Abstract
Two phase I studies were conducted to assess the plasma pharmacokinetics of telbivudine and potential drug-drug interactions between telbivudine (200 or 600 mg/day) and lamivudine (100 mg/day) or adefovir dipivoxil (10 mg/day) in healthy subjects. Study drugs were administered orally. The pharmacokinetics of telbivudine were characterized by rapid absorption with biphasic disposition. The maximum concentrations in plasma (Cmax) were reached at median times ranging from 2.5 to 3.0 h after dosing. Mean single-dose Cmax and area under the plasma concentration-time curve from time zero to infinity (AUC0-∞) were 1.1 and 2.9 μg/ml and 7.4 and 21.8 μg · h/ml for the 200- and 600-mg telbivudine doses, respectively. Steady state was reached after daily dosing for 5 to 7 days. The mean steady-state Cmax and area under the plasma concentration-time curve over the dosing interval (AUCτ) were 1.2 and 3.4 μg/ml and 8.9 and 27.5 μg · h/ml for the 200- and 600-mg telbivudine repeat doses, respectively. The steady-state AUCτ of telbivudine was 23 to 57% higher than the single-dose values. Concomitant lamivudine or adefovir dipivoxil did not appear to significantly alter the steady-state plasma pharmacokinetics of telbivudine; the geometric mean ratios and associated 90% confidence interval (CI) for the AUCτ of telbivudine alone versus in combination were 106.3% (92.0 to 122.8%) and 98.6% (86.4 to 112.5%) when coadministered with lamivudine and adefovir dipivoxil, respectively. Similarly, the steady-state plasma pharmacokinetics of lamivudine or adefovir were not markedly affected by the coadministration of telbivudine; the geometric mean ratios and associated 90% CI, alone versus in combination with telbivudine, were 99.0% (87.1 to 112.4%) and 92.2% (84.0 to 101.1%), respectively, for the lamivudine and adefovir AUCτ values. Moreover, the combination regimens studied were well tolerated in all subjects. The results from these studies provide pharmacologic support for combination therapy or therapy switching involving telbivudine, lamivudine, and adefovir dipivoxil for the treatment of chronic hepatitis B virus infection.
Within the past decade, a number of new immunomodulatory and antiviral therapies have been studied for the treatment of chronic hepatitis B (CHB). At present, interferon alfa-2b, lamivudine, adefovir dipivoxil (hereafter referred to as adefovir), entecavir, and pegylated interferon alfa-2a are approved treatments for hepatitis B (15, 20). Furthermore, a number of agents have shown promise and therapeutic potential in CHB, namely, telbivudine, tenofovir, clevudine, and emtricitabine (1, 7, 14).
Despite these advances, the question of how best to treat patients remains a challenge. Currently approved therapies can be limited by low rates of sustained response, the emergence of resistant strains, and adverse events (15, 18). Studies in vitro and in the woodchuck model have suggested that combination therapy for CHB may improve overall efficacy and minimize the risk of developing resistance due to prolonged exposure to a single agent (11, 17).
There are several important considerations when initiating combination therapy: whether the combined therapy has synergistic or antagonistic effects, different uptake and activation pathways (which might result in altered pharmacokinetics and activity), and altered safety and tolerability profiles (18). Colledge et al. evaluated the antiviral effects of combinations of penciclovir, lamivudine, and adefovir and observed that, whereas synergy was observed at low total drug concentration, slight antagonism was observed at higher total drug concentrations (3). A study by Seignères et al. indicated that combinations of emtricitabine, amdoxovir, and clevudine resulted in superior activity versus any of the agents alone (19). Delaney et al. have reported that in vitro adefovir exerted additive antiviral effects when combined with lamivudine, emtricitabine, or telbivudine and moderately synergistic effects in combination with entecavir or tenofovir (6). A number of clinical trials have been conducted to evaluate the combination therapy with pegylated interferon and lamivudine in patients with CHB (2, 9, 16).
Telbivudine (β-l-2′-deoxythymidine) is under clinical development for the treatment of chronic hepatitis B virus (HBV) infection (12, 13). Telbivudine, a synthetic l-nucleoside analogue, is phosphorylated to its active metabolite, 5′-triphosphate, by cellular kinases. The telbivudine 5′-triphosphate inhibits HBV DNA polymerase (a reverse transcriptase) by competing with the natural substrate, dTTP. Incorporation of the 5′-triphosphorylated telbivudine into viral DNA causes DNA chain termination, resulting in inhibition of HBV replication (1). Recent clinical trials in adults with chronic HBV have established that once-daily telbivudine is well tolerated by patients and exhibits dose-dependent potent antiviral activity (12, 13, 21).
Pharmacokinetic studies in healthy subjects and in HBV-infected patients demonstrated that after oral dosing, telbivudine is absorbed rapidly, with maximum plasma concentrations (Cmax) reached within 1 to 3 h (21, 22). Telbivudine exhibits dose-proportional pharmacokinetics (21). The binding of telbivudine to plasma protein is low (3.3%). Systemic telbivudine is primarily eliminated via renal pathway as unchanged drug, and its renal clearance is in the normal range of creatinine clearance (23), suggesting that passive diffusion is the main mechanism mediating the renal clearance of telbivudine. Recovery within 7 days is 42.0% in urine and 49.6% in feces for a total of 91.6% of the administered dose. Telbivudine is not a substrate or an inhibitor of human hepatic CYP450 isozymes. No telbivudine metabolites were detected in human plasma, urine, or feces (unpublished data).
The objectives of the current studies were to further assess the pharmacokinetics of telbivudine after a single dose and repeat doses and to evaluate the potential for steady-state pharmacokinetic drug-drug interactions between telbivudine and lamivudine and between telbivudine and adefovir in healthy adult volunteers. These studies also evaluated the safety of telbivudine treatment as a single agent and in combination with either lamivudine or adefovir.
MATERIALS AND METHODS
Results from two open-label, multidose studies in healthy adult subjects are reported. The first study evaluated the pharmacokinetics of telbivudine (200 mg/day) and lamivudine (100 mg/day), alone and in combination. The second study evaluated the pharmacokinetics of telbivudine (600 mg/day) and adefovir (10 mg/day), alone and in combination. These studies were conducted in accordance with Good Clinical Practice procedures as described in ICH Harmonized Tripartite Guidelines, the U.S. Code of Federal Regulations (CRF), the principles of the Declaration of Helsinki, and U.S. Food and Drug Administration regulations. Approval for the studies was obtained from the appropriate local Institutional Review Boards.
The telbivudine-lamivudine study was conducted at MDS Pharma Services in Phoenix, Ariz. The first subject was dosed on 7 May 2001, and the last subject completed the final dose on 22 May 2001. The telbivudine-adefovir study was conducted at MDS Pharma Services in Lincoln, Nebr. The first subject was dosed on 29 September 2003, and the last subject completed the final dose on 22 October 2003.
Study populations.
The telbivudine-lamivudine study enrolled only male subjects, while both male and female subjects participated in the telbivudine-adefovir study. All subjects gave written informed consent after the nature of the studies was fully explained. Healthy adults from the general population who had voluntarily consented to participate in the studies were included if they met the following major inclusion criteria: aged 18 to 65 years; body weight within 15% of normal for their size and frame; and no evidence of clinically significant abnormalities on medical history, physical examination, 12-lead electrocardiogram, or clinical laboratory testing during screening. Female subjects were required to be practicing a double-barrier method of contraception plus systemic contraceptives or be surgically incapable of pregnancy or postmenopausal for at least 1 year. Pregnant or lactating female subjects were excluded from the studies. Subjects were also excluded if they had a history of clinically significant disease that, in the opinion of the investigator, might put them at risk; tested positive for human immunodeficiency virus, hepatitis C virus, or HBV; tested positive for drugs of abuse; had a history of alcohol abuse; or had participated in a clinical study within 30 days prior to study drug administration.
Study design.
Both studies used an open-label, multidose, randomized, parallel-group design.
In the telbivudine-lamivudine study, 16 male subjects were randomized to two parallel groups, with 8 subjects in each group. Subjects in group 1 received telbivudine (200 mg/day; two 100-mg tablets) as a single agent on days 1 to 7 and then in combination (telbivudine at 200 mg/day plus lamivudine at 100 mg/day) on days 8 to 14. Subjects in group 2 received lamivudine (100 mg/day) as a single agent on days 1 to 7 and then in combination (telbivudine at 200 mg/day plus lamivudine at 100 mg/day) on days 8 to 14. Lamivudine was taken at the same time with telbivudine. Similarly, the telbivudine-adefovir study enrolled 16 healthy male and female subjects who were randomized to two parallel groups of 8 subjects each (designated group 3 and group 4). Subjects randomized to group 3 received telbivudine as a single agent (600 mg/day; three 200-mg tablets) on day 1. This dosing was repeated on days 3 to 16, thus resulting in 14 consecutive daily doses. Adefovir (10 mg/day, taken at the same time with telbivudine) was dosed in combination with telbivudine on days 10 to 16 (telbivudine at 600 mg/day plus adefovir at 10 mg/day). Subjects randomized to group 4 received adefovir (10 mg/day) as a single agent on days 1 to 7 and then in combination (telbivudine at 600 mg/day plus adefovir at 10 mg/day) on days 8 to 14.
For both studies, the subjects discontinued over-the-counter medications (including aspirin, vitamins, and herbal supplements) and any prescription medications except for oral contraceptives within 2 days prior to reporting to the clinics. Study medications were given on an empty stomach after a fasting period of approximately 10 h prior to each dose, and for an additional 4 h postdosing. All meals and beverages served during the studies were xanthine-free and caffeine-free. Grapefruit juice was not allowed from 48 h before the first dose until discharge from the studies. For each dose, 240 ml (8 fluid oz.) of water were given with the study medication dose(s). No other liquid intake was permitted from 1 h prior to dosing until 2 h postdosing. Water was permitted ad libitum 2 h postdosing. Subjects were asked to remain upright (sitting or standing) for the first 4 h after dosing and not to engage in any strenuous activity during their stay at the study centers.
In the telbivudine-lamivudine study, intensive pharmacokinetic sampling was performed over a period of 24 h after dosing on day 1 (group 1) and on days 7 and 14 (both groups). Serial blood samples (5 ml at each time point) for measuring plasma telbivudine and/or lamivudine were drawn into heparin-containing Vacutainer tubes at the following time points: 0 (predose), 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 10, 12, 16, and 24 h. Blood samples for monitoring trough levels of telbivudine and/or lamivudine in plasma were collected prior to daily dosing on days 5, 6, 12, and 13 for both groups. In the telbivudine-adefovir study, intensive pharmacokinetic sampling was performed over a period of 48 h after dosing on days 1 and 16 in group 3 and on day 14 in group 4 and over 24 h on day 9 in group 3 and on day 7 in group 4. Serial blood samples (5 ml at each time point) for measuring plasma telbivudine and/or adefovir were drawn into heparin-containing Vacutainer tubes at the following time points: 0 (predose), 0.5, 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24 h (group 3 on day 9 and group 4 on day 7) and 28, 36, and 48 h (group 3 on days 1 and 16 and group 4 on day 14). Blood samples for monitoring trough plasma levels of telbivudine and/or adefovir were drawn prior to daily dosing on days 4 to 8 and 11 to 15 for group 3 and on days 1 to 6 and 9 to 13 for group 4, respectively.
Plasma was obtained by centrifugation and stored frozen at −20°C or below until analysis. The total durations encompassing sample collection and completion of bioanalysis were 35 days in the telbivudine-lamivudine study and up to 182 days in the telbivudine-adefovir study. All three drugs have been shown to remain stable during storage and assay. The short-term stability of the analytes in plasma has been documented when spiked samples were subjected to two to three freeze-thaw cycles (−22°C to room temperature) and storage at ambient temperature for 19 to 26 h.
Plasma sample analysis.
Plasma samples were analyzed for telbivudine, lamivudine, and adefovir by using validated high-performance liquid chromatography and tandem mass spectrometry (MS/MS) methodologies.
Study drugs were assessed simultaneously in the telbivudine-lamivudine study. The same assay was also used to measure plasma telbivudine in the telbivudine-adefovir study. Briefly, to 100 μl of calibration standards (10 to 5,000 ng/ml for telbivudine and 10 to 2,519 ng/ml for lamivudine), quality controls (QCs; 30 to 4,000 ng/ml for telbivudine and 30 to 2,000 ng/ml for lamivudine) and unknown plasma samples were added 40 μl of internal standard (β-l-2′-deoxyadenosine [LdA] at 650 ng/ml) containing thymidine phosphorylase (EC 2.4.2.4 [Sigma Chemical Co., St. Louis, Mo.]; >1 U/μl). The mixture was vortexed thoroughly and incubated at 37°C for 1 h to digest any endogenous thymidine that may interfere with telbivudine assay. After incubation, acetonitrile (200 μl) was added to precipitate protein. Samples were centrifuged, and the supernatant was transferred to injection vials. Extraction recovery for both analytes was nearly complete. Chromatography was performed on a TSK-GEL Amide-80 column (4.6 by 150 mm, 5 μm; Tosoh Bioscience, Montgomeryville, Pa.). Elution was carried out isocratically at 1 ml/min with a mobile phase of 90:10 (vol/vol) methanol-25 mM ammonium formate (pH 3.5). Under these conditions, the retention times were approximately 1.68, 1.73, and 1.73 min for telbivudine, lamivudine, and LdA, respectively. Telbivudine, lamivudine, and LdA were monitored by using a PE Sciex API 3000 MS/MS mass analyzer at mass transition of 243.0 to 127.1 m/z, 230.0 to 112.1 m/z, and 252.0 to 136.0 m/z, respectively. There was no interference at the specific mass transitions indicating the absence of matrix effect and lack of influence from concomitant drug. The mass analyzer was operated under positive mode using atmospheric pressure chemical ionization. This assay has a lower limit of quantitation of 10 ng/ml for both drugs with calibration curve ranging from 10 to 5,000 ng/ml for telbivudine and from 10 to 2,519 ng/ml for lamivudine. The intra- and interday precisions (CVs) and accuracies (percent deviation) were from 2.3 to 5.6% and −4.2 to 1.4%, respectively, based on QC samples with spiked concentrations ranging from 30 to 4,000 ng/ml for telbivudine, and were from 1.5 to 4.2% and −1.1 to 8.1%, respectively, based on QC samples with spiked concentrations ranging from 30 to 2,000 ng/ml for lamivudine.
Plasma adefovir was quantitated by using a proprietary high-pressure liquid chromatography-MS/MS methodology developed and validated at MDS Pharma Services (Saint Laurent, Montréal, Quebec, Canada). The adefovir assay has a lower limit of quantitation of 1 ng/ml, a calibration curve ranging from 1 to 200 ng/ml, and intra- and interday precisions (CVs) and accuracies (percent deviation) ranging from 1.9 to 6.6% and −1.2 to 5.0%, respectively, as well as an extraction recovery from 92.6 to 98.6%, based on QC samples ranging from 3 to 160 ng/ml. Adefovir was monitored at a mass transition of 274.1 to 162.1 m/z. This method is highly specific with no matrix effect or influence from coadministered telbivudine.
Pharmacokinetic analysis.
The plasma concentration time data were analyzed by using model-independent approaches with the WinNonlin software (version 4.1; Pharsight Corp., Mountain View, Calif.). The maximum plasma drug concentration (Cmax) and time to Cmax (Tmax) were directly obtained from the plasma concentration time profiles. The observed elimination half-life (t1/2) was calculated as 0.693/lz, where lz is the slope of the apparent elimination phase of the natural logarithmic (ln) transformation of the plasma drug concentration time curve estimated by using linear regression. The area under the plasma concentration-time curve from time zero to t (AUC0−t), where t is the time of last measurable sample, was calculated according to the linear trapezoidal rule. The AUC from time zero to infinity (AUC0−∞) was estimated as AUC0−t + Ct/lz, where Ct is the plasma concentration of the last measurable sample. AUCτ, a measure of the extent of exposure over the dose interval (τ = 24 h), was also calculated. Oral apparent total plasma clearance (CL/F) was calculated as dose/AUC0−∞ after a single dose and as dose/AUCτ at steady state.
Statistical analysis.
In the telbivudine-lamivudine study, attainment of steady state for telbivudine and lamivudine on days 7 and 14 was evaluated by regressing the ln-transformed trough levels on days 5, 6, and 7 and on days 12, 13, and 14 over time, respectively. Similarly, in the telbivudine-adefovir study, attainment of telbivudine steady state on days 9 and 16 in group 3 and on day 14 in group 4 was evaluated by regressing the ln-transformed trough levels on days 8 to 10 and days 11 to 17 (group 3) and on days 13 to 15 (group 4) over time. Attainment of adefovir steady state on day 16 in group 3 and on days 7 and 14 in group 4 was evaluated by regressing the ln-transformed trough levels on days 14 to 17 (group 3) and on days 5 to 7 and days 9 to 15 (group 4) over time. Steady state was concluded if the slope was not statistically different from zero (P > 0.05).
Plasma pharmacokinetic drug-drug interaction was assessed by comparing combination with a single agent for the principal parameters underlying plasma exposure (AUCτ and Cmax). A parametric (normal-theory) general linear model was applied to the ln-transformed AUCτ and Cmax. An analysis of variance with subject and treatment as factors was performed by using the GLM procedure in SAS (SAS Institute, Inc., Cary, NC). The results for AUCτ and Cmax were reported as 90% confidence intervals (CI) surrounding the ratio (percentage) of the geometric means of the pharmacokinetic measures with or without the second drug. It was concluded that no statistically significant plasma drug-drug interaction exists if the 90% CI of the ratio of geometric means were to lie within 80 and 125% for AUCτ and Cmax.
Safety analysis.
The safety analysis included all subjects who received at least one dose of study medication. Safety measurements included clinical history, physical examination, laboratory measurements (hematology, serum chemistries, and urinalysis), vital signs, and assessments of adverse events (AEs). AEs, listed by preferred term and body system, were coded by using MedDRA version 3.3 or higher.
RESULTS
Demographics.
As summarized in Table 1, subjects in the two groups of each study had comparable demographics with respect to age, weight, and race. All subjects completed the studies and were included in the safety analysis. One subject in group 2 of the telbivudine-lamivudine study vomited within 2 h postdosing on day 14 and the day 14 data were excluded from pharmacokinetic analysis.
TABLE 1.
Demographics
| Patient parameter | Telbivudine-lamivudine study
|
Telbivudine-adefovir study
|
||||
|---|---|---|---|---|---|---|
| Group 1 (n = 8) | Group 2 (n = 8) | Total (n = 16) | Group 3 (n = 8) | Group 4 (n = 8) | Total (n = 16) | |
| Mean age (yr) ± SD | 35.5 ± 8.3 | 29.5 ± 6.6 | 32.5 ± 7.9 | 39.0 ± 9.4 | 28.4 ± 12.1 | 33.7 ± 11.8 |
| Mean wt (kg) ± SD | 79.0 ± 7.0 | 81.5 ± 13.2 | 80.3 ± 10.3 | 69.6 ± 4.6 | 73.9 ± 7.1 | 71.8 ± 6.2 |
| No. (%) male | 8 (100) | 8 (100) | 16 (100) | 6 (75) | 8 (100) | 14 (88) |
| No. (%) Caucasian | 7 (87) | 4 (50) | 11 (69) | 7 (87) | 5 (63) | 12 (75) |
Single-dose, steady-state pharmacokinetics of telbivudine and interaction with lamivudine or adefovir.
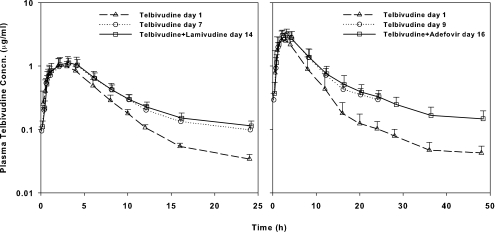
The single-dose pharmacokinetics of telbivudine were evaluated over 24 h after a 200-mg dose on day 1 in the telbivudine-lamivudine study and over 48 h after a 600-mg dose on day 1 in the telbivudine-adefovir study. After oral dosing, telbivudine was rapidly absorbed, with the median Tmax ranging from 2.5 to 3.0 h. Mean Cmax and AUC0−∞ were 1.1 μg/ml and 7.4 μg · h/ml, respectively, for a 200-mg dose and 2.9 μg/ml and 21.8 μg · h/ml, respectively, after a 600-mg dose; an ∼3-fold increase from the 200-mg to the 600-mg dose, which is suggestive of dose proportionality. The plasma disposition of telbivudine was apparently biphasic, with an initial distribution-elimination phase, followed by a more gradual elimination phase starting at roughly 12 to 20 h after dosing. In the telbivudine-lamivudine study, the mean t1/2 estimated based on the observed elimination phase from 16 to 24 h was 12.6 h compared to a mean of 19.9 h from the telbivudine-adefovir study, with a longer sampling period of up to 48 h. For both studies, however, the telbivudine levels in plasma at 24 h after a single dose (34.3 ± 6.1 and 102.8 ± 30.7 ng/ml, respectively, for the 200- and 600-mg doses) represented only ca. 3 to 4% of Cmax. Furthermore, based on comparison of the mean single-dose AUCτ with the AUC0−∞, it was estimated that the contribution from plasma telbivudine beyond 24 h to total exposure was minimal (only ∼10%). Despite the differences in sampling period, the calculated oral total plasma clearance (CL/F) was in excellent agreement: 27.3 and 29.6 liters/h for a 200- and a 600-mg telbivudine dose, respectively. Mean single-dose plasma concentration-time profiles of telbivudine at 200 and 600 mg are shown in Fig. 1.
FIG. 1.
Single-dose and steady-state plasma concentration-time curves for telbivudine as a single agent and in combination with lamivudine (left panel) or adefovir (right panel) in healthy subjects. The mean plus the standard deviation is shown.
Subjects in both studies continued daily dosing. Steady state was reached after five to seven daily doses for telbivudine, lamivudine, and adefovir (the slope ranged from −0.06 to 0.03; the P value ranged from 0.09 to 0.99). The pharmacokinetics of telbivudine, alone and in combination with lamivudine or adefovir, were therefore evaluated when all three drugs reached steady state.
As depicted in Fig. 1, mean steady-state plasma concentration-time curves of telbivudine were essentially superimposable when telbivudine at 200 mg/day was administered alone and in combination with lamivudine at 100 mg/day and when telbivudine at 600 mg/day was administered alone and with concomitant adefovir at 10 mg/day. In the telbivudine-lamivudine study, the mean steady-state Cmax and AUCτ of telbivudine at 200 mg/day alone and in the presence of lamivudine were comparable: 1.2 versus 1.2 μg/ml and 8.9 versus 9.4 μg · h/ml, respectively. The geometric mean ratios (percentage) and associated 90% CIs were 106.7% (88.5 to 128.7%) and 106.3% (92.0 to 122.8%) for the telbivudine Cmax and AUCτ, respectively. The median Tmax (3.0 h) was identical for telbivudine alone and in combination with lamivudine. In the telbivudine-adefovir study, the mean steady-state Cmax and AUCτ of telbivudine at 600 mg/day alone and in the presence of adefovir were comparable: 3.4 versus 3.2 μg/ml and 27.5 versus 27.3 μg · h/ml, respectively. The geometric mean ratios and associated 90% CIs were 92.7% (80.9 to 106.3%) and 98.6% (86.4 to 112.5%) for the telbivudine Cmax and AUCτ, respectively. The median Tmax was similar for telbivudine alone (3.0 h) and in combination with adefovir (2.5 h). In addition, the mean CL/F of telbivudine, consistent for the two telbivudine doses (23.5 liters/h for the 200-mg/day repeat doses and 22.5 liters/h for the 600-mg/day repeat doses), was not altered by coadministered lamivudine (21.8 liters/h) or adefovir (23.0 liters/h). Therefore, concomitant lamivudine or adefovir did not appear to significantly alter the plasma pharmacokinetic profiles telbivudine. The results for the pharmacokinetic and statistical analyses of telbivudine are summarized in Table 2.
TABLE 2.
Pharmacokinetic parameters and statistical results for telbivudinea
| Studyb | Treatmentc | Cmax (μg/ml) | Tmax (h) | AUCτ (μg · h/ml) | AUC0−∞ (μg · h/ml) | t1/2 (h) | CL/F (liters/h) | Geometric mean ratio (%) (90% CI)
|
|
|---|---|---|---|---|---|---|---|---|---|
| Cmax | AUCτ | ||||||||
| LdT/Lam | LdT, SD | 1.1 ± 0.2 | 3.0 (2.0-4.0) | 6.7 ± 0.7 | 7.4 ± 0.6 | 12.6 ± 3.2 | 27.3 ± 2.3 | ||
| LdT, SS | 1.2 ± 0.4 | 3.0 (1.0-4.0) | 8.9 ± 2.0 | 23.5 ± 5.7 | 106.7 (88.5-128.7) | 106.3 (92.0-122.8) | |||
| LdT/Lam, SS | 1.2 ± 0.2 | 3.0 (2.0-4.0) | 9.4 ± 1.5 | 21.8 ± 3.7 | |||||
| LdT/Adv | LdT, SD | 2.9 ± 1.0 | 2.5 (2.0-4.0) | 19.0 ± 6.3 | 21.8 ± 6.0 | 19.9 ± 14.7 | 29.6 ± 8.4 | ||
| LdT, SS | 3.4 ± 0.6 | 3.0 (2.0-4.0) | 27.5 ± 5.3 | 22.5 ± 3.6 | 92.7 (80.9-106.3) | 98.6 (86.4-112.5) | |||
| LdT/Adv, SS | 3.2 ± 0.5 | 2.5 (1.0-4.0) | 27.3 ± 6.2 | 23.0 ± 5.4 | |||||
Parameters are presented as means ± the standard deviations except for Tmax, where the median(range) values are shown.
LdT, telbivudine; Lam, lamivudine; Adv, adefovir.
LdT, SD = single-dose telbivudine in group 1 (telbivudine-lamivudine study) and group 3 (telbivudine-adefovir study) on day 1; LdT, SS = steady-state telbivudine as a single agent in group 1 on day 7 (telbivudine-lamivudine study) and group 3 on day 9 (telbivudine-adefovir study); LdT/Lam, SS = steady-state telbivudine in combination with lamivudine in group 1 on day 14; LdT/Adv, SS = steady-state telbivudine in combination with adefovir in group 3 on day 16.
After repeat daily dosing, telbivudine exhibited a slight accumulation, as evidenced by higher steady-state plasma exposure compared to the single-dose data. After 200-mg and 600-mg repeat dosing, the plasma trough concentrations for telbivudine alone after the seventh consecutive dose averaged 98.5 ± 15.3 and 297.0 ± 73.8 ng/ml, respectively, with an ∼3-fold increase from the single-dose values (accumulation index). At steady state, there were a mean 8 to 25% increase in Cmax and a mean 23 to 57% increase in AUCτ.
Effects of telbivudine on the pharmacokinetics of lamivudine and adefovir.
The mean steady-state Cmax and AUCτ of lamivudine alone and in the presence of telbivudine were comparable: 1.2 versus 1.1 μg/ml and 4.3 versus 4.2 μg · h/ml, respectively. The geometric mean ratios and associated 90% CIs were 86.0% (71.2 to 104.0%) and 99.0% (87.1 to 112.4%) for the lamivudine Cmax and AUCτ, respectively. The median Tmax, 0.7 versus 0.8 h for lamivudine alone and in combination with telbivudine, respectively, was also comparable. Similarly, the mean steady-state Cmax and AUCτ of adefovir alone and in the presence of telbivudine were comparable: 19.5 versus 18.5 ng/ml and 214.2 versus 198.2 ng · h/ml, respectively. The geometric mean ratios and associated 90% CIs were 94.4% (89.9 to 99.2%) and 92.2% (84.0 to 101.1%) for the adefovir Cmax and AUCτ, respectively. The median Tmax, 1.0 h, was identical for adefovir alone and in combination with telbivudine. Therefore, based on statistical analyses, the coadministration of telbivudine did not appear to significantly alter the plasma pharmacokinetic profiles of lamivudine or adefovir. The results for the pharmacokinetic and statistical analyses are summarized in Table 3.
TABLE 3.
Pharmacokinetic parameters and statistical results for lamivudine and adefovira
| Studyb | Treatmentc | Cmax (μg/ml) | Tmax (h) | AUCτ (μg · h/ml) | Geometric mean ratio (%) (90% CI)
|
|
|---|---|---|---|---|---|---|
| Cmax | AUCτ | |||||
| LdT/Lam | Lam, SS | 1.2 ± 0.3 | 0.7 (0.5-1.0) | 4.3 ± 0.3 | 86.0 (71.2-104.0) | 99.0 (87.1-112.4) |
| LdT/Lam, SS | 1.1 ± 0.2 | 0.8 (0.7-2.0) | 4.2 ± 0.6 | |||
| LdT/Adv | Adv, SS | 19.5 ± 2.6 | 1.0 (0.5-2.0) | 214.2 ± 41.1 | 94.4 (89.9-99.2) | 92.2 (84.0-101.1) |
| LdT/Adv, SS | 18.5 ± 3.2 | 1.0 (0.5-1.0) | 198.2 ± 45.1 | |||
Parameters are presented as means ± the standard deviations except for Tmax, where median (range) values are shown. The Cmax and AUCτ for adefovir are expressed in ng/ml and ng · h/ml, respectively.
LdT, telbivudine; Lam, lamivudine; Adv, adefovir.
Lam, SS = steady-state lamivudine alone in group 2 on day 7; LdT/Lam, SS = steady-state lamivudine in combination with telbivudine in group 2 on day 14; Adv, SS = steady-state adefovir alone in group 4 on day 7; LdT/Adv, SS = steady-state adefovir in combination with telbivudine in group 4 on day 14.
Safety results.
Twenty-eight and twenty-four treatment-emergent AEs were reported in the telbivudine-lamivudine and telbivudine-adefovir studies, respectively. Most of the AEs reported were mild. The most commonly reported AEs included arthralgia, dizziness, headache, and nausea. No serious AEs were reported, and none of the subjects discontinued therapy as a result of AEs. Furthermore, there were no clinically relevant changes in the vital signs, electrocardiograms, clinical laboratory analyses, or physical examinations of any of the subjects.
DISCUSSION
The advent of new treatments for CHB may provide the opportunity to improve clinical outcomes by combining or alternating therapeutic agents. Before such treatment strategies could be explored in patients, it was essential to examine potential drug-drug interaction that might impact therapeutic response and/or safety.
The telbivudine-lamivudine interaction study was conducted in parallel with a first-in-human phase I/II dose escalation trial in patients (13, 21). The choice of the telbivudine dose in this interaction study was therefore dictated by the progress of the dose escalation trial, which, at the time of the study, was limited to pharmacokinetic and safety data available through 200 mg. The dose escalation study later proved that telbivudine was well tolerated through 800 mg/day, with a near-maximum antiviral effect reached in the dose range of 400 to 800 mg/day. A dose of 600 mg/day was selected as the intended clinical dose of telbivudine based on results from phase I (13, 21) and II (12) trials, and this was the dose used in the telbivudine-adefovir interaction study.
Both studies reported here contained an arm with telbivudine as a single agent, allowing evaluation of single-dose and steady-state pharmacokinetics of telbivudine. After a single oral dose of 200 and 600 mg, telbivudine was rapidly absorbed, with Cmax reached within median Tmax from 2.5 to 3.0 h. The 600-mg telbivudine exposure was threefold higher than that of the 200-mg dose, indicating good dose proportionality and confirming previous findings (21). Telbivudine reached steady-state exposure following five to seven repeated daily dosing. At steady state, trough, peak, and total exposure were higher compared to single dose, indicating a slight accumulation. The degree of accumulation was comparable between the 200- and 600-mg doses and was consistent with previous findings in HBV-infected patients (21). Such an accumulation resulted in sustained plasma exposure upon daily dosing. With the intended clinical dose regimen of 600 mg/day, steady-state troughs were around 300 ng/ml. Because of the low protein binding of telbivudine (3.3% [unpublished data]), the trough concentrations of unbound telbivudine are expected to be consistently above the in vitro 50% effective concentration (∼48 ng/ml) observed in the 2.2.15 cell line (1). In a previous study of HBV-infected patients with a sampling duration of only 8 h, the observed postpeak disposition of plasma telbivudine was apparently monophasic (21). With longer sampling durations of 24 to 48 h, a biphasic disposition profile was revealed with the observed elimination phase starting at 12 to 20 h. Plasma levels fell to ca. 3 to 4% of Cmax 24 h after dosing. Accordingly, plasma exposure beyond 24 h contributed only ca. 10% to the total AUC. These results suggest that a sampling period of 24 h is adequate for assessing plasma exposure of telbivudine, although a longer sampling period would be needed for characterizing the true terminal phase. With sampling periods from 24 to 48 h, the terminal phase was partially observed in the current studies; in the telbivudine-lamivudine study, only two datum points (16 and 24 h) were used in calculating the t1/2. The incomplete observation of the terminal phase, together with the lack of samples on the observed portion (the telbivudine-lamivudine study), therefore resulted in a less accurately estimated t1/2 and also likely accounts for the slightly higher values of CL/F from the single-dose data due to an underestimation of the extrapolated portion of the area under the curve. The availability of the single-dose and steady-state trough plasma concentrations nonetheless allowed for an indirect approximation of the terminal phase rate constant (λ) and half-life by using the following equation: accumulation index = 1/(1 − e−λ · τ) (8). With an accumulation index of 3 upon once-daily dosing (τ = 24 h), the terminal phase rate constant was ca. 0.017 h−1 and the associated half-life was 41 h. These approximations are consistent with the results of compartmental analysis in HBV-infected patients with estimated half-lives ranging from 29.5 to 41.3 h (21) and also in agreement with results from more recent studies with prolonged sampling up to 120 to 168 h postdosing. These latter studies fully characterized the biphasic plasma disposition profile of telbivudine with an estimated terminal-elimination-phase half-life and oral clearance of approximately 40 h and 25 liters/h, respectively, after a single dose of 600 mg of telbivudine (unpublished data).
In the telbivudine-lamivudine study, although the 90% CIs narrowly missed the target range of 80 to 125% for Cmax, a point estimate known to be inherently more variable than AUC, the geometric mean ratios were rather close to 100%. The AUCτ, a measure of overall exposure, remained unaffected for both drugs. The 90% CIs for the AUCτ were within the target range for both drugs. Although the telbivudine dosage used for pharmacokinetic assessments with lamivudine was lower than the intended clinical dose (200 mg versus 600 mg, respectively), it is unlikely that clinically significant interactions would occur between the two agents based on the lack of impact on AUCτ observed with telbivudine in combination with lamivudine. Coadministration of telbivudine and adefovir did not markedly affect the pharmacokinetics of either drug. The 90% CIs for Cmax and AUCτ for both agents were within the 80 to 125% target range. In addition, values of pharmacokinetic parameters of lamivudine and adefovir found in the current studies are in full agreement with previously reported data (4, 5, 10).
It should, however, be pointed out that the studied drugs are all nucleoside or nucleotide analogues that require intracellular phosphorylation to their respective pharmacologically active 5′-triphosphate. Therefore, potential intracellular interactions, although unlikely since their phosphorylation processes involve different cellular kinases (1, 5, 10), remain to be elucidated.
Finally, telbivudine in combination with lamivudine or adefovir appeared to be safe and well tolerated in healthy subjects. The long-term safety of telbivudine in combination with lamivudine has recently been demonstrated in a phase II trial in patients with CHB (12).
In conclusion, coadministration of telbivudine with either lamivudine or adefovir did not appear to significantly alter the plasma pharmacokinetics of either drug, hence providing pharmacologic support for combination therapy and/or therapy alternating between these drugs.
Acknowledgments
We thank the healthy volunteers and the staff of MDS Pharma Services. We are also grateful to R. Boehme for his critical review of the manuscript and helpful suggestions.
REFERENCES
- 1.Bryant, M. L., E. G. Bridges, L. Placidi, A. Faraj, A.-G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J.-L. Imbach, B. Hernandez, A. Juodawlkis, B. Tennant, B. Korba, P. Cote, P. Marion, E. Cretton-Scott, R. F. Schinazi, and J.-P. Sommadossi. 2001. Antiviral l-nucleosides specific for hepatitis B virus infection. Antimicrob. Agents Chemother. 45:229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan, H. L., N. W. Leung, A. Y. Hui, V. W. Wong, C. T. Liew, A. M. L. Chim, F. K. Chan, L. C. Hung, Y. T. Lee, J. S. Tam, C. W. Lam, and J. J. Sung. 2005. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-α2b and lamivudine with lamivudine alone. Ann. Intern. Med. 142:240-250. [DOI] [PubMed] [Google Scholar]
- 3.Colledge, D., G. Civitico, S. Locarnini, and T. Shaw. 2000. In vitro antihepadnaviral activities of combinations of penciclovir, lamivudine, and adefovir. Antimicrob. Agents Chemother. 44:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cundy, K. C. 1999. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin. Pharmacokinet. 36:127-143. [DOI] [PubMed] [Google Scholar]
- 5.Dando, T. M., and G. Plosker. 2003. Adefovir dipivoxil: a review of its use in chronic hepatitis B. Drugs 63:2215-2234. [DOI] [PubMed] [Google Scholar]
- 6.Delaney, W. E., IV, H. Yang, M. D. Miller, C. S. Gibbs, and S. Xiong. 2004. Combinations of adefovir with nucleoside analogs produce additive antiviral effects against hepatitis B virus in vitro. Antimicrob. Agents Chemother. 48:3702-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghany, M. G., and E. C. Doo. 2004. Management of chronic hepatitis B. Gastroenterol. Clin. N. Am. 33:563-579. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi, M., and D. Perrier. 1982. Multiple dosing, p. 113-144. In J. Swarbrick (ed.), Drugs and the pharmaceutical sciences, vol. 15, 2nd ed. Marcel Dekker, Inc., New York, N.Y. [Google Scholar]
- 9.Janssen, H. L. A., M. van Zonneveld, H. Senturk, S. Zeuzem, U. S. Akarca, Y. Cakaloglu, C. Simon, T. M. K. So, G. Gerken, R. A. De Man, H. G. M. Niesters, P. Zondervan, B. Hansen, S. W. Schalm, et al. 2005. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 365:123-129. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, M. A., K. H. Moore, G. J. Yuen, A. Bye, and G. E. Pakes. 1999. Clinical pharmacokinetics of lamivudine. Clin. Pharmacokinet. 36:41-66. [DOI] [PubMed] [Google Scholar]
- 11.Korba, B. E., P. Cote, W. Hornbuckle, R. Schinazi, J. D. Gangemi, B. C. Tennant, and J. L. Gerin. 2000. Enhanced antiviral benefit of combination therapy with lamivudine and alpha interferon against WHV replication in chronic carrier woodchucks. Antivir. Ther. 5:95-104. [PubMed] [Google Scholar]
- 12.Lai, C. L., N. W. Y. Leung, E. K. Teo, M. Tong, F. Wong, H. W. Hann, S. Han, T. Poynard, M. Myers, G. Chao, D. Lloyd, N. Brown, et al. 2005. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 129:528-536. [DOI] [PubMed] [Google Scholar]
- 13.Lai, C. L., S. G. Lim, N. A. Brown, X. J. Zhou, D. M. Lloyd, Y. M. Lee, M. F. Yuen, G. C. Chao, and. M. W. Myers. 2004. A dose-finding study of once-daily oral telbivudine in HBeAg-positive patients with chronic hepatitis B virus infection. Hepatology 40:719-726. [DOI] [PubMed] [Google Scholar]
- 14.Lok, A. S. F. 2004. New treatment of hepatitis B. Semin. Liver Dis. 24(Suppl. 1):77-82. [DOI] [PubMed] [Google Scholar]
- 15.Lok, A. S. F., and B. J. McMahon. 2004. Chronic hepatitis B: update of recommendations. Hepatology 39:857-861. [DOI] [PubMed] [Google Scholar]
- 16.Marcellin, P., G. K. K. Lau, F. Bonino, P. Farci, S. Hadziyannis, R. Jin, Z.-M. Lu, T. Piratvisuth, G. Germanidis, C. Yurdaydin, M. Diago, S. Gurel, M.-Y. Lai, P. Button, N. Pluck, et al. 2004. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 351:1206-1217. [DOI] [PubMed] [Google Scholar]
- 17.Menne, S., C. A. Roneker, B. C. Tennant, B. E. Korba, J. L. Gerin, and P. J. Cote. 2002. Immunogenic effects of woodchuck hepatitis virus surface antigen vaccine in combination with antiviral therapy: breaking of humoral and cellular immune tolerance in chronic woodchuck hepatitis virus infection. Intervirology 45:237-250. [DOI] [PubMed] [Google Scholar]
- 18.Perillo, R. P. 2004. Overview of treatment of hepatitis B: key approaches and clinical challenges. Semin. Liver Dis. 24(Suppl. 1):23-29. [DOI] [PubMed] [Google Scholar]
- 19.Seignères, B., P. Martin, B. Werle, O. Schorr, C. Jamard, L. Rimsky, C. Trépo, and F. Zoulim. 2003. Effects of pyrimidine and purine analog combinations in the duck hepatitis B virus infection model. Antimicrob. Agents Chemother. 47:1842-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. 2005. CDER drug and biologic approvals for calendar year 2005. [Online.] http://www.fda.gov/cder/rdmt/ndaaps05cy.htm.
- 21.Zhou, X. J., S. G. Lim, D. M. Lloyd, G. C. Chao, N. A. Brown, and C. L. Lai. 2006. Pharmacokinetics of telbivudine following oral administration of escalating single and multiple doses in patients with chronic hepatitis B virus infection: pharmacodynamic implications. Antimicrob. Agents Chemother. 50:874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou, X. J., S. G. Lim, C. L. Lai, D. M. Pow, N. A. Brown, and M. W. Myers. 2001. Pharmacokinetics of β-l-deoxythymidine (LdT) in healthy subjects and patients with chronic hepatitis B virus infection, p. 92, abstr. 100. HEP DART 2001. Frontiers in Drug Development for Viral Hepatitis. Elsevier Science B.V., Amsterdam, The Netherlands.
- 23.Zhou, X. J., M. Myers, G. Chao, G. Dubuc, and N. A. Brown. 2004. Clinical pharmacokinetics of telbivudine, a potent antiviral for hepatitis B, in subjects with impaired hepatic or renal function. 55th Annu. Meet. Am. Assoc. Study Liver Dis., abstr. 1166. Hepatology 40(Suppl. 1):672A. [Google Scholar]