Abstract
Tenofovir is an acyclic nucleotide analog with activity against human immunodeficiency virus (HIV) and hepatitis B virus (HBV). Tenofovir disoproxil fumarate (tenofovir DF), a bis-alkoxyester prodrug of tenofovir, is approved for the treatment of HIV and is currently being developed to treat chronic hepatitis B. In this report, we further characterize the in vitro activity of tenofovir against HBV as well as its metabolism in hepatic cells. We show that tenofovir is efficiently phosphorylated to tenofovir diphosphate (TFV-DP) in both HepG2 cells and primary human hepatocytes. TFV-DP has a long intracellular half-life (95 h) and is a potent and competitive inhibitor of HBV polymerase (Ki = 0.18 μM). Tenofovir has a 50% effective concentration of 1.1 μM against HBV in cell-based assays, and potency is improved >50-fold by the addition of bis-isoproxil progroups. Tenofovir has previously demonstrated full activity against lamivudine-resistant HBV in vitro and clinically. Here we show that the major adefovir resistance mutation, rtN236T, confers three- to fourfold-reduced susceptibility to tenofovir in cell culture; the clinical significance of this susceptibility shift has not yet been determined. The rtA194T HBV polymerase mutation recently identified in tenofovir DF-treated HIV/HBV-coinfected patients did not confer in vitro resistance to tenofovir as a single mutation or in a lamivudine-resistant viral background. Overall, the antiviral and metabolic profile of tenofovir supports its development for the treatment of chronic hepatitis B.
Tenofovir belongs to a class of acyclic phosphonate nucleotide analogs which have demonstrated clinical utility against a broad spectrum of viral infections (reviewed by De Clercq and Holý [3]). Tenofovir has selective activity against retroviruses and hepadnaviruses and is currently approved for the treatment of human immunodeficiency virus (HIV) as the bis-alkoxyester prodrug tenofovir disoproxil fumarate (tenofovir DF). Tenofovir DF is orally bioavailable, and the promoieties are cleaved during adsorption to release tenofovir into systemic circulation (reviewed by Kearney et al. [11]). With respect to hepadnaviruses, tenofovir was shown to be active against duck and human hepatitis B viruses (HBV) in vitro (7) and more recently against woodchuck hepatitis virus in vivo (15). Clinically, 48 weeks of tenofovir DF treatment in HIV/HBV-coinfected patients has resulted in serum HBV DNA reductions of 4.7 to 5.5 log10 copies/ml in patients with either wild-type or lamivudine-resistant HBV infection (5, 28). The clinical activity seen against lamivudine-resistant viruses is consistent with in vitro studies which have demonstrated that tenofovir is active against all major patterns of lamivudine resistance mutations (35).
There are now three oral antiviral agents approved to treat chronic hepatitis B: the nucleotide adefovir dipivoxil (ADV) and the nucleosides lamivudine and entecavir. While each of these agents produces multilog suppression of serum HBV DNA, they induce only modest rates of HBV surface antigen seroconversion and thus require long-term administration to control disease in most patients. The need for long-term therapy necessitates drug safety and the ability to delay or manage the emergence of resistant HBV strains. While lamivudine is a well-tolerated drug, resistance emerges in approximately 20% of patients per year of monotherapy (13). In contrast, long-term studies with ADV and more limited experience with entecavir indicate that the selection of drug resistance is considerably slower with these agents. ADV retains clinical efficacy against lamivudine-resistant mutants (17, 18) but selects the mutation rtN236T and, less frequently, rtA181V (14). Entecavir shows reduced susceptibility to lamivudine-resistant mutants in vitro and clinically and an accelerated development of entecavir resistance mutations (rtI169T, rtT184S/G, rtS202I, and rtM250V) in lamivudine-resistant patients (27, 35). Since resistance leads to loss of virologic suppression and the resumption of liver disease, new agents and treatment modalities will be necessary to adequately treat patients as new mutations emerge.
On a molar basis, the in vitro antiviral activity of tenofovir is similar to adefovir. However, tenofovir DF is used clinically at higher doses (300 mg) without the reversible nephrotoxicity observed using high doses of ADV (30 mg or greater) (8-10). Preliminary clinical data suggest that 300 mg of tenofovir DF results in a significantly greater serum HBV DNA suppression than the approved 10-mg dose of ADV (28). Based on long-term safety data and initial efficacy data in patients with wild-type or lamivudine-resistant HBV infection, tenofovir DF is undergoing clinical development for the treatment of chronic hepatitis B. We therefore profiled the in vitro properties of tenofovir which are relevant for the treatment of HBV infection. Specifically, we sought to (i) confirm the antiviral mechanism of action of tenofovir and its prodrug against HBV, (ii) evaluate the activity of tenofovir against additional clinically relevant drug resistance mutations, and (iii) investigate the intracellular metabolism of tenofovir to its active diphosphate species in hepatic cells.
MATERIALS AND METHODS
Cell culture.
HepG2 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in humidified incubators at 37°C and 5% CO2. HepG2 cells were grown in minimal essential medium (American Type Culture Collection) supplemented with 100 units/ml penicillin, 10 μg/ml streptomycin, and 10% fetal bovine serum (Irvine Scientific, Santa Ana, CA). 2.2.15 cells were kindly provided by Brent Korba (Georgetown University, Washington, D.C.) and maintained under conditions identical to HepG2 cells. Freshly plated human primary hepatocytes were obtained from BD Biosciences (Bedford, MA) and maintained in 12-well tissue culture plates with the supplier's recommend medium (Hepato-STIM; BD Biosciences).
Compounds.
Tenofovir, tenofovir diphosphate (TFV-DP), tenofovir DF, adefovir, adefovir diphosphate (AFV-DP), and ADV were synthesized by Gilead Sciences (Foster City, CA). Lamivudine was obtained from Moravek Biochemicals (Brea, CA).
HBV polymerase assays.
The generation and purification of active HBV polymerase using recombinant baculovirus has been described previously (33, 34). HBV DNA polymerase activity was measured by incorporation of α-33P-labeled dATP into acid-precipitatible products using activated calf thymus DNA as a template as described previously (33, 34). Inhibition constants (Ki) were determined by fitting initial rates to Lineweaver-Burk plots based on the competitive inhibition equation 1/V = 1/Vmax + Km/Vmax(1 + [I]/Ki) (1/S).
2.2.15 antiviral assays.
2.2.15 cells were seeded in 48-well plates and treated with freshly prepared medium containing compound every 2 days for a 2-week period. Seven doses of each compound were used at concentrations ranging from 0.001 μM to 10 μM in half-log increments. Following the treatment period, cells were lysed by the addition of 200 μl phosphate-buffered saline containing 0.5% NP-40 (Sigma, St. Louis, MO) per well. Lysates were transferred to microcentrifuge tubes and centrifuged for 10 min at maximum speed to pellet nuclei. Supernatants were transferred to clean tubes, and cytoplasmic DNA was extracted using QiaAmp DNA blood mini extraction kits (QIAGEN, Valencia, CA). Purified DNA was transferred to nylon membranes using a slot blot manifold as described previously (12). Viral DNA was detected by nucleic acid hybridization using a 33P-labeled HBV probe and quantified using a Storm 860 PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Data were fit to the logistic dose-response equation y = a/[1+(x/b)c], and 50% effective concentration (EC50) values were calculated from the resulting equations as described previously (4).
Generation and cloning of HBV encoding drug resistance mutations.
The plasmid pFBHBV-wt encodes a terminally redundant (1.3 unit-length), replication-competent HBV genome (genotype A; GenBank accession number AF305422) in the pFastBacDual vector backbone (Invitrogen, Carlsbad, CA). The rtA194T, rtL180M, and rtM204V mutations were introduced by site-directed mutagenesis using the following primers: rtA194T, 5′-TCA GTG GTT CGT AGG ACT TTC CCC CAC TGT TTG-3′; rtL180M, 5′-AGT CCG TTT CTC ATG GCT CAG TTT ACT-3′; rtM204V, 5′-GCT TTC AGC TAT GTG GAT GAT GTG GTA-3′ (bolded sequence indicates the mutated codon). The reverse transcriptase domains of all constructs were sequenced to confirm that no additional mutations had been introduced. Patient baseline and rtN236T-containing viruses were cloned by full-length PCR amplification as described by Gunther et al. (6), followed by SapI digestion and ligation into the HBV expression vector pHY106 as previously described (36).
Transient antiviral assays.
HepG2 cells were seeded into six-well culture dishes at a density of 1 × 106 cells/dish. Sixteen hours postseeding, cells were transfected with 5 μg of plasmid DNA encoding the HBV genome of interest using the Fugene 6 transfection reagent (Roche, Indianapolis, IN). The following day, cells were fed fresh medium containing compound. Cells were treated for 1 week, after which intracellular HBV replicative intermediates were isolated as previously described (4). Viral DNA was fractionated on 1% agarose gels and transferred to nylon membranes (Roche) using standard Southern blotting procedures (25). Viral DNA was quantified, and EC50 values were calculated as described above.
Analysis of intracellular tenofovir metabolites.
HepG2 cells were seeded in 12-well tissue culture plates and grown to confluence (average of 8.8 × 105 cells/well). Fresh primary hepatocytes were purchased in 12-well tissue culture plates. Uptake and phosphorylation experiments were initiated by the addition of 10 μM tenofovir to cell culture medium. At time points of 2, 6, and 24 h, drug-containing medium was aspirated and cells were washed two times with 2.5 ml ice-cold phosphate-buffered saline. Cells were then scraped into 500 μl 70% methanol and stored at −20°C to facilitate the extraction of metabolites. Cellular debris was removed by centrifugation for 10 min at 15,000 rpm in a microcentrifuge, and supernatants were dried by speed vacuum. Samples were resuspended in 10 μl tetrabutyl ammonium acetate containing 2 picomole of [5-(6-amino-purin-9-yl)-2,5-dihydro-furan-2-yloxymethyl] phosphonic acid diphosphate (as an internal standard) per 200,000 cells. Transient ion-pairing high-performance liquid chromatography coupled to positive ion electrospray tandem mass spectrometery (LC/MS/MS) was used to analyze tenofovir and its phosphorylated metabolites. Comparative experiments performed with adefovir were conducted as described above using 10 μM adefovir as the concentration for cell incubation. Details of the LC/MS/MS conditions have recently been published (22).
RESULTS
Tenofovir is a competitive inhibitor of HBV polymerase.
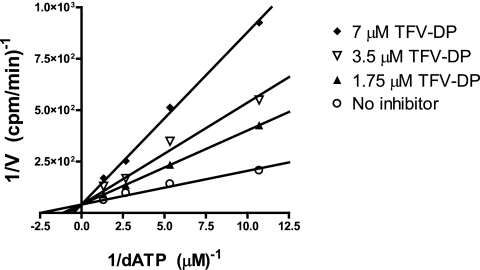
Tenofovir is a nucleotide analog, suggesting that it may inhibit viral replication by competing with natural nucleotides for binding to the active site of HBV polymerase. To test this hypothesis, we used a previously established quantitative HBV DNA polymerase assay (33, 34). The catalytic activity of HBV polymerase was measured in the absence or presence of three different concentrations of TFV-DP. HBV polymerase activity was inhibited in a dose-dependent manner without a change in Vmax, confirming that TFV-DP acts by competitive inhibition with respect to the natural substrate, dATP (Fig. 1). The Ki of TFV-DP was determined to be 0.18 μM, which is 2.1-fold lower than the Km of dATP (0.38 μM) (33).
FIG. 1.
Competitive inhibition of HBV polymerase by TFV-DP. HBV polymerase was incubated with template (activated calf thymus DNA) deoxynucleoside triphosphates and α-33P-labeled dATP. Inhibition of labeled dATP incorporation was measured in the presence of the indicated concentrations of TFV-DP. Data are presented as a Lineweaver-Burk plot.
Activities of tenofovir and tenofovir DF against HBV in 2.2.15 cells.
We used the HepG2 2.2.15 cell line, which stably expresses wild-type HBV, to assay the anti-HBV activities of tenofovir and its bis-alkoxyester prodrug, tenofovir DF, in cell culture. Adefovir, ADV (bis-alkoxyester prodrug of adefovir), and lamivudine were also tested in parallel. 2.2.15 cells were treated with drug for 2 weeks, and replicating cytoplasmic DNA was then extracted, quantified by Southern blotting, and used to calculate EC50 values (Table 1). Tenofovir and adefovir had similar antiviral activities in 2.2.15 cells (EC50 values were 1.1 μM and 0.8 μM, respectively). The potency of tenofovir increased approximately 50-fold to 0.02 μM by the addition of the bis-isoproxil promoieties. The activity of adefovir increased about 10-fold with the addition of bis-pivoxylmethyl progroups.
TABLE 1.
Anti-HBV activities of tenofovir and related compounds in HepG2 2.2.15 cells
| Compound | EC50 (μM)a | n |
|---|---|---|
| Tenofovir | 1.1 ± 0.3 | 5 |
| Tenofovir DF | 0.02 ± 0.01 | 2 |
| Adefovir | 0.8 ± 0.2 | 3 |
| ADV | 0.1 ± 0.01 | 3 |
| Lamivudine | 0.06 ± 0.01 | 3 |
Mean ± standard deviation from the indicated number of independent experiments (n).
Activity of tenofovir against adefovir-resistant HBV.
The major resistance mutation to adefovir dipivoxil is an asparagine-to-threonine change at position 236 of HBV polymerase (rtN236T). To investigate potential cross-resistance, we tested baseline and postbreakthrough HBV isolates from two patients who developed rtN236T while on adefovir dipivoxil therapy. In transient-cell-based antiviral assays, rtN236T conferred 3- to 4.2-fold resistance to tenofovir (Table 2). The same clinical isolates had 7.3- to 13.8-fold susceptibility shifts to adefovir and 2.3- to 3.5-fold susceptibility shifts to lamivudine.
TABLE 2.
In vitro activities of tenofovir, adefovir, and lamivudine against HBV, isolated from two patients, encoding the adefovir resistance mutation rtN236T
| Patient no. and drug | Pretherapya,b
|
Posttherapyb
|
Fold change | ||
|---|---|---|---|---|---|
| EC50 | n | EC50 | n | ||
| Patient 1 | |||||
| Tenofovir | 0.33 ± 0.11 | 7 | 1.01 ± 0.84 | 8 | 3.0 |
| Adefovir | 0.26 ± 0.17 | 7 | 3.64 ± 2.78 | 8 | 13.8c |
| Lamivudine | 0.04 ± 0.01 | 3 | 0.12 ± 0.06 | 3 | 3.5c |
| Patient 2 | |||||
| Tenofovir | 0.13 ± 0.03 | 2 | 0.55 ± 0.07 | 5 | 4.2 |
| Adefovir | 0.21 ± 0.01 | 6 | 1.55 ± 0.91 | 7 | 7.3 |
| Lamivudine | 0.03 ± 0.02 | 3 | 0.07 ± 0.2 | 5 | 2.3 |
Pretherapy isolates were confirmed not to contain rtN236T.
Mean ± standard deviation from the indicated number of independent experiments (n).
Previously reported (1).
The rtA194T mutation does not confer resistance to tenofovir in vitro.
A recent study identified rtA194T as an emerging mutation in two HBV/HIV-coinfected patients receiving long-term tenofovir DF therapy; in vitro analysis indicated this HBV mutation resulted in a 7.6-fold reduction in tenofovir susceptibility (26). To study this mutation, we engineered rtA194T into wild-type and lamivudine-resistant (rtL180M+rtM204V) laboratory strains of HBV and assayed susceptibility to tenofovir and lamivudine using transient-replication assays. Our data indicated that the rtA194T mutation resulted in a 1.5-fold increase in the EC50 of tenofovir (Table 3). The lamivudine-resistant mutant rtL180M+rtM204V HBV displayed a 2.1-fold increase in the EC50 for tenofovir. The addition of rtA194T to the lamivudine-resistant background did not significantly change the susceptibility of the virus to tenofovir compared to the rtL180M+rtM204V mutant (Table 3).
TABLE 3.
In vitro susceptibilities of wild-type, rtA194T, rtL180M+rtM204V, and rtA194T+rtL180M+rtM204V HBV to tenofovir in a transient-cell-based antiviral assay
| HBV | EC50 (μM)a | n | Fold resistanceb |
|---|---|---|---|
| Wild type | 0.13 ± 0.043 | 5 | 1.0 |
| rtA194T | 0.19 ± 0.059 | 4 | 1.5 |
| rtL180M+rtM204V | 0.27 ± 0.16 | 5 | 2.1 |
| rtA194T+rtL180M+rtM204V | 0.31 ± 0.041 | 4 | 2.4 |
Mean ± standard deviation from the indicated number of experiments (n).
The fold resistance is the ratio of the mutant EC50 and the wild-type EC50. The reported value is the mean of three or more independent experiments.
Tenofovir is efficiently phosphorylated in hepatic cells.
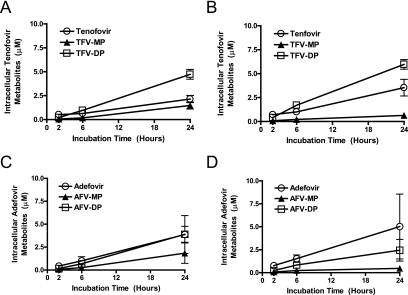
To investigate the phosphorylation of tenofovir in hepatic cells, we incubated HepG2 cells and primary human hepatocytes with 10 μM tenofovir for 2, 6, or 24 h and then quantified intracellular metabolites by LC/MS/MS (Fig. 2A and B). In both cell types, there were time-dependent increases in the amount of intracellular tenofovir, with the highest levels being reached at 24 h; this is consistent with the low inherent permeability of charged nucleotides. Time-dependent increases were observed for both tenofovir monophosphate (TFV-MP) and TFV-DP. Notably, TFV-DP, the species active against HBV polymerase, was efficiently formed and achieved higher intracellular concentrations than tenofovir. TFV-DP reached levels of 4.7 μM and 6 μM in primary hepatocytes and HepG2 cells, respectively. TFV-MP was the least abundant metabolite detected (<1.5 μM), suggesting that once formed it was efficiently converted into diphosphate.
FIG. 2.
Phosphorylation kinetics of tenofovir to mono- and diphosphate species in HepG2 cells and primary human hepatocytes. Primary human hepatocytes (A) and HepG2 cells (B) were treated with 10 μM tenofovir. At the indicated time points, cells were washed and lysed and the amounts of intracellular tenofovir, TFV-MP, and TFV-DP were quantified by LC/MS/MS. Parallel experiments were performed with 10 μM adefovir in primary hepatocytes (C) and HepG2 cells (D) to determine levels of adefovir, AFV-MP, and AFV-DP.
We also performed parallel experiments with adefovir in both cell types (Fig. 2C and D). Adefovir achieved slightly lower diphosphate levels in each cell type (3.9 μM and 2.4 μM in primary human hepatocytes and HepG2 cells, respectively). Unlike tenofovir, AFV-DP levels were equal to (in primary human hepatocytes) or lower than (in HepG2 cells) adefovir levels, suggesting a lower overall phosphorylation efficiency than tenofovir. Like tenofovir, AFV-MP was the least abundant species, suggesting it was efficiently converted to diphosphate.
TFV-DP has a long half-life in hepatic cells.
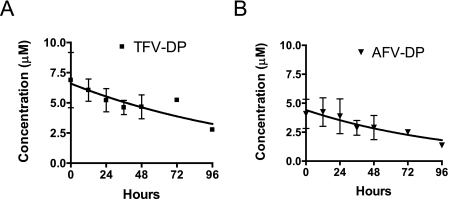
To determine the half-life of TFV-DP in hepatic cells, we incubated primary human hepatocytes in 10 μM tenofovir for 24 h and then measured TFV-DP concentrations over a 4-day period following drug removal (Fig. 3A). Consistent with the previous experiment, approximately 7 μM TFV-DP was formed after a 24-h incubation with tenofovir. TFV-DP remained detectable throughout the 4-day period after drug withdrawal, and a half-life of 95 ± 6 h was calculated using a single exponential decay model. We also determined the half-life of AFV-DP in parallel (Fig. 3B). Following a 24-h incubation with adefovir, 3.2 μM AFV-DP was formed and the diphosphate had an intracellular half-life of 75 ± 1 h.
FIG. 3.
Intracellular half-life of TFV-DP in primary human hepatocytes. Primary human hepatocytes were incubated with 10 μM tenofovir (A) or 10 μM adefovir (B) for 24 h to accumulate diphosphate. Cells were then washed, fed fresh medium without drug, and then lysed at the indicated times for quantification of intracellular diphosphate levels by LC/MS/MS. Using a single exponential decay model, intracellular diphosphate half-lives of 95 ± 6 and 75 ± 1 h were calculated for tenofovir and adefovir, respectively.
DISCUSSION
The anti-HIV properties of tenofovir and tenofovir DF have been well characterized both in vitro and clinically. While in vitro and preliminary clinical data have demonstrated that tenofovir is a potent anti-HBV agent, detailed studies on its mechanism of action, resistance profile against newer mutations, and metabolism in hepatic cells have not been reported. Furthermore, while tenofovir is structurally similar to adefovir (differing only by addition of a single methyl group in the acyclic linker region), it cannot be assumed that the two molecules will behave similarly with respect to antiviral activity, resistance profile, or metabolism. The aim of our studies was to provide a specific in vitro evaluation of the properties of tenofovir relevant to its development as an anti-HBV agent.
Our enzymatic studies confirmed tenofovir inhibits HBV replication through competitive inhibition of the viral polymerase. The Ki of TFV-DP for HBV polymerase (0.18 μM) is similar to that of AFV-DP (0.10 μM) and slightly lower than the Km of the natural substrate, dATP (0.38 μM) (33). This is analogous to what was observed for HIV reverse transcriptase, where TFV-DP had a Ki of 0.16 μM, AFV-DP had a Ki of 0.07 μM, and the Km of dATP was 0.33 μM (16, 32). Overall, tenofovir appears to inhibit HIV and HBV by the same mechanism (inhibition of viral DNA polymerization). However, it should be noted that assays to study inhibition in greater detail (e.g., individual contributions of nucleotide incorporation and excision) are not currently available for HBV (31).
In cell culture, tenofovir had an EC50 of 1 μM against wild-type HBV using the stable cell line HepG2.2.15; this is similar to previous reports which have used either 2.2.15 cells or other stable cell lines (7, 35). Addition of the two soproxil progroups to tenofovir lowers the EC50 to 0.02 μM, a 50-fold increase in activity. This improvement in potency is likely due to significantly improved cellular permeability as the two hydroxyl groups of the phosphonic acid are covered with uncharged lipophilic promoieties. Indeed, analysis of permeability using a Caco-2 assay indicated that tenofovir DF had moderate cell permeability while tenofovir had low cell permeability (data not shown).
Clinical experience with famciclovir, lamivudine, ADV, and entecavir has indicated that resistance can be selected to all these compounds. Although resistant mutants emerge relatively slowly during ADV and entecavir therapy, it can be expected that resistance will become an increasing problem, since most patients require long-term therapy to adequately control disease. The cross-resistance profiles of all new agents should be carefully evaluated to guide the development of rational treatment regimens. Like adefovir, tenofovir retains activity against all of the major patterns of lamivudine resistance mutations in vitro and clinically (17, 18, 35). Our results indicated that tenofovir showed a small but reproducible decrease in susceptibility (3- to 4.2-fold) to clinical HBV isolates bearing rtN236T, the most common adefovir-associated resistance mutation. These findings are in agreement with a recent report by Brunelle et al., who observed a 4.5-fold shift in the tenofovir EC50 after introduction of rtN236T into a laboratory strain of HBV (2).
It is important to note that the in vitro rtN236T susceptibility shift observed for tenofovir is smaller than that of adefovir (7.3- to 13.8-fold) and that tenofovir DF is administered clinically at a dose 30 times higher than adefovir dipivoxil (300 mg versus 10 mg, respectively). The combination of a smaller susceptibility shift and a significantly higher dose may enable tenofovir DF to effectively suppress serum HBV DNA in patients with the rtN236T mutant. Indeed, two recent reports indicated that two patients with rtN236T had serum HBV DNA reductions of ≥4 log10 copies/ml when switched from ADV to tenofovir DF therapy (20, 30). Similarly, patients with rtN236T also respond to therapy with 100 mg of lamivudine despite a 2.1- to 3.5-fold in vitro decrease in lamivudine susceptibility (19). Future studies are needed to determine the best treatment options for patients with rtN236T; however, early clinical data indicate that tenofovir DF and lamivudine should each be explored.
We also studied the impact of the rtA194T mutation, which was recently reported to emerge in two HIV/HBV-coinfected patients receiving tenofovir DF plus lamivudine as part of their antiretroviral treatment regimen (26). Our phenotypic analysis indicated that rtA194T did not cause a significant change in tenofovir susceptibility either alone or when expressed in combination with lamivudine resistance mutations (EC50 values changed 1.5- to 2.5-fold). These results do not agree with those reported by Sheldon et al., who observed a 7.6-fold change with the single rtA194T mutation and a >10-fold increase in the EC50 when rtA194T was expressed with lamivudine resistance mutations (26). The discrepancy might be explained by differences between the EC50 assays used in the two labs. Our results were obtained using standard Southern blotting procedures to quantify intracellular HBV replication, whereas Sheldon et al. used a PCR assay to quantify extracellular HBV DNA following transient transfection. Examining the clinical data does not provide a clear association of rtA194T with viral load rebound: one patient had a transient viral load increase of 1.5 logs after the mutation emerged, while the second patient had continuous viral load decline after the emergence of rtA194T and a >9 log10 decline in serum HBV DNA after the initiation of tenofovir DF plus lamivudine therapy. We are not aware of any additional patients who have developed this mutation under tenofovir DF therapy. Nevertheless, this residue should be monitored closely during the clinical development of tenofovir DF for chronic hepatitis B.
Studies of the anabolism of tenofovir to its active diphosphate form were previously restricted to lymphoid cells to support the HIV indication of tenofovir DF (21, 24). Here we have shown that tenofovir phosphorylation occurs efficiently in a human hepatoblast cell line (HepG2) and in primary human hepatocytes. In the CEM (lymphoid) cell line, TFV-DP achieved levels of 5.2 μM, which is similar to the levels we observed in HepG2 cells (6.0 μM). However, TFV-DP formation in primary human hepatocytes was significantly greater (4.7 μM) than in primary lymphocytes (1.0 μM levels reached in peripheral blood mononuclear cells) (21). In parallel experiments, the efficiency of TFV-DP formation was greater than that of adefovir in both cell types that we tested; however, the difference in primary human hepatocytes (<2-fold) was smaller than in HepG2 cells (2.5-fold).
Tenofovir diphosphate increased in a linear manner over the 24-hour incubation period. Linear increases over the same time period were also observed for adefovir diphosphate when run in parallel during these experiments as well as in previous studies (22). The linear accumulation of adefovir and tenofovir diphosphates over 24 hours is in contrast to most nucleoside analogs, which usually reach a maximal intracellular concentration after 8 to 12 h. However, this result is consistent with the reduced permeability of adefovir and tenofovir due to the presence of the two negative charges on the phosphonate moiety. Similar studies conducted on T cells in our laboratory indicate that tenofovir diphosphate levels begin to reach maximal concentrations after 48 hours of incubation (data not shown). The diphosphates of both tenofovir and adefovir had very long intracellular half-lives, which is consistent with earlier studies demonstrating prolonged in vitro antiviral effects with tenofovir and adefovir after drug removal (38). Overall, the efficient phosphorylation and long half-life we observed for tenofovir agree with the clinical results indicating that a single daily dose of tenofovir DF will be phosphorylated to levels sufficient to exert a potent antiviral effect in the liver.
Due to its favorable safety profile and efficacy, tenofovir DF is a recommended first-line treatment option for HIV infection (37). Multiple small investigator studies have demonstrated that the 300-mg dose of tenofovir DF approved for HIV also results in a potent antiviral suppression of serum HBV DNA (>4 log10 reduction in serum HBV copies/ml) in coinfected patients (5, 23, 28, 29). The in vitro data presented here have confirmed that tenofovir inhibits HIV and HBV by similar mechanisms (competitive inhibition of DNA polymerization) and that tenofovir DF has potent cell-based anti-HBV activity (EC50, 0.02 μM). Tenofovir also has a favorable metabolic profile in hepatic cells, the target compartment for antiviral activity in vivo. Accordingly, enrollment has recently begun for phase III studies of tenofovir DF in HBV e antigen-negative and e antigen-positive chronic hepatitis B patients. We have also shown that tenofovir is more efficacious than adefovir against the rtN236T adefovir resistance mutation in vitro. Since significantly higher doses of tenofovir DF are being used clinically, these data suggest tenofovir should also be explored for the treatment of adefovir-resistant HBV.
Acknowledgments
We thank Jennifer Vela for her technical assistance during metabolism studies. We also thank Katyna Borroto-Esoda, Steven Chuck, Mick Hitchcock, Herve Mommeja-Marin, and Joe Quinn for critical reading of the manuscript.
REFERENCES
- 1.Angus, P., R. Vaughan, S. Xiong, H. Yang, W. Delaney, C. Gibbs, C. Brosgart, D. Colledge, R. Edwards, A. Ayres, A. Bartholomeusz, and S. Locarnini. 2003. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 125:292-297. [DOI] [PubMed] [Google Scholar]
- 2.Brunelle, M. N., A. C. Jacquard, C. Pichoud, D. Durantel, S. Carrouee-Durantel, J. P. Villeneuve, C. Trepo, and F. Zoulim. 2005. Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir. Hepatology 41:1391-1398. [DOI] [PubMed] [Google Scholar]
- 3.De Clercq, E., and A. Holy. 2005. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4:928-940. [DOI] [PubMed] [Google Scholar]
- 4.Delaney, W. E., IV, R. Edwards, D. Colledge, T. Shaw, J. Torresi, T. G. Miller, H. C. Isom, C. T. Bock, M. P. Manns, C. Trautwein, and S. Locarnini. 2001. Cross-resistance testing of antihepadnaviral compounds using novel recombinant baculoviruses which encode drug-resistant strains of hepatitis B virus. Antimicrob. Agents Chemother. 45:1705-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dore, G. J., D. A. Cooper, A. L. Pozniak, E. DeJesus, L. Zhong, M. D. Miller, B. Lu, and A. K. Cheng. 2004. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J. Infect. Dis. 189:1185-1192. [DOI] [PubMed] [Google Scholar]
- 6.Gunther, S., B. C. Li, S. Miska, D. H. Kruger, H. Meisel, and H. Will. 1995. A novel method for efficient amplification of whole hepatitis B virus genomes permits rapid functional analysis and reveals deletion mutants in immunosuppressed patients. J. Virol. 69:5437-5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heijtink, R. A., J. Kruining, G. A. de Wilde, J. Balzarini, E. De Clercq, and S. W. Schalm. 1994. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob. Agents Chemother. 38:2180-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izzedine, H., J. S. Hulot, V. Launay-Vacher, P. Marcellini, S. J. Hadziyannis, G. Currie, C. L. Brosgart, C. Westland, S. Arterbrun, and G. Deray. 2004. Renal safety of adefovir dipivoxil in patients with chronic hepatitis B: two double-blind, randomized, placebo-controlled studies. Kidney Int. 66:1153-1158. [DOI] [PubMed] [Google Scholar]
- 9.Izzedine, H., J. S. Hulot, D. Vittecoq, J. E. Gallant, S. Staszewski, V. Launay-Vacher, A. Cheng, and G. Deray. 2005. Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol. Dial. Transplant. 20:743-746. [DOI] [PubMed] [Google Scholar]
- 10.Kahn, J., S. Lagakos, M. Wulfsohn, D. Cherng, M. Miller, J. Cherrington, D. Hardy, G. Beall, R. Cooper, R. Murphy, N. Basgoz, E. Ng, S. Deeks, D. Winslow, J. J. Toole, and D. Coakley. 1999. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: a randomized controlled trial. JAMA 282:2305-2312. [DOI] [PubMed] [Google Scholar]
- 11.Kearney, B. P., J. F. Flaherty, and J. Shah. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 43:595-612. [DOI] [PubMed] [Google Scholar]
- 12.Korba, B. E., and G. Milman. 1991. A cell culture assay for compounds which inhibit hepatitis B virus replication. Antivir. Res. 15:217-228. [DOI] [PubMed] [Google Scholar]
- 13.Lai, C. L., J. Dienstag, E. Schiff, N. W. Leung, M. Atkins, C. Hunt, N. Brown, M. Woessner, R. Boehme, and L. Condreay. 2003. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. 36:687-696. [DOI] [PubMed] [Google Scholar]
- 14.Locarnini, S., X. Qi, S. Arterburn, A. Snow, C. L. Brosgart, G. Currie, M. Wulfsohn, M. Miller, and S. Xiong. 2005. Incidence and predictors of emergence of HBV mutations associated with ADV resistance during 4 years of ADV therapy for patients with chronic hepatitis B. J. Hepatol. 42:17. [Google Scholar]
- 15.Menne, S., P. J. Cote, B. E. Korba, S. D. Butler, A. L. George, I. A. Tochkov, W. E. T. Delaney, S. Xiong, J. L. Gerin, and B. C. Tennant. 2005. Antiviral effect of oral administration of tenofovir disoproxil fumarate in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 49:2720-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, M. D., P. D. Lamy, M. D. Fuller, A. S. Mulato, N. A. Margot, T. Cihlar, and J. M. Cherrington. 1998. Human immunodeficiency virus type 1 reverse transcriptase expressing the K70E mutation exhibits a decrease in specific activity and processivity. Mol. Pharmacol. 54:291-297. [DOI] [PubMed] [Google Scholar]
- 17.Perrillo, R., H. W. Hann, D. Mutimer, B. Willems, N. Leung, W. M. Lee, A. Moorat, S. Gardner, M. Woessner, E. Bourne, C. L. Brosgart, and E. Schiff. 2004. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology 126:81-90. [DOI] [PubMed] [Google Scholar]
- 18.Peters, M. G., H. Hann Hw, P. Martin, E. J. Heathcote, P. Buggisch, R. Rubin, M. Bourliere, K. Kowdley, C. Trepo, D. Gray, M. Sullivan, K. Kleber, R. Ebrahimi, S. Xiong, and C. L. Brosgart. 2004. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 126:91-101. [DOI] [PubMed] [Google Scholar]
- 19.Qi, X., A. Snow, V. Thibault, Y. Zhu, M. Curtis, S. Hadziyannis, C. L. Brosgart, G. Currie, S. Arterburn, C. Gibbs, M. Miller, and S. Xiong. 2004. Long-term incidence of adefovir dipivoxil (ADV) resistance in chronic hepatitis B (CHB) patients after 144 weeks of therapy. J. Hepatol. 40:20-21. [Google Scholar]
- 20.Ratziu, V., V. Thibault, Y. Benhamou, and T. Poynard. 2006. Successful rescue therapy with tenofovir in a patient with hepatic decompensation and adefovir resistant HBV mutant. Comp. Hepatol. 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray, A. S., F. Myrick, J. E. Vela, L. Y. Olson, E. J. Eisenberg, K. Borroto-Esodo, M. D. Miller, and A. Fridland. 2005. Lack of a metabolic and antiviral drug interaction between tenofovir, abacavir and lamivudine. Antivir. Ther. 10:451-457. [PubMed] [Google Scholar]
- 22.Ray, A. S., J. E. Vela, L. Olson, and A. Fridland. 2004. Effective metabolism and long intracellular half life of the anti-hepatitis B agent adefovir in hepatic cells. Biochem. Pharmacol. 68:1825-1831. [DOI] [PubMed] [Google Scholar]
- 23.Ristig, M. B., J. Crippin, J. A. Aberg, W. G. Powderly, M. Lisker-Melman, L. Kessels, and P. Tebas. 2002. Tenofovir disoproxil fumarate therapy for chronic hepatitis B in human immunodeficiency virus/hepatitis B virus-coinfected individuals for whom interferon-alpha and lamivudine therapy have failed. J. Infect. Dis. 186:1844-1847. [DOI] [PubMed] [Google Scholar]
- 24.Robbins, B. L., C. K. Wilcox, A. Fridland, and J. H. Rodman. 2003. Metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharmacotherapy 23:695-701. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Analysis and cloning of eukaryotic genomic DNA, p. 9.1-9.62. In N. Ford, C. Nolan, and M. Ferguson (ed.), Molecular cloning, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sheldon, J., N. Camino, B. Rodes, A. Bartholomeusz, M. Kuiper, F. Tacke, M. Nunez, S. Mauss, T. Lutz, G. Klausen, S. Locarnini, and V. Soriano. 2005. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir. Ther. 10:727-734. [PubMed] [Google Scholar]
- 27.Tenney, D. J., S. M. Levine, R. E. Rose, A. W. Walsh, S. P. Weinheimer, L. Discotto, M. Plym, K. Pokornowski, C. F. Yu, P. Angus, A. Ayres, A. Bartholomeusz, W. Sievert, G. Thompson, N. Warner, S. Locarnini, and R. J. Colonno. 2004. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob. Agents Chemother. 48:3498-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Bommel, F., T. Wunsche, S. Mauss, P. Reinke, A. Bergk, D. Schurmann, B. Wiedenmann, and T. Berg. 2004. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology 40:1421-1425. [DOI] [PubMed] [Google Scholar]
- 29.van Bommel, F., T. Wunsche, D. Schurmann, and T. Berg. 2002. Tenofovir treatment in patients with lamivudine-resistant hepatitis B mutants strongly affects viral replication. Hepatology 36:507-508. [DOI] [PubMed] [Google Scholar]
- 30.Villeneuve, J.-P., B. Willems, and F. Zoulim. 2005. Efficacy of tenofovir in patients with chronic hepatitis B and resistance or sub-optimal response to adefovir. Hepatology 42:588A.16037944 [Google Scholar]
- 31.White, K. L., N. A. Margot, J. K. Ly, J. M. Chen, A. S. Ray, M. Pavelko, R. Wang, M. McDermott, S. Swaminathan, and M. D. Miller. 2005. A combination of decreased NRTI incorporation and decreased excision determines the resistance profile of HIV-1 K65R RT. AIDS 19:1751-1760. [DOI] [PubMed] [Google Scholar]
- 32.White, K. L., N. A. Margot, T. Wrin, C. J. Petropoulos, M. D. Miller, and L. K. Naeger. 2002. Molecular mechanisms of resistance to human immunodeficiency virus type 1 with reverse transcriptase mutations K65R and K65R+M184V and their effects on enzyme function and viral replication capacity. Antimicrob. Agents Chemother. 46:3437-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong, X., C. Flores, H. Yang, J. J. Toole, and C. S. Gibbs. 1998. Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology 28:1669-1673. [DOI] [PubMed] [Google Scholar]
- 34.Xiong, X., H. Yang, C. E. Westland, R. Zou, and C. S. Gibbs. 2000. In vitro evaluation of hepatitis B virus polymerase mutations associated with famciclovir resistance. Hepatology 31:219-224. [DOI] [PubMed] [Google Scholar]
- 35.Yang, H., X. Qi, A. Sabogal, M. D. Miller, S. Xiong, and W. E. Delaney IV. 2005. Cross-resistance testing of next-generation nucleoside and nucleotide analogs against lamivudine-resistant HBV. Antivir. Ther. 10:625-633. [PubMed] [Google Scholar]
- 36.Yang, H., C. Westland, S. Xiong, and W. E. Delaney. 2004. In vitro antiviral susceptibility of full-length clinical hepatitis B virus isolates cloned with a novel expression vector. Antivir. Res. 61:27-36. [DOI] [PubMed] [Google Scholar]
- 37.Yeni, P. G., S. M. Hammer, M. S. Hirsch, M. S. Saag, M. Schechter, C. C. Carpenter, M. A. Fischl, J. M. Gatell, B. G. Gazzard, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2004. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA panel. JAMA 292:251-265. [DOI] [PubMed] [Google Scholar]
- 38.Ying, C., E. De Clercq, and J. Neyts. 2000. Lamivudine, adefovir and tenofovir exhibit long-lasting anti-hepatitis B virus activity in cell culture. J. Viral Hepat. 7:79-83. [DOI] [PubMed] [Google Scholar]