Vollum 1B (pXO1+ pXO2+), and UM23-Cl2 (pXO1− pXO2−; double-cured derivative of the Sterne strain). All strains were obtained from the culture collection at the Defense Science and Technology Laboratory (Porton Down, Salisbury, United Kingdom). The Ames and Vollum strains of B. anthracis were handled in Advisory Committee on Dangerous Pathogens (ACDP) 3 containment facilities both as vegetative strains and as spores. B. anthracis UM23-Cl2, B. thuringiensis, and B. cereus strains were handled at ACDP 2 containment facilities, and B. subtilis was handled at ACDP 1 containment facilities. All Bacillus cultures were maintained on Mueller-Hinton agar plates or broth (Oxoid).
Detection of extracellular protease activity.
Bacillus species were grown to late stationary phase in Mueller-Hinton broth at 37°C to allow maximal expression of extracellular proteases (23). Mueller-Hinton broth was chosen to allow comparison of the results with those obtained by the modified microtiter broth dilution method (48) for determination of LL-37 susceptibility. However, the protease secretion profiles in Mueller-Hinton broth were comparable for each of the strains when the strains were grown in a range of other culture media, including L broth and New Sporulation media (data not shown). The cultures (5 ml) were pelleted in a microcentrifuge, and the supernatant was passed through 0.45-μm-pore-size filters and then 0.2-μm-pore-size filters (Whatman International Ltd., Maidstone, United Kingdom) to remove all bacteria. The protease activity present in the culture supernatant was determined following the degradation of a universal protease substrate, resorufin-labeled casein, according to the manufacturer's instructions (Roche Diagnostics Ltd., Lewes, United Kingdom). The release of resorufin-labeled peptides by proteolytic activity was measured spectrophotometrically at 574 nm. Protease activity was quantified by using purified protease from Streptomyces caespitosus as a standard (Sigma-Aldrich Company Ltd., Poole, United Kingdom).
Peptide synthesis.
The peptides used in this study, including LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES), were produced by Alta Bioscience, Birmingham, United Kingdom, by using standard 9-fluorenylmethoxy carbonyl protection chemistry. The purity and molecular weight were confirmed by high-pressure liquid chromatography and matrix-assisted laser desorption ionization mass spectrometry. The peptides were lyophilized and stored at 4°C. Stock solutions were prepared in phosphate-buffered saline (PBS) for the evaluation of antimicrobial activity and were stored frozen at −20°C in 1-ml aliquots to be ready for use.
In vitro assay for antimicrobial activity.
The antimicrobial activity of LL-37 was determined as the concentration of LL-37 required to inhibit bacterial growth by 50% relative to that of the (no-peptide) control (GI50). The GI50 values were measured by the modified microtiter broth dilution method (49). Briefly, liquid cultures were grown as described above to midexponential phase and then diluted to 1 × 104 CFU/well. Microtiter plates were prepared immediately prior to use, with peptide concentrations, arranged in triplicate, ranging from 0.5 to 200 μg/ml of LL-37 suspended in 0.02% acetic acid and 0.4% bovine serum albumin (Sigma-Aldrich Company Ltd.). A suspension containing 100 μl of diluted bacteria was added to each well of a 96-well microtiter plate, and then the plate was incubated at 37°C for 16 h. Bacterial growth was determined by measuring the absorbance at 620 nm with a Thermo multiskan EX microplate reader (Thermo Electron Corp., Basingstoke, United Kingdom.). Inhibition levels (GI50s) were defined as growth less than or equal to one-half of the growth observed in control wells in which no peptide was present. Assays were also carried out in the presence of protease inhibitors: 5 μM pepstatin (Sigma-Aldrich Company Ltd.), which inhibits aspartic proteases; 10 μM E-64 (Sigma-Aldrich Company Ltd.), which inhibits cysteine proteases; 4 mM Pefabloc (Roche Diagnostics Ltd.), which inhibits serine proteases); and 0.5 mM EDTA, 0.1 mM 1,10-phenanthroline, and 10 μM phosphoramidon (Sigma-Aldrich Company Ltd.), which inhibit metalloproteases.
Measurement of proteolytic degradation of LL-37.
Bacillus species were grown to late stationary phase in Mueller-Hinton broth at 37°C to allow maximal expression of extracellular proteases. The cultures (5 ml) were pelleted in a microcentrifuge, and the supernatant was passed through 0.45-μm-pore-size filters and then 0.2-μm-pore-size filters (Whatman International Ltd.) to remove all bacteria. Untreated filtered supernatant was analyzed as a control, alongside supernatant treated at 37°C for 1 h with 5 mM EDTA (to inhibit metalloprotease activity) and supernatant heated to 70°C for 1 h (to inhibit all enzymatic activity). To each 100 μl of treated supernatant, 5 μl of 1 mg/ml LL-37 was added. The supernatants were then incubated at 37°C, duplicate samples were taken at various time intervals, and the reactions were stopped by boiling (5 min). The samples were separated on 8 to 25% precast polyacrylamide Phast gel (Amersham Biosciences, Little Chalfont, United Kingdom) and then Western blotted onto nitrocellulose membranes. The membranes were blocked overnight for 16 h with 2% bovine serum albumin (Sigma-Aldrich Company Ltd.) in PBS and were then incubated for a further hour, following the addition of mouse anti-LL-37 antibody (1 μg/ml; Hycult Biotechnology, Uden, The Netherlands). The membranes were washed (three times for 5 min each time) in PBS containing 0.05% Tween 20 and were then incubated for 1 h with peroxidase-conjugated anti-mouse antibody (Sigma-Aldrich Company Ltd.), following the manufacturer's instructions, and then washed as described above. The labeled bands were visualized with 3,3′-diaminobenzidine (Sigma-Aldrich Company Ltd.). Once the membranes dried, they were analyzed to quantify protein degradation by using a Gene genius gel documentation system (Syngene, Cambridge, United Kingdom).
Preparation of B. anthracis spores.
B. anthracis UM23-Cl2 was cultured on the surface of Roux bottles containing New Sporulation medium (3.0 g Difco tryptone; 6.0 g Oxoid bacteriological peptone, 3. 0 g Oxoid yeast extract, 1.5 g Oxoid Lab Lemco, 1 ml 0.1% MnCl2 · 4H2O, and 25 g Difco Bacto agar made up to 1 liter with H2O) at 37°C until the cultures contained more than 95% phase-bright spores, as measured by phase-contrast microscopy; typically, incubation of between 7 and 10 days resulted in > 95% phase-bright spores. The spores were harvested by centrifugation at 10,000 × g for 10 min and then washed 10 times with ice-cold distilled water to remove any vegetative cells and cell debris. The spore preparation was heated at 70°C for 1 h to kill any remaining vegetative cells, and then the final spore concentration was determined by serial dilution. Spore preparations were stored at −20°C until they were required.
Germination assay.
B. anthracis spores (1 × 105 CFU/ml) were germinated in Mueller-Hinton broth at 37°C. Duplicate samples were taken at intervals throughout the germination process (0, 1.5, 3, 4.5, 6, and 7.5 h) and were then serially diluted in sterile distilled water. The first set of samples was plated out immediately and represented the total cell count (vegetative cells and spores). The second dilution series was incubated at 70°C for 1 h to kill any vegetative bacilli (spores only). From these values the percentage of vegetative bacteria in relation to the numbers of spores was determined over time.
RESULTS
Antimicrobial activity of LL-37 for Bacillus species.
LL-37 has attracted attention as a novel antimicrobial due to its broad spectrum of activity, its presence at epithelial cell surfaces, and its ability to stimulate the innate immune system (3, 4, 16). Initially, we determined the GI50 values for LL-37 for a range of Bacillus strains which show different potentials to cause severe disease in humans. The bacteria were incubated with 0 to 200 μg/ml of LL-37, and bacterial growth was measured by spectrometry following a 16-h overnight incubation. The antimicrobial activity of LL-37 differed markedly between different Bacillus species (Table 1). LL-37 showed high levels of activity (GI50s, 1 to 2 μg/ml) against B. atrophaeus subsp. globgii and B. subtilis. In comparison, opportunistic pathogens, such as B. thuringiensis var. israelensis, B. cereus 569, and B. cereus ATCC 10876, demonstrated lower levels of susceptibility (GI50s, 33, 37, and 35 μg/ml, respectively) to growth inhibition by LL-37. The GI50 of LL-37 for B. anthracis Ames, an obligate pathogen, was 66 μg/ml, approximately 2-fold higher than that for B. cereus and 33-fold higher than that for B. subtilis.
TABLE 1.
LL-37 susceptibilities of various Bacillus species
| Species | Strain | GI50 (μg/ml)a |
|---|---|---|
| B. subtilis | 168 | 1 |
| B. brevis | NCTC 2611 | 1 |
| B. subtilis | NCTC 3610 | 2 |
| B. atrophaeus | B. atrophaeus subsp. globigii | 2 |
| B. cereus | NCTC 10334 | 31 |
| B. cereus | NCTC 9946 | 33 |
| B. cereus | ATCC 10876 | 35 |
| B. cereus | NCTC 9939 | 35 |
| B. cereus | 569 | 37 |
| B. cereus | NCTC 11143 | 38 |
| B. thuringiensis | B. thuringiensis subsp. israelensis | 33 |
| B. thuringiensis | B. thuringiensis subsp. kurstaki | 38 |
| B. anthracis | Ames pXO1+ pXO2+ | 66 |
| B. anthracis | Sterne pXO1+ pXO2− | 68 |
| B. anthracis | UM23-C12 pXO1− pXO2− | 63 |
| B. anthracis | Vollum pXO1+ pXO2+ | 40 |
The GI50 values are the minimum concentrations of LL-37 necessary to reduce the optical densities of cultures incubated overnight at 37°C by 50% in relation to those of the negative control cultures. These results were taken from two separate experiments, each containing triplicate samples; the standard deviation never exceeded 0.01.
Effects of LL-37 against B. anthracis.
The activity of LL-37 against a range of B. anthracis strains that differed in their pXO1 and/or pXO2 plasmid contents was determined by measuring the GI50 of the peptide as described above. The bacteria were incubated for 16 h overnight in the presence of increasing concentrations of LL-37 (0 to 200 μg/ml), the turbidity of the suspension was measured as described above, and the GI50 of LL-37 was calculated (Table 1). The GI50s of LL-37 for B. anthracis strain Ames (pXO1+ pXO2+), strain Sterne (pXO1+ pXO2−), and strain UM23-Cl2 (pXO1− pXO2−) did not differ significantly (66 μg/ml, 68 μg/ml, and 63 μg/ml, respectively). This finding suggests that the genes that confer resistance to LL-37 are chromosomally located rather than plasmid borne. However, the level of resistance of B. anthracis strain Vollum to LL-37 was only two-thirds those of B. anthracis Sterne, Ames, or UM23-C12, with a GI50 value of 40 μg/ml.
Metalloprotease inhibitors increase the susceptibility of B. anthracis to killing by LL-37.
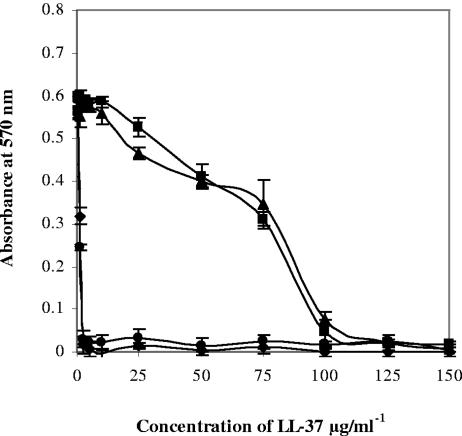
We hypothesized that the resistance of B. anthracis to LL-37 may be mediated by extracellular proteases. Thus, the GI50 of LL-37 for B. anthracis UM23-Cl2 was determined in the presence or absence of protease inhibitors. In the presence of metalloprotease inhibitors such as EDTA, resistance to killing by LL-37 was virtually eliminated (GI50, 1.25 μg/ml) (Fig. 1). In comparison, serine, cysteine, and aspartate protease inhibitors had no effect on the susceptibility of B. anthracis to LL-37 (data not shown).
FIG. 1.
Direct antimicrobial activity of LL-37 against B. anthracis UM23-Cl2 measured by a 96-well plate assay to determine the extent of growth inhibition in the presence of LL-37 and a variety of chemical protease inhibitors: no inhibitors (▪), the metalloprotease inhibitor 5 mM EDTA (⧫), the serine protease inhibitor 4 mM Pefabloc combined with metalloprotease inhibitor 5 mM EDTA (▴), and the serine protease inhibitor 4 mM Pefabloc (•). Each experiment was performed at least three times; the error bars represent the standard deviation calculated from the mean values.
Metalloprotease inhibitors decrease extracellular protease activity in B. anthracis.
The effects of chemical protease inhibitors on the extracellular proteolytic activity of a range of B. anthracis strains were determined. The total proteolytic activity was determined by measuring the degradation of resorufin-labeled casein. The percent reduction in proteolytic activity due to the activities of protease inhibitors was determined (Table 2). The total protease activity of B. anthracis Vollum was fourfold lower than that of B. anthracis Ames. Addition of 0.5 mM EDTA reduced the extracellular protease activities of B. anthracis Ames, Sterne, and UM23-Cl2 by 80 to 90%. However, in comparison, the protease activity of the B. anthracis Vollum strain was inhibited by 20%. In the presence of a serine protease inhibitor, all strains of B. anthracis showed decreases in proteolytic activity of between 4 and 12%. Use of a combination of metalloprotease and serine protease inhibitors resulted in an almost complete inhibition of proteolysis. In three separate experiments, each with triplicate samples, we found that the proteases produced by B. anthracis Vollum were more resistant to inactivation by EDTA. It is known that B. anthracis produces a range of proteases, some of which are inhibited by ion chelators (2). Clearly, our results suggest that not only different levels or proteases but also different spectrums of proteases are produced by the B. anthracis Vollum, Ames, and Sterne strains. Synergies between different classes of protease inhibitors are well documented (38, 45), and this is the likely explanation for the ability of a combination of EDTA and Pefabloc to abolish 98% of the protease activity of B. anthracis Vollum.
TABLE 2.
Extracellular protease secretion in B. anthracis strains
| Strain | Total proteolytic activity (mg/h/liter/OD600)a | % Reduction of proteolytic activityb
|
||
|---|---|---|---|---|
| EDTA | Pefabloc | EDTA + Pefabloc | ||
| Ames | 1.05 | 89 | 10 | 93 |
| Sterne | 0.90 | 83 | 6 | 99 |
| UM23-Cl2 | 0.85 | 88 | 4 | 94 |
| Vollum | 0.25 | 20 | 12 | 98 |
The proteolytic activity was determined in cell-free culture supernatant of stationary-phase B. anthracis cultures. Total protease activity was measured by the degradation of resorufin-labeled casein (Roche Diagnostics Ltd.) at 37°C for 4 h and measured as (mg/h/liter/OD600, where OD600 is the optical density at 600 nm) compared to that of a set of protease standards.
The percent reduction in protease activity by various inhibitors was measured as the percent decrease in comparison to the total protease activity; the inhibitors included 0.5 mM EDTA, 4 mM Pefabloc, and a combination of 0.5 mM EDTA and 4 mM Pefabloc.
Culture supernatant from B. anthracis degrades LL-37.
The effect of the culture supernatant on the stability of LL-37 was determined in vitro. LL-37 was incubated in filtered culture supernatant from B. anthracis UM23-Cl2 or B. subtilis 168, alongside a culture supernatant that had been pretreated with 5 mM EDTA (to inhibit metalloprotease activity) or heated to 70°C for 1 h (to inhibit all enzymatic activity). Samples were taken over 48 h and then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with an anti-LL-37 antibody. The degradation of LL-37 was determined as a percentage of the initial LL-37 concentration (Fig. 2).
FIG. 2.
Degradation of LL-37 by cell-free culture supernatant from overnight cultures of B. subtilis 168 (A) and B. anthracis UM23-Cl2 (B). LL-37 (5 mg/ml) was incubated in 100 μl of culture supernatant (▪), culture supernatant pretreated with 5 mM EDTA (⧫), or culture supernatant pretreated by heating for 1 h at 70°C (▴). Samples were evaluated by Western blotting with anti-LL-37 antibody, and the percentage of LL-37 present compared with the initial amount of LL-37 present at time zero was determined over time.
In the presence of the culture supernatant from B. subtilis, LL-37 was stable, with over 90% of the full-length peptide detectable after 48 h. Conversely, in the presence of the culture supernatant from B. anthracis UM23-Cl2, LL-37 was rapidly degraded, with only 5% of the full-length LL-37 detectable after 6 h. Heat pretreatment of the B. anthracis supernatant resulted in increased LL-37 stability, with the levels being comparable to those observed with B. subtilis. Metalloprotease inhibitors also protected LL-37 from degradation, with 85% of the initial LL-37 detectable after 48 h. This was slightly lower than that with heat treatment, suggesting degradation by other protease classes. These data suggest that B. anthracis, unlike B. subtilis, secretes a metalloprotease which is capable of rapidly degrading extracellular LL-37.
B. anthracis culture supernatant increases resistance of B. subtilis to killing by LL-37.
We next investigated whether the B. anthracis culture supernatant could protect other Bacillus species from killing by LL-37. B. anthracis UM23-Cl2 or B. subtilis cells were grown to exponential phase, pelleted, washed, and resuspended in the filtered culture supernatant; and their susceptibilities to LL-37 were determined. Figure 3 shows that B. subtilis was more resistant to growth inhibition by LL-37 when it was resuspended in the culture supernatant from B. anthracis, with the GI50 increasing from 1 to 3.75 μg/ml. However, no significant difference in the resistance of B. anthracis to LL-37 was determined when it was resuspended in the B. subtilis supernatant (data not shown).
FIG. 3.
Protective effects of the B. anthracis UM23-Cl2 culture supernatant on B. subtilis 168 in the presence of LL-37. The culture supernatant from overnight cultures was filtered, while the bacterial cells were washed three times in sterile medium before being resuspended in the filtered culture supernatant. B. subtilis resuspended in B. subtilis culture supernatant (▪); B. subtilis resuspended in B. anthracis culture supernatant (⧫). Each experiment was performed at least three times; the error bars represent the standard deviations calculated from the mean values.
LL-37 is active against newly germinated spores.
The GI50 assays reported above describe the effects of LL-37 against B. anthracis cells harvested during exponential growth in broth culture. Extracellular protease secretion in bacilli is initiated during the transition from exponential phase into stationary phase (34), conditions which are unlikely to be achieved in a mammalian host until late in the infection process. Anthrax is usually a consequence of the entry of spores rather than the entry of vegetative cells into host tissues. Therefore, in the time period immediately after spore germination, the vegetative bacilli will not have initiated protease secretion and may therefore be increasingly susceptible to LL-37-mediated killing.
B. anthracis UM23-Cl2 spores (1 × 105 CFU/ml) were germinated in Mueller-Hinton medium at 37°C, and samples were taken at various time intervals to determine the proportion of germinated Bacillus spores. At each time point, the GI50 values were calculated to determine susceptibility to LL-37. Figure 4 shows that 90% of the spores germinated within 1.5 h. At the same time, the susceptibility to LL-37 was a GI50 of 7 μg/ml, which is significantly lower than that of the exponentially growing bacteria (GI50, 63 μg/ml). Resistance to LL-37 increased gradually at the onset of vegetative growth, reaching a GI50 of 20 μg/ml after 6 h. However, the onset of exponential growth corresponded to a significant increase in LL-37 resistance, with GI50 values of 65 μg/ml after 7.5 h.
FIG. 4.
Effects of growth phase on susceptibility of B. anthracis UM23-Cl2 to LL-37. (A) Germination of 1 × 105 B. anthracis spores in Mueller-Hinton broth at 37°C as a percentage of the total number of spores in culture (▪) and as a percentage of the total number of vegetative cells in culture (□); (B) logarithmic growth (optical density at 600 nm [OD 600 nm]) of germinated vegetative B. anthracis (⧫); (C) susceptibilities of B. anthracis cells to LL-37 (GI50 in μg/ml) (▴).
DISCUSSION
In humans, LL-37 is expressed within mast cells, within neutrophils, and by epithelial cells at a wide range of sites, including the skin, lung, gastrointestinal tract, urogenital tract, and oral tract (14, 17). LL-37 is widely secreted in wound, sweat, and airway surface fluids. At mucosal surfaces the baseline level of LL-37 is approximately 2 μg/ml. However, expression is markedly upregulated during infection, during inflammation, or following exposure to proinflammatory mediators (29). Studies that have used gene therapy and gene-knockout studies have demonstrated that LL-37 performs a key function in the innate immune response against invading microorganisms (4, 28).
LL-37 is described as a membrane-active agent. The direct antimicrobial activity is mediated by disruption of the target cell lipid bilayer via toroidal pore formation, leading to osmotic lysis and cell death (20). LL-37 is active against the cell membranes of a broad spectrum of bacteria, fungi, and enveloped viruses (3). However, in addition to direct antimicrobial activity, LL-37 also performs a variety of other functions with the innate immune system, including the neutralization of bacterial lipopolysaccharide (24); chemotaxis of neutrophils, mast cells, monocytes, and T cells (27, 50); and activation of both airway epithelial cells and dendritic cells (8, 36).
Pathogens have a range of strategies that they use to resist the activities of antimicrobial peptides (31). These include modulation of the cell wall charge (9, 32), efflux pumps (39), and alteration of the fluidity of the cell membrane and cleavage by extracellular proteases (15, 30, 35, 40). Evidence in the scientific literature suggests that the proteases secreted by pathogenic organisms, including P. aeruginosa, E. faecalis, and S. pyogenes, confer resistance to antimicrobial peptides such as LL-37 (35). Here we have shown that B. anthracis cultures in vitro secrete metalloproteases, which provide resistance to the bactericidal effects of LL-37. These metalloproteases may therefore play a role in the virulence of B. anthracis, especially in infections caused by the airborne route.
The classical description of virulence in B. anthracis is based primarily on the activities of two plasmid-borne virulence determinants: the anthrax toxins and the antiphagocytic capsule. In this study we demonstrate that B. anthracis also possesses a chromosomally located gene(s) that encodes extracellular proteases. This proteolytic activity is able to degrade the host antimicrobial peptide LL-37 and may therefore contribute to virulence.
Although, B. anthracis is more resistant to LL-37 than other members of the Bacillus genus, LL-37 was bactericidal for B. anthracis at concentrations greater than 125 μg/ml. Infections with B. anthracis are mediated by spores, which lack protease secretion and, as such, are extremely susceptible to LL-37-mediated killing at this early stage in the infection process.
Antimicrobial peptides such as LL-37 show considerable potential for the treatment of infectious diseases. However, as with all antimicrobial therapeutics, the effects of pathogen resistance must be investigated. The escalating prevalence of bacterial strains resistant to conventional antibiotics has prompted a search for new therapeutic agents. LL-37 has many advantages over conventional antibiotic regimens, including a broad spectrum of activity and a rapid rate of killing, and fewer problems associated with classical antibiotic resistance mechanisms.
REFERENCES
- 1.Agerberth, B. H., J. Gunne, P. Odeberg, H. Kogner, H. G. Boman, and G. H. Gudmundsson. 1995. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl. Acad. Sci. USA 92:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson, A. I., C. Bell, and B. Fulroth. 2005. Plasmid-encoded regulator of extracellular proteases in Bacillus anthracis. J. Biol. Chem. 187:3133-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R. 2000. Epithelial antimicrobial peptides in host defense against infection. Respir. Res. 1:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., D. J. Weiner, A. D. Moscioni, R. L. Meegalla, and J. M. Wilson. 1999. Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide. Infect. Immun. 67:6084-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowdish, D. M. E., D. J. Davidson, M. G. Scott, and R. E. W. Hancock. 2005. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 49:1727-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradaric, N., and V. Pundapolic. 1992. Cutaneous anthrax due to penicillin-resistant Bacillus anthracis transmitted by an insect bite. Lancet 340:306-307. [DOI] [PubMed] [Google Scholar]
- 8.Davidson, D. J., A. J. Currie, G. S. D. Reid, D. M. E. Bowdish, K. L. MacDonald, R. C. Ma, R. E. W. Hancock, and D. P. Speert. 2004. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell proliferation. J. Immunol. 172:1146-1156. [DOI] [PubMed] [Google Scholar]
- 9.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 10.Fellows, P. F. 1996. A survey of worldwide strains of Bacillus anthracis. Salisbury Med. Bull. Special Suppl. 87:31-33. [Google Scholar]
- 11.Fouet, A., J.-C. Sirard, and M. Mock. 1995. Virulence gene determinants. Salisbury Med. Bull. Special Suppl. 87:84-85. [Google Scholar]
- 12.Frohm, M., B. Agerberth, G. Ahangari, M. Stahle-Backdahl, S. Liden, H. Wigzell, H., and G. H. Gudmundsson. 1997. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 272:15258-15263. [DOI] [PubMed] [Google Scholar]
- 13.Green, B. D., L. Battisti, T. M. Koehler, C. B. Thorne, and B. F. Ivins. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun. 49:291-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudmundsson, G. H., and B. Agerberth. 1999. Neutrophil antibacterial peptides, multifunctional effector molecules in the mammalian immune system. J. Immunol. Methods 232:45-54. [DOI] [PubMed] [Google Scholar]
- 15.Guina, T., E. C. Yi, H. L. Wang, M. Hackett, and S. I. Miller. 2000. A PhoP-regulated outer membrane protease of Salmonella enterica serovar Typhimurium promotes resistance to α-helical antimicrobial peptides. J. Bacteriol. 182:4077-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock, R. E. W. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, R. E. W., and G. Diamond. 2000. Cationic antimicrobial peptides: a major element in mammalian innate immune defences against infection. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 18.Hancock, R. E. W., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock, R. E. W., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88. [DOI] [PubMed] [Google Scholar]
- 20.Henzler-Wildman, K. A., G. V. Martinez, M. F. Brown, and A. Ramamoorthy. 2004. Perturbation of the hydrophobic core of lipid bilayers by human antimicrobial peptide LL-37. Biochemistry 43:8459-8469. [DOI] [PubMed] [Google Scholar]
- 21.Inglesby, T. V., D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Hauer, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 1999. Anthrax as a biological weapon—medical and public health management. JAMA 281:1735-1745. [DOI] [PubMed] [Google Scholar]
- 22.Ivins, B. E., P. F. Fellows, and G. O. Nelson. 1994. Efficacy of a standard human anthrax vaccine against Bacillus anthracis spore challenge in guinea-pigs. Vaccine 12:872-874. [DOI] [PubMed] [Google Scholar]
- 23.Kunst, F., T. Msadek, J. Bignon, and G. Rapoport. 1994. The DegS/DegU and ComP/ComA two-component systems are part of a network controlling degradative enzyme synthesis and competence in Bacillus subtilis. Res. Microbiol. 145:393-402. [DOI] [PubMed] [Google Scholar]
- 24.Larrick, J., M. Hirata, R. Balint, J. Lee, J. Zhong, and S. Wright. 1995. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect. Immun. 63:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikesell, P., B. E. Ivins, J. D. Ristroph, and T. M. Dreier. 1983. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect. Immun. 39:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi, S., and S. Shinoda. 2000. Microbial metalloproteases and pathogenesis. Microbes Infect. 2:91-98. [DOI] [PubMed] [Google Scholar]
- 27.Niyonsaba, F., K. Iwabuchi, A. Someya, M. Hirata, H. Matsuda, H. Ogawa, and I. Nagaoka. 2002. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology 106:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 29.Ong, P. Y., T. Ohtake, C. Brandt, I. Strickland, M. Boguniewicz, T. Ganz, R. L. Gallo, and D. Y. Leung. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151-1160. [DOI] [PubMed] [Google Scholar]
- 30.Park, P. W., G. B. Pier, M. T. Hinkes, and M. Bernfield. 2001. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature 411:98-102. [DOI] [PubMed] [Google Scholar]
- 31.Peschel, A. 2002. How do bacteria resist human antimicrobial peptides? Trends Microbiol. 10:179-186. [DOI] [PubMed] [Google Scholar]
- 32.Peschel, A., and L. V. Collins. 2001. Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin. Peptides 22:1651-1659. [DOI] [PubMed] [Google Scholar]
- 33.Popov, S. G., T. G. Popova, S. Hopkins, R. S. Weinstein, R. MacAfee, K. J. Fryxell, V. Chandhoke, C, Bailey, and K. Alibek. 2005. Effective antiprotease-antibiotic treatment of experimental anthrax. BMC Infect. Dis. 5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaffer, P. 1969. Sporulation and the production of antibiotics, enzymes and exotoxins. Bacteriol. Rev. 33:48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidtchen, A., I.-M. Frick, E. Andersson, H. Tapper, and L. Björck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157-168. [DOI] [PubMed] [Google Scholar]
- 36.Scott, M. G., D. J. Davidson, M. R. Gold, D. Bowdish, and R. E. W. Hancock. 2002. The human antimicrobial peptide LL-37 is a multifunctional modulator of the innate immune responses. J. Immunol. 169:3883-3891. [DOI] [PubMed] [Google Scholar]
- 37.Scott, M. G., and R. E. W. Hancock. 2000. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 20:407-431. [PubMed] [Google Scholar]
- 38.Semenov, A., J. E. Olson, and P. J. Rosenthal. 1998. Antimalarial synergy of cysteine and aspartic protease. Antimicrob. Agents Chemother. 42:2254-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafer, W. M., X. D. Qu, A. J. Waring, and R. I. Lehrer. 1998. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA 95:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sieprawska-Lupa, M., P. Mydel, K. Krawczyk, K. Wojcik, M. Puklo, B. Lupa, P. Suder, J. Silberring, M. Reed, J. Pohl, W. Shafer, F. McAleese, T. Foster, J. Travis, and J. Potempa. 2004. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob. Agents Chemother. 48:4673-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorensen, O., K. Arnljots, J. B. Cowland, D. F. Bainton, and N. Borregaard. 1997. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90:2796-2803. [PubMed] [Google Scholar]
- 42.Stepanov, A. S., K. R. Klimpel, and S. H. Leppla. 1996. Extracellular proteases in Bacillus anthracis. Salisbury Med. Bull. Special Suppl. 87:87. [Google Scholar]
- 43.Stepanov, A. S., N. I. Mikshis, and M. F. Bolotnikova. 1996. Role of chromosomally-encoded factors in virulence of Bacillus anthracis for mice and guinea pigs. Salisbury Med. Bull. Special Suppl. 87:86. [Google Scholar]
- 44.Travis, J., and J. Potempa. 2000. Bacterial proteinases as targets for the development of second-generation antibiotics. Biochim. Biophys. Acta 1477:35-50. [DOI] [PubMed] [Google Scholar]
- 45.Tremblay, C., D. P. Merrill, T. C. Chou, and M. S. Hirsch. 1999. Interactions among combinations of two and three protease inhibitors against drug-susceptible and drug-resistant HIV-1 isolates. J. Acquir. Immune Defic. Syndr. 15:430-436. [DOI] [PubMed] [Google Scholar]
- 46.Turnbull, P. C. B., and J. M. Kramer. 1995. Bacillus, p. 349-356. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 47.Uchida, I., T. Sekizaki, K. Hashimoto, and N. Terakado. 1985. Association of the encapsulation of Bacillus anthracis with a 60 megadalton plasmid. J. Gen. Microbiol. 131:363-367. [DOI] [PubMed] [Google Scholar]
- 48.Welkos, S. L., N. J. Vietri, and P. H. Gibbs. 1993. Non-toxigenic derivatives of the Ames strain of Bacillus anthracis are fully virulent for mice: role of plasmid pX02 and chromosome in strain-dependent virulence. Microb. Pathog. 14:381-388. [DOI] [PubMed] [Google Scholar]
- 49.Wu, M., and R. E. W. Hancock. 1999. Improved derivatives of bactenecin, a cyclic dodecameric antimicrobial cationic peptide. Antimicrob. Agents Chemother. 43:1274-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, D., Q. Chen, A. P., Schmidt, G. M. Anderson, J. M. Wang, J. Wooters, J. J. Oppenheim, and O. Chertov. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilises formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]