Abstract
The inhibitory activities (50% inhibitory concentrations [IC50s]) of gatifloxacin and other quinolones against both DNA gyrase and topoisomerase IV of the wild-type Streptococcus pneumoniae IID553 were determined. The IC50s of 10 compounds ranged from 4.28 to 582 μg/ml against DNA gyrase and from 1.90 to 35.2 μg/ml against topoisomerase IV. The inhibitory activity against DNA gyrase was more varied than that against topoisomerase IV among fluoroquinolones. The IC50s for DNA gyrase of the 8-methoxy quinolones gatifloxacin and AM-1147 were approximately seven times lower than those of their 8-H counterparts AM-1121 and ciprofloxacin, whereas the IC50s for topoisomerase IV were 1.5 times lower. Moreover, the IC50 ratios (IC50 for DNA gyrase/IC50 for topoisomerase IV) of gatifloxacin, AM-1147, and moxifloxacin, which possess 8-methoxy groups, were almost the same. The 8-methoxy quinolones showed higher antibacterial activity and less mutant selectivity against IID553 than their 8-H counterparts. These results suggest that the 8-methoxy group enhances both target inhibition, especially for DNA gyrase, leading to potent antipneumococcal activity and dual inhibition against both DNA gyrase and topoisomerase IV in the bacterial cell.
Gatifloxacin shows potent activity against various respiratory pathogens, including Streptococcus pneumoniae (5, 11, 22), and is known to be a respiratory quinolone. Gatifloxacin is similar to ciprofloxacin in its chemical structure, except for substitutions of the methoxy group at position 8 (8-methoxy group) and the 3′-methyl group of the piperazinyl ring at position 7 (3′-methyl group) of the quinolone ring. In addition, gatifloxacin shows more potent activity and lower resistance selectivity against S. pneumoniae than ciprofloxacin (5, 7). Based on the antibacterial activity of gatifloxacin and its structurally related compounds as well as genetic analysis of quinolone-resistant mutants, we have hypothesized that the 8-methoxy group of gatifloxacin potentiates target inhibition, especially against DNA gyrase, and that 8-methoxy quinolones, including gatifloxacin, inhibit DNA gyrase at nearly the same level as topoisomerase IV inhibition in S. pneumoniae bacterial cells, leading to potent antibacterial activity and low mutant selectivity (7). Some investigators also have reported that introduction of the 8-methoxy group of quinolone ensures strong antibacterial activity, bactericidal activity, and/or prevention of the emergence of mutant strains in Escherichia coli (14, 26), Staphylococcus aureus (12, 13, 25), S. pneumoniae (7), and mycobacteria (2, 19, 24).
In this study, we cloned and expressed the gyrA, gyrB, parC, and parE genes of S. pneumoniae in E. coli and purified GyrA, GyrB, ParC, and ParE subunits to reconstitute DNA gyrase and topoisomerase IV in vitro. The inhibitory activities of quinolones, including gatifloxacin (8-methoxy, 7-piperazinyl-3′-methyl) and its structurally related compounds (AM-1121 [8-H, 7-piperazinyl-3′-methyl], AM-1147 [8-methoxy, 7-piperazinyl-3′-H], and ciprofloxacin [8-H, 7-piperazinyl-3′-H]), against the purified DNA gyrase and topoisomerase IV of S. pneumoniae were determined.
MATERIALS AND METHODS
Fluoroquinolones.
Gatifloxacin and the other quinolones, except ciprofloxacin, were synthesized at Kyorin Pharmaceutical Co., Ltd. (Tokyo, Japan). Ciprofloxacin was purchased from Apin Chemicals Co. (Abingdon, United Kingdom). AM-1121 (8-H, 7-piperazinyl-3′-methyl) and AM-1147 (8-methoxy, 7-piperazinyl-3′-H) are analogues of gatifloxacin (8-methoxy, 7-piperazinyl-3′-methyl) (Table 1).
TABLE 1.
Chemical structures of gatifloxacin and its structurally related compounds ciprofloxacin, AM-1121, and AM-1147
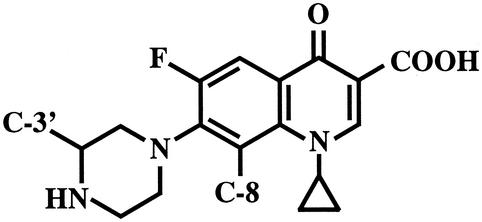
| Quinolone | Substitution at:
|
|
|---|---|---|
| C-8 | C-3′ | |
| Gatifloxacin | OCH3 | CH3 |
| AM-1147 | OCH3 | H |
| AM-1121 | H | CH3 |
| Ciprofloxacin | H | H |
Determination of MICs.
Quinolone-susceptible wild-type S. pneumoniae IID553 (a type strain collected at the Institute of Medical Science, University of Tokyo) was provided through the Japanese Society for Bacteriology. The MICs were determined by agar dilution methods with Mueller-Hinton agar (Difco Laboratories, Detroit, Mich.) containing 5% defibrinated horse blood. The MIC was defined as the lowest concentration of an antibacterial agent that inhibited visible growth after incubation for 20 h at 35°C.
Mutation frequencies.
Overnight cultures of IID553 on Mueller-Hinton agar plates containing 5% defibrinated horse blood were suspended in saline. The mutant strains were selected by plating the bacterial suspension (approximately 109 CFU) on Mueller-Hinton agar containing 5% defibrinated horse blood with quinolones at one, two, four, and eight times the MICs. The selection plates were incubated at 35°C for 72 h before being scored for the number of bacterial colonies. The incidence of the appearance of resistant strains was calculated as the ratio of the number of colonies that emerged to the number of bacteria inoculated (in CFU).
Determination of MPCs.
IID553 was incubated in Todd-Hewitt broth (Difco Laboratories) for 6 h. Cultures were centrifuged at 3,000 × g for 15 min and resuspended in saline. Mutant strains were selected by plating the bacterial suspension (approximately 1010 CFU for each concentration of quinolones) on Mueller-Hinton agar containing 5% defibrinated horse blood with quinolones at 1, 2, 4, 8, and 16 times the MICs. The selection plates were incubated at 35°C for 72 h before being scored for the appearance of bacterial colonies. The mutant prevention concentration (MPC) was recorded as the lowest concentration of quinolone that allowed no growth of mutant strains.
Preparation of type II topoisomerases.
The gyrA, gyrB, parC, and parE genes of S. pneumoniae were amplified from S. pneumoniae IID553 genomic DNA by PCR on a Hybaid thermal cycler with ELONGase Enzyme Mix (GIBCO BRL, Gaithersburg, Md.) and appropriate oligonucleotide primers (Table 2). The basic PCR parameters were 30 cycles of 95°C for 1 min, 55°C for 1 min, and 70°C for 2 min. The DNA fragments of gyrA, parC, and parE and pGEX-2T (Amersham Pharmacia Biotech) were digested with BamHI, ligated, and transformed into E. coli DH5α (Toyobo, Tokyo, Japan). The DNA fragments of gyrB and pGEX-2T were digested with EcoRI and BamHI, ligated, and transformed into E. coli DH5α. The nucleotide sequences of gyrA, gyrB, parC, and parE gene fragments that include regions corresponding to quinolone resistance-determining regions were identical to those of the wild-type strain of S. pneumoniae published previously in the National Center for Biotechnology Information database (NC 003098). The GyrA, GyrB, ParC, and ParE proteins of DNA gyrase and topoisomerase IV were purified separately as fusion proteins with glutathione S-transferase from overproducing strains of E. coli. Transformants were cultured in 2× YTG medium (tryptone, 16 g/liter; yeast extract, 10 g/liter; NaCl, 5 g/liter; glucose, 2% [pH 7.0]) containing 100 μg of ampicillin/ml at 37°C and induced at the mid-exponential phase by the addition of isopropyl-1-thio-β-d(−)-galactopyranoside to a final concentration of 0.05 mM. After incubation for 16 to 17 h at 25°C, the cells were harvested and stored at −80°C until used. The cells were suspended in sonication buffer (50 mM Tris-HCl [pH 8.0], 50 mM NaCl, 1 mM EDTA, and 1 mM dithiothreitol [DTT]) and sonicated on ice. Triton X-100 was added to a final concentration of 1%, and the suspension was centrifuged at 30,000 × g for 30 min The supernatant was loaded onto a glutathione-Sepharose 4B column previously equilibrated with sonication buffer containing 0.5% Triton X-100. The column was washed with 30 volumes of sonication buffer (with 0.5% Triton X-100) and 10 volumes of phosphate-buffered saline. The proteins were eluted with 1 volume of thrombin solution (80 U of thrombin/ml in phosphate-buffered saline). Concentration and buffer exchange were carried out with a Centriprep-10 (Millipore, Beverly, Mass.) with 50 mM Tris-HCl (pH 8.0), 10% glycerol, 1 mM EDTA, and 1 mM DTT, and the proteins were stored at −80°C.
TABLE 2.
DNA primers used for PCR amplification of DNA gyrase and topoisomerase IV of S. pneumoniae
| Primer | Sequencea |
|---|---|
| gyrA | |
| Forward | 5′-GGCATGGATCCATGCAGGATAAAAATTTAG-3′ |
| Reverse | 5′-GTAATATCAGGATCCCTGCTAGGATATTTGTC-3′ |
| gyrB | |
| Forward | 5′-GAAAAAGGGGATCCATGACAGAAGAAATCAAAAATCTGC-3′ |
| Reverse | 5′-CTTGAAGAATTCGTAACGATTATATCATATTTTTTAGACC-3′ |
| parC | |
| Forward | 5′-GCTTTGGGATCCATGTCTAACATTCAAAACATGTCCCTGG-3′ |
| Reverse | 5′-CTTCGGATCCCTTATTTATCTTCAGTAACTACTTCC-3′, |
| parE | |
| Forward | 5′-GAATAGGGGATCCCTTGTGTCAAAAAAGGAAATC-3′ |
| Reverse | 5′-CACATAGGGATCCACATCCGACTCTAATTTCCAG-3′ |
Restriction sites are underlined.
Topoisomerase reactions.
Both S. pneumoniae DNA gyrase and topoisomerase IV were reconstituted by incubation of each A and B subunit of the enzymes (GyrA-GyrB or ParC-ParE) on ice for at least 2 h. The topoisomerase activity was measured electrophoretically.
(i) Supercoiling activity of DNA gyrase.
Reaction mixtures (10 μl) containing 50 mM Tris-HCl (pH 7.5), 8 mM MgCl2, 30 mM KCl, 4 mM ATP, 5 mM DTT, 2 mM spermidine HCl, 200 mM potassium glutamate, 50 μg of bovine serum albumin/ml, 40 μg of tRNA/ml, 1 U of reconstituted DNA gyrase, 50 ng of relaxed pBR322 DNA, and various concentrations of the quinolones tested were incubated at 37°C for 1 h. The maximum concentrations of quinolones in the reaction mixtures were as follows: AM-1121, ciprofloxacin, sparfloxacin, and norfloxacin, 1,000 μg/ml; levofloxacin, 600 μg/ml; gatifloxacin, AM-1147, moxifloxacin, and trovafloxacin, 200 μg/ml; and clinafloxacin, 50 μg/ml. Each quinolone was added to the reaction mixture after serial dilutions from maximum concentrations. The reaction was terminated by the addition of 1.4% sodium dodecyl sulfate and 90 μg of proteinase K/ml. After an additional 10 min of incubation at 37°C, one-fifth volume of a loading dye was added.
One unit of activity was defined as the amount of enzyme required to fully show supercoiling in the reaction under the above-mentioned conditions.
(ii) Decatenating activity of topoisomerase IV.
The decatenating activity of topoisomerase IV, that is, the conversion of kinetoplast DNA (Topogene Inc., Columbus, Ohio) to the monomer, was measured as follows. Reaction mixtures (10 μl) containing 40 mM Tris-HCl (pH 7.5), 4 mM MgCl2, 30 mM KCl, 2 mM ATP, 5 mM DTT, 2 mM spermidine HCl, 100 mM potassium glutamate, 50 μg of bovine serum albumin/ml, 1 U of reconstituted topoisomerase IV, 150 ng of kinetoplast DNA, and various concentrations of the quinolones tested were incubated at 37°C for 1 h. The maximum concentrations of quinolones in the reaction mixtures were as follows: Sparfloxacin and norfloxacin, 100 μg/ml; gatifloxacin, AM-1147, AM-1121, ciprofloxacin, and levofloxacin, 50 μg/ml; and moxifloxacin, trovafloxacin, and clinafloxacin, 25 μg/ml. Each quinolone was added to the reaction mixture after serial dilutions from maximum concentrations. The reaction was terminated by the addition of 1.4% sodium dodecyl sulfate and 90 μg of proteinase K/ml. After an additional 10 min of incubation at 37°C, one-fifth volume of a loading dye was added.
One unit of activity was defined as the amount of enzyme required to fully show decatenation in the reaction under the above-mentioned conditions.
(iii) Inhibitory effects of quinolones.
The reaction mixtures were electrophoresed on a 1% agarose gel. Quantification of DNA in agarose gels was carried out after ethidium bromide staining. The brightness of the bands corresponding to supercoiled pBR322 DNA and decatenated kinetoplast DNA were determined by densitometric analysis with a FMBIO II Multi View fluorescent image analyzer (Hitachi Software Engineering Co., Ltd., Yokohama, Japan). The inhibitory effect of each quinolone against topoisomerases was assayed by determining the concentrations required to inhibit 50% of the enzyme reaction (IC50s).
RESULTS
Inhibitory activities of fluoroquinolones against type II topoisomerases of S. pneumoniae.
The IC50s of 10 quinolones, including AM-1121 and AM-1147, against DNA gyrase and topoisomerase IV of S. pneumoniae are shown in Table 3. The IC50s for DNA gyrase were more varied than those for toposiomerase IV among the quinolones. The IC50s of the 10 compounds ranged from 4.28 to 582 μg/ml against DNA gyrase and from 1.90 to 35.2 μg/ml against topoisomerase IV. Also, the IC50 ratios (ratios of IC50s for DNA gyrase to those for topoisomerase IV) of the 10 quinolones ranged from 2.25 to 20.1. This variation was caused by the variations in IC50s for both type II topoisomerases, especially those for DNA gyrase.
TABLE 3.
Inhibitory activities for type II topoisomerases and antibacterial activities of gatifloxacin and the other quinolones
| Quinolone | MIC (μg/ml) against IID553 | IC50 (μg/ml) against:
|
IC50 ratioa | Target preference | |
|---|---|---|---|---|---|
| DNA gyrase | Topoisomerase IV | ||||
| Gatifloxacin | 0.25 | 25.9 | 7.27 | 3.56 | DNA gyrase |
| AM-1147 | 0.25 | 19.4 | 4.05 | 4.79 | DNA gyrase |
| AM-1121 | 0.5 | 181 | 11.7 | 15.5 | Topoisomerase IV |
| Ciprofloxacin | 0.5 | 138 | 6.85 | 20.1 | Topoisomerase IV |
| Clinafloxacin | 0.063 | 4.28 | 1.90 | 2.25 | DNA gyrase |
| Moxifloxacin | 0.25 | 21.8 | 6.04 | 3.61 | DNA gyrase |
| Sparfloxacin | 0.25 | 145 | 8.28 | 17.5 | DNA gyrase |
| Trovafloxacin | 0.125 | 17.7 | 3.07 | 5.77 | Topoisomerase IV |
| Levofloxacin | 1 | 89.3 | 15.9 | 5.62 | Topoisomerase IV |
| Norfloxacin | 4 | 582 | 35.2 | 16.5 | Topoisomerase IV |
IC50 ratio, IC50 against DNA gyrase/IC50 against topoisomerase IV.
Gatifloxacin, moxifloxacin, and AM-1147, which possess an 8-methoxy group, have comparable inhibitory activities against type II topoisomerases, with IC50s of 19.4 to 25.9 μg/ml for DNA gyrase and 4.05 to 7.27 μg/ml for topoisomerase IV. Moreover, the IC50s of the 8-methoxy quinolones gatifloxacin and AM-1147 against DNA gyrase are approximately 7 times lower than those of their 8-H counterparts AM-1121 and ciprofloxacin, whereas the IC50s of these 8-methoxy quinolones against topoisomerase IV are approximately 1.5 times lower. These results indicate that the 8-methoxy quinolones show higher inhibitory activity against both target enzymes than their 8-H counterparts.
Resistance selection.
The selectivities of mutant strains of fluoroquinolones were evaluated (Table 4). Sparfloxacin selected mutant strains at 16 times the MIC, and trovafloxacin and norfloxacin were selected at 4 times the MICs. AM-1121, ciprofloxacin, and levofloxacin also selected at two times the MICs. In contrast, gatifloxacin, AM-1147, clinafloxacin, and moxifloxacin selected no mutant strains at the MIC.
TABLE 4.
Frequencies of appearance of mutant strains by selection with various quinolones
| Quinolone | Frequency at the following multiple of the MICa:
|
MPC (μg/ml) | |||||
|---|---|---|---|---|---|---|---|
| 1× | 2× | 4× | 8× | 16× | 32× | ||
| Gatifloxacin | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 | NDc | ND | 1 |
| AM-1147b | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | ND | 1 |
| AM-1121b | >2.4 × 10−6 | >2.4 × 10−6 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | ND | 8 |
| Ciprofloxacinb | >2.4 × 10−6 | >2.4 × 10−6 | <2.4 × 10−9 | <2.4 × 10−9 | <2.4 × 10−9 | ND | 8 |
| Clinafloxacin | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 | ND | ND | 0.25 |
| Moxifloxacin | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 | <5.9 × 10−10 | ND | ND | 1 |
| Sparfloxacinb | ND | >2.2 × 10−6 | >2.2 × 10−6 | >2.2 × 10−6 | 1.1 × 10−7 | <1.1 × 10−9 | 4 |
| Trovafloxacinb | ND | >2.2 × 10−6 | 6.2 × 10−8 | <1.1 × 10−9 | <1.1 × 10−9 | <1.1 × 10−9 | 1 |
| Levofloxacinb | ND | 1.5 × 10−7 | <1.1 × 10−9 | <1.1 × 10−9 | <1.1 × 10−9 | <1.1 × 10−9 | 4 |
| Norfloxacinb | ND | 2.6 × 10−7 | 2.2 × 10−9 | <1.1 × 10−9 | <1.1 × 10−9 | <1.1 × 10−9 | 64 |
MPC.
The MPCs of 10 quinolones were also determined (Table 4). The MPC is a novel concept for evaluation of the mutant selectivities of drugs. The MPCs of gatifloxacin, AM-1147, clinafloxacin, and moxifloxacin were same or lower than those of the other quinolones tested. The MPCs of gatifloxacin and AM-1147 were 1 μg/ml, which were lower than those of their 8-H counterparts, AM-1121 and ciprofloxacin.
DISCUSSION
On the basis of genetic analysis, it has been reported that the target preference in S. pneumoniae differs among the quinolones. Trovafloxacin, ciprofloxacin, AM-1121, levofloxacin, and norfloxacin prefer topoisomerase IV, whereas clinafloxacin, AM-1147, moxifloxacin, sparfloxacin, and gatifloxacin prefer DNA gyrase (5, 7, 15, 18). We have previously reported that the target preferences of the quinolones in S. aureus can be anticipated based on determinations of the IC50 ratios (21). Our data also generally show that the IC50 ratios (IC50 for DNA gyrase/IC50 for topoisomerase IV) of quinolones preferring DNA gyrase are lower than those of quinolones preferring topoisomerase IV (Table 3). These results suggest that the IC50 ratios are related to the target preference in S. pneumoniae, as they are in S. aureus.
It has been proposed that the MICs of quinolones are determined by the inhibitory activity against the preferential target (6, 9, 23). Gatifloxacin and AM-1147 showed twice the antibacterial activity of their 8-H counterparts, AM-1121 and ciprofloxacin, against wild-type strain IID553. It has been reported that the primary target of the 8-methoxy quinolones (AM-1147 and gatifloxacin) was DNA gyrase, although that of their 8-H counterparts (ciprofloxacin and AM-1121) was topoisomerase IV (7). Also, the IC50s of the 8-methoxy quinolones gatifloxacin and AM-1147 against DNA gyrase are approximately 7 times lower than those of their 8-H counterparts AM-1121 and ciprofloxacin, whereas the IC50s of these 8-methoxy quinolones against topoisomerase IV are approximately 1.5 times lower (Table 3). Furthermore, the MICs of the 8-methoxy quinolones for gyrA mutants were higher than those for the wild-type strain, although the MICs for parC mutants were almost the same as those for wild-type strain. Hence, increases of the MICs of the 8-H counterparts for parC mutants were higher than those for gyrA mutants (7). These results suggest that the increase in the inhibitory activities of the 8-methoxy quinolones against DNA gyrase is one of the possible mechanisms underlying the potent antipneumococcal activity of these quinolones.
In this study, the IC50s of sparfloxacin for both enzymes were similar to those of ciprofloxacin, although sparfloxacin had more potent antibacterial activity than ciprofloxacin against IID553. Furthermore, the IC50 ratio of sparfloxacin was 17.5, which was similar to those of norfloxacin and ciprofloxacin. However, sparfloxacin seems to prefer DNA gyrase, as this agent selected gyrA mutant strains with high frequency and showed no decrease in antibacterial activity against parC mutant strains compared with the wild-type strain (5). There are some discrepancies between inhibitory activity against target enzymes and either antipneumococcal activity or target preference. Other investigators have already observed these discrepancies in sparfloxacin (10, 16, 17); however, possible causes of these discrepancies remain unclear.
Gatifloxacin, AM-1147, moxifloxacin, and clinafloxacin showed less mutant selectivity than the other quinolones (Table 4). It has been reported that some fluoroquinolones selected the mutant strains of S. pneumoniae and S. aureus with low frequency, as these agents seem to inhibit both DNA gyrase and topoisomerase IV at nearly the same level in bacterial cells (dual-targeting property) (5, 7, 8, 15). As such, these four quinolones seem to possess the dual-targeting property, even though the target preference of these quinolones seems to be DNA gyrase (5, 15, 18). Furthermore, gatifloxacin and AM-1147 selected no mutant strains at the MIC or higher concentrations, whereas their 8-H counterparts, AM-1121 and ciprofloxacin, selected the resistant mutants at two times the MIC. These results suggest that the 8-methoxy group of quinolones contributes to enhancement of the inhibition of DNA gyrase, leading to the dual-targeting property of the agents in bacterial cells.
Recently, in order to evaluate drug activity, the novel concept designated MPC has been proposed. The MPC has been defined as the minimal drug concentration that allows no mutant recovery when approximately 1010 cells are applied to drug-containing agar (3). The MPCs of gatifloxacin, AM-1147, clinafloxacin, and moxifloxacin were the same or lower than those of the other quinolones tested (Table 4). These results suggest that gatifloxacin, AM-1147, clinafloxacin, and moxifloxacin are expected to restrict the selection of resistant mutants in a clinical setting. Dong et al. reported that the 8-methoxy quinolones possessed lower MPCs against mycobacteria (3, 4) and S. aureus (3). Blondeau et al. (1) determined the MPCs against clinical isolates of S. pneumoniae and also suggested that gatifloxacin and moxifloxacin restricted the selection of resistant mutants. Our results for the MPCs agree with those studies and suggest that both the dual-targeting property and potent antipneumococcal activity of 8-methoxy quinolones contribute to low MPCs.
The IC50 ratios of gatifloxacin, AM-1147, clinafloxacin, and moxifloxacin were found to be 3.56, 4.79, 2.25, and 3.61, respectively. We have previously hypothesized that the IC50 ratio is important for predicting target preference, including the dual-targeting property, in bacterial cells (21). Similar findings were observed in whole-cell assays with gyrase mutant and topoisomerase IV mutant of S. aureus. Roychoudhury et al. (20) have shown that the cell growth inhibition of quinolones against gyrase mutant was correlated to the IC50 for topoisomerase IV of the wild-type strain and have suggested that the dual-targeting property could be anticipated by the ratio of the cell growth inhibition against each mutant. In the present study, gatifloxacin, AM-1147, clinafloxacin, and moxifloxacin showed almost the same IC50 ratios, suggesting that this range of IC50 ratios might represent the dual-targeting property of quinolones in S. pneumoniae bacterial cells.
In these enzyme studies, we have clarified that the 8-methoxy group of gatifloxacin and AM-1147 contributes to an enhancement of both target inhibition, especially for DNA gyrase, leading to potent antipneumococcal activity and the dual-targeting property of 8-methoxy quinolones in S. pneumoniae bacterial cells. Further studies on the contributions of the other substituents at position 8 of the quinolone ring to target inhibition will give us useful information for the discovery of favorable quinolones against S. pneumoniae.
REFERENCES
- 1.Blondeau, J. M., X. Zhao, G. Hansen, and K. Drlica. 2001. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong, Y., C. Xu, X. Zhao, J. Domagala, and K. Drlica. 1998. Fluoroquinolone action against mycobacteria: effects of C-8 substituents on growth, survival, and resistance. Antimicrob. Agents Chemother. 42:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong, Y., X. Zhao, J. Domagala, and K. Drlica. 1999. Effect of fluoroquinolone concentration on selection of resistant mutants of Mycobacterium bovis BCG and Staphylococcus aureus. Antimicrob. Agents Chemother. 43:1756-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong, Y., X. Zhao, B. N. Kreiswirth, and K. Drlica. 2000. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukuda, H., and K. Hiramatsu. 1999. Primary targets of fluoroquinolones in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:410-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda, H., S. Hori, and K. Hiramatsu. 1998. Antibacterial activity of gatifloxacin (AM-1155, CG5501, BMS-206584), a newly developed fluoroquinolone, against sequentially acquired quinolone-resistant mutants and the norA transformant of Staphylococcus aureus. Antimicrob. Agents Chemother. 42:1917-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda, H., R. Kishii, M. Takei, and M. Hosaka. 2001. Contributions of the 8-methoxy group of gatifloxacin to resistance selectivity, target preference, and antibacterial activity against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:1649-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaton, V. J., J. E. Ambler, and L. M. Fisher. 2000. Potent antipneumococcal activity of gemifloxacin is associated with dual targeting of gyrase and topoisomerase IV, an in vivo target preference for gyrase, and enhanced stabilization of cleavable complexes in vitro. Antimicrob. Agents Chemother. 44:3112-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper, D. C. 2000. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 31:S24-S28. [DOI] [PubMed] [Google Scholar]
- 10.Hooper, D. C. 2001. Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin. Infect. Dis. 32:S9-S15. [DOI] [PubMed] [Google Scholar]
- 11.Hosaka, M., T. Yasue, H. Fukuda, H. Tomizawa, H. Aoyama, and K. Hirai. 1992. In vitro and in vivo antibacterial activities of AM-1155, a new 6-fluoro-8-methoxy quinolone. Antimicrob. Agents Chemother. 36:2108-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ince, D., and D. C. Hooper. 2001. Mechanisms and frequency of resistance to gatifloxacin in comparison to AM-1121 and ciprofloxacin in Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2755-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito, T., M. Matsumoto, and T. Nishino. 1995. Improved bactericidal activity of Q-35 against quinolone-resistant staphylococci. Antimicrob. Agents Chemother. 39:1522-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu, T., X. Zhao, and K. Drlica. 1999. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic and bactericidal activities by the C-8-methoxy group. Antimicrob. Agents Chemother. 43:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan, X. S., and L. M. Fisher. 1998. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan, X. S., and L. M. Fisher. 1999. Streptococcus pneumoniae DNA gyrase and topoisomerase IV: overexpression, purification, and differential inhibition by fluoroquinolones. Antimicrob. Agents Chemother. 43:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan, X. S., G. Yague, and L. M. Fisher. 2001. Quinolone resistance mutations in Streptococcus pneumoniae GyrA and ParC proteins: mechanistic insights into quinolone action from enzymatic analysis, intracellular levels, and phenotypes of wild-type and mutant proteins. Antimicrob. Agents Chemother. 45:3140-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pestova, E., J. J. Millichap, G. A. Noskin, and L. R. Peterson. 2000. Intracellular targets of moxifloxacin: a comparison with other fluoroquinolones. J. Antimicrob. Chemother. 45:583-590. [DOI] [PubMed] [Google Scholar]
- 19.Renau, T. E., J. W. Gage, J. A. Dever, G. E. Roland, E. T. Joannides, M. A. Shapiro, J. P. Sanchez, S. J. Gracheck, J. M. Domagala, M. R. Jacobs, and R. C. Reynolds. 1996. Structure-activity relationships of quinolone agents against mycobacteria: effect of structural modifications at the 8 position. Antimicrob. Agents Chemother. 40:2363-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roychoudhury, S., C. E. Catrenich, E. J. McIntosh, H. D. McKeever, K. M. Makin, P. M. Koenigs, and B. Ledoussal. 2001. Quinolone resistance in staphylococci: activities of new nonfluorinated quinolones against molecular targets in whole cells and clinical isolates. Antimicrob. Agents Chemother. 45:1115-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takei, M., H. Fukuda, R. Kishii, and M. Hosaka. 2001. Target preference of 15 quinolones against Staphylococcus aureus, based on antibacterial activities and target inhibition. Antimicrob. Agents Chemother. 45:3544-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsurumaki, Y., H. Manda, M. Takei, and M. Hosaka. 2000. In vitro antimicrobial activity of gatifloxacin against 873 clinical isolates from respiratory tract, urinary tract and surgical infections during 1997-1998 in Japan. J. Antimicrob. Chemother. 45:685-689. [DOI] [PubMed] [Google Scholar]
- 23.Yamagishi, J., T. Kojima, Y. Oyamada, K. Fujimoto, H. Hattori, S. Nakamura, and M. Inoue. 1996. Alterations in the DNA topoisomerase IV grlA gene responsible for quinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1157-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao, B. Y., R. Pine, J. Domagala, and K. Drlica. 1999. Fluoroquinolone action against clinical isolates of Mycobacterium tuberculosis: effects of a C-8 methoxyl group on survival in liquid media and in human macrophages. Antimicrob. Agents Chemother. 43:661-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao, X., J. Y. Wang, C. Xu, Y. Dong, J. Zhou, J. Domagala, and K. Drlica. 1998. Killing of Staphylococcus aureus by C-8-methoxy fluoroquinolones. Antimicrob. Agents Chemother. 42:956-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao, X., C. Xu, J. Domagala, and K. Drlica. 1997. DNA topoisomerase targets of the fluoroquinolones: a strategy for avoiding bacterial resistance. Proc. Natl. Acad. Sci. USA 94:13991-13996. [DOI] [PMC free article] [PubMed] [Google Scholar]


