Abstract
The potencies of several alkoxyalkyl esters of acyclic nucleoside phosphonates against vaccinia virus and cowpox virus were evaluated in cell monolayers and three-dimensional epithelial raft cultures. Prodrugs were at least 20-fold more active than their parent compounds. Octadecycloxyethyl-(S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine emerged as the most potent derivative.
Smallpox has been declared eradicated since 1980 (5). However, due to the potential threat of variola virus as a weapon for bioterrorism (4) and the increasing number of infections with monkeypox virus (11), it is essential that adequate antiviral drugs be developed. Cidofovir (CDV) has already proven to be active against orthopoxviruses (9, 10, 14, 16, 18). However, this drug needs to be administered intravenously and is potentially nephrotoxic. Recently, it was shown that alkoxyalkyl and alkyl esters of CDV and cyclic cidofovir (cCDV) were more active against vaccinia virus (VV) and cowpox virus (CPV) in human foreskin fibroblasts than their parent compounds due in part to an increased penetration through the cell membrane (13, 15). In vivo studies indicate that the prodrugs are highly orally bioavailable and are as effective as parenterally administered CDV for the treatment of VV- or CPV-infected mice (6, 7, 17).
The activities of various alkoxyalkyl esters of the acyclic nucleoside phosphonate (ANP) analogues CDV [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine; HPMPC; Vistide], cCDV (cHPMPC), and (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine (HPMPA) against VV (strain Lederle-Chorioallentoic; ATCC VR-118) and CPV (strain Brighton; ATCC VR-302) were evaluated. Stock solutions of the parent compounds were made in phosphate-buffered saline (PBS) at a concentration of 2 mg/ml and stored at 4°C. The following alkoxyalkyl esters were used: 1-O-octadecyl-2-O-benzyl-glyceryl-CDV (ODBG-CDV), oleyloxyethyl-CDV (OLE-CDV), hexadecycloxypropyl-CDV (HDP-CDV), octadecycloxyethyl-CDV (ODE-CDV), oleyloxypropyl-CDV (OLP-CDV), oleyloxyethyl-cCDV (OLE-cCDV), hexadecycloxypropyl-HPMPA (HDP-HPMPA), and octadecycloxyethyl-HPMPA (ODE-HPMPA). The structures and the synthesis of these compounds were described elsewhere (3, 12, 15). Stock solutions in a concentration of 2 to 10 mg/ml were made in PBS/dimethyl sulfoxide (3:1) and stored at −20°C. Antiviral assays with monolayers of both human embryonic lung (HEL) fibroblasts (ATCC CCL-137) and primary human keratinocyte (PHK) cells isolated from neonatal foreskins were carried out as described before (18). The viral cytopathic effect (CPE) was recorded, and the 50% effective concentration (EC50) was defined as the compound concentration required to reduce the viral CPE by 50% compared to that for the untreated control. Cytotoxicity assays were carried out as reported previously (18). The 50% cytotoxic concentration (CC50) was defined as the concentration required to reduce the cell number by 50% compared to that for the untreated controls. The selectivity index (SI) was defined as the ratio of the CC50 for cell growth to the EC50 for viral CPE. All the prodrugs tested were far more potent than the parent compounds against VV and CPV in HEL fibroblasts (Table 1). ODE-CDV and OLE-CDV emerged as the most active CDV derivatives. ODBG-CDV exhibited the weakest activity but was still at least 130-fold more active than CDV. OLE-cCDV was 2,800-fold more active than its parent compound against VV, while ODE-HPMPA was up to 15,300-fold more active than HPMPA. These strong activities result in high degrees of selectivity of the prodrugs, despite their higher cytotoxicities, in comparison with those of the original compounds. OLP-CDV appeared to be the most selective prodrug of CDV, while ODE-HPMPA showed up to 30-fold more selectivity than HPMPA. The minimal cytotoxic concentration (MCC) reflects the effects of the compounds on the change in the structure of the monolayer due to ballooning and detachment of the cells. The highest concentrations of the ANP derivatives that were used to test the HEL fibroblasts did not alter the morphology of the cells (Table 1). Since poxviruses have an epithelial tropism, the antiviral activities of the ANP derivatives were analyzed by using epithelial cell monolayers (Table 2). As in HEL fibroblasts, all prodrugs were more potent than the parent compounds. The differences in the activities in comparison to those of the parent compounds were, however, not as pronounced as in the HEL fibroblasts. The order of the decreasing activities of the CDV derivatives was different when PHK cells were used. ODBG-CDV, OLE-CDV, and ODE-CDV were the most active compounds. The decrease in EC50 varied from 290-fold for the most active compounds to 20-fold for the least potent compounds. ODE-HPMPA was clearly the most potent prodrug. Its activity was increased up to 750-fold in comparison to that of HPMPA. Due to the high sensitivity of growing PHK cells to the compounds, no selectivity could be seen. Most prodrugs affected the morphology of PHK cells at only the highest concentrations used (Table 2).
TABLE 1.
Activities of alkoxyalkyl esters of CDV, cCDV, and HPMPA against VV and CPV in HEL fibroblast monolayers
| Compound | VV
|
CPV
|
Cytotoxicity (μg/ml)
|
SIe
|
||||
|---|---|---|---|---|---|---|---|---|
| EC50 (μg/ml)a | Fold decreaseb | EC50 (μg/ml)a | Fold decreaseb | MCC (μg/ml)c | CC50 (μg/ml)d | VV | CPV | |
| HDP-CDV | 0.013 ± 0.006 | 360 | 0.035 ± 0.004 | 390 | >5 | 0.37 ± 0.09 | 28 | 11 |
| ODE-CDV | 0.0051 ± 0.0043 | 910 | 0.014 ± 0.001 | 900 | >5 | 0.16 ± 0.11 | 31 | 11 |
| OLP-CDV | 0.019 ± 0.012 | 260 | 0.032 ± 0.000 | 420 | >5 | 1.04 ± 0.51 | 55 | 33 |
| ODBG-CDV | 0.044 ± 0.017 | 130 | 0.069 ± 0.004 | 230 | >5 | 0.30 ± 0.09 | 7 | 4 |
| OLE-CDV | 0.0083 ± 0.0029 | 570 | 0.018 ± 0.004 | 720 | >5 | 0.18 ± 0.09 | 22 | 10 |
| CDV | 2.52 ± 1.45 | 6.83 ± 0.34 | >50 | 37.9 ± 0.7 | 15 | 6 | ||
| OLE-cCDV | 0.0035 ± 0.0034 | 2,800 | 0.011 ± 0.005 | 1,500 | >5 | 0.26 ± 0.18 | 74 | 24 |
| cCDV | 5.25 ± 1.23 | 8.97 ± 1.46 | >50 | 42.4 ± 12.2 | 8 | 5 | ||
| HDP-HPMPA | 0.00051 ± 0.00044 | 670 | 0.0017 ± 0.0022 | 250 | >2 | 0.044 ± 0.003 | 86 | 26 |
| ODE-HPMPA | 0.000023 ± 0.000023 | 15,300 | 0.000062 ± 0.000035 | 7,200 | >2 | 0.01 ± 0.00 | 435 | 161 |
| HPMPA | 0.19 ± 0.10 | 0.24 ± 0.13 | >20 | 2.80 ± 0.07 | 15 | 12 | ||
Concentration required to inhibit 50% of virus-induced CPE. The EC50 values of each compound represent the mean ± standard deviation of the EC50 values of at least two independent experiments.
Fold decrease in EC50 relative to that of the parental compound based on molar values, given the following molecular weights: HDP-CDV, 583.68; ODE-CDV, 581.71; OLP-CDV, 609.71; ODBG-CDV, 717.85; OLE-CDV, 595.68; CDV, 315.12; OLE-cCDV, 559.65; cCDV, 297.2; HDP-HPMPA, 585.72; ODE-HPMPA, 599.38; HPMPA, 325.
Minimum cytotoxic concentration required to alter cell morphology.
Concentration required to reduce cell growth by 50%. The CC50 values of each compound represent the mean ± standard deviation of the CC50 values of at least two independent experiments.
Selectivity index (ratio of CC50 to EC50).
TABLE 2.
Activities of alkoxyalkyl esters of CDV, cCDV, and HPMPA against VV and CPV in PHK monolayers
| Compound | VV
|
CPV
|
Cytotoxicity (μg/ml)
|
SIe
|
||||
|---|---|---|---|---|---|---|---|---|
| EC50 (μg/ml)a | Fold decreaseb | EC50 (μg/ml)a | Fold decreaseb | MCC (μg/ml)c | CC50 (μg/ml)d | VV | CPV | |
| HDP-CDV | 0.48 ± 0.52 | 20 | 0.32 ± 0.19 | 30 | 20 | 0.18 ± 0.18 | 0.4 | 0.6 |
| ODE-CDV | 0.081 ± 0.079 | 130 | 0.053 ± 0.037 | 180 | 20 | 0.012 ± 0.011 | 0.2 | 0.3 |
| OLP-CDV | 0.40 ± 0.44 | 30 | 0.33 ± 0.23 | 30 | 20 | 0.034 ± 0.027 | 0.1 | 0.1 |
| ODBG-CDV | 0.058 ± 0.070 | 230 | 0.072 ± 0.071 | 170 | >5 | 0.012 ± 0.011 | 0.2 | 0.2 |
| OLE-CDV | 0.065 ± 0.037 | 170 | 0.035 ± 0.022 | 290 | >20 | 0.014 ± 0.013 | 0.2 | 0.4 |
| CDV | 5.8 ± 4.2 | 5.3 ± 2.1 | >50 | 7.2 ± 6.7 | 1.2 | 1.4 | ||
| OLE-cCDV | 0.31 ± 0.32 | 110 | 0.12 ± 0.17 | 160 | >20 | 0.055 ± 0.080 | 0.2 | 0.5 |
| cCDV | 18.9 ± 12.9 | 9.9 ± 5.7 | >50 | 41.7 ± 34.9 | 2.2 | 4.2 | ||
| HDP-HPMPA | 0.079 ± 0.059 | 30 | 0.022 ± 0.023 | 50 | 2 | 0.021 ± 0.025 | 0.3 | 1.0 |
| ODE-HPMPA | 0.005 ± 0.000 | 560 | 0.0015 ± 0.0008 | 750 | 2 | 0.0078 ± 0.0110 | 2 | 5.2 |
| HPMPA | 1.5 ± 0.9 | 0.61 ± 0.28 | 20 | 0.53 ± 0.05 | 0.4 | 0.9 | ||
Concentration required to inhibit 50% of virus-induced CPE. The EC50 values of each compound represent the mean ± standard deviation of the EC50 values of at least two independent experiments.
Fold decrease in EC50 relative to that of the parental compound based on molar values, given the following molecular weights: HDP-CDV, 583.68; ODE-CDV, 581.71; OLP-CDV, 609.71; ODBG-CDV, 717.85; OLE-CDV, 595.68; CDV, 315.12; OLE-cCDV, 559.65; cCDV, 297.2; HDP-HPMPA, 585.72; ODE-HPMPA, 599.38; HPMPA, 325.
Minimum cytotoxic concentration required to alter cell morphology.
Concentration required to reduce cell growth by 50%. The CC50 values of each compound represent the mean ± standard deviation of the CC50 values of at least two independent experiments.
Selectivity index (ratio of CC50 to E50).
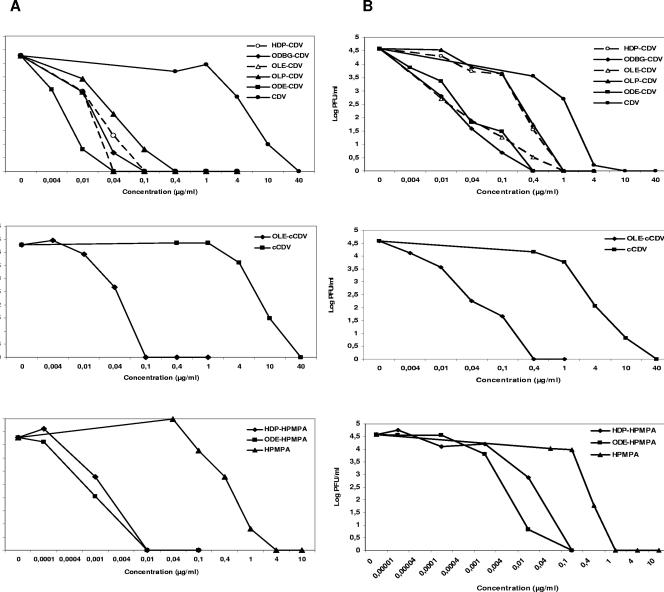
The more potent activity of the alkoxyalkyl derivatives against VV was confirmed in a virus yield reduction assay carried out as described before (1). HEL fibroblasts and PHK cells were infected with VV (850 PFU/well), and at 3 days postinfection samples were taken to determine the virus yield. Results showing the extracellular VV production are depicted in Fig. 1. Similar data were obtained for intracellular virus production (data not shown).
FIG. 1.
Effects of alkoxyalkyl esters of CDV, cCDV, and HPMPA on extracellular VV production in HEL fibroblast monolayers (A) and PHK monolayers (B).
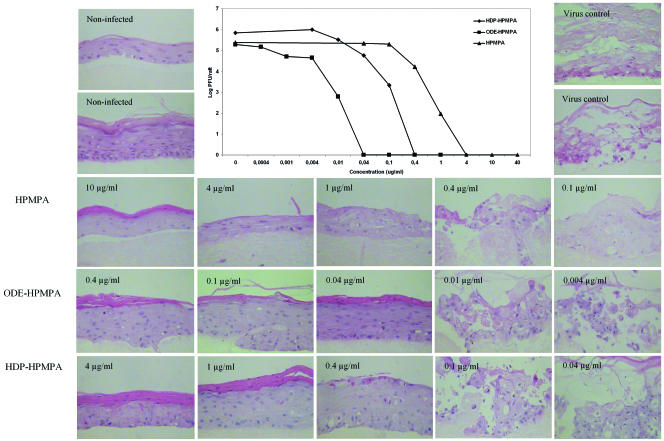
Because three-dimensional organotypic epithelial raft cultures more closely mimic the differentiated epithelium, data concerning the activities of the prodrugs against the dermotropic poxviruses in this ex vivo model would be more representative than the results obtained with keratinocyte monolayers. As shown before, the lesions in the raft cultures infected with VV, which can be seen after histological examination, are similar to the ones seen in the clinic (18). These three-dimensional epithelial culture systems have recently been successfully used to determine the activities of ANP derivatives against VV, orf virus, and herpesviruses (2, 8, 18). Organotypic cultures were prepared as described previously (2, 18). The cultures were infected in duplicate with VV at day 7 after the raft cultures were lifted, and the compounds were added to the medium. Every other day, the medium was removed and replenished with growth medium containing the compounds. At day 12 after the raft cultures were lifted, the organotypic cultures were harvested. One series was fixed in 10% buffered formalin for histological examination. The other series of the rafts was frozen in PBS and thawed to release the virus from the infected epithelium. Virus yield was determined by a plaque assay with HEL fibroblasts. The results obtained with CDV, ODBG-CDV, and OLE-CDV are shown in Fig. 2. ODBG-CDV was the most active CDV derivative, showing full protection against VV at a concentration of 0.1 μg/ml, which was confirmed in the virus yield assay and which is reflected by EC90 and EC99 values of <0.04 μg/ml. The order of increasing activity of the CDV derivatives obtained with the organotypic epithelial cultures was comparable to that obtained with the PHK cell monolayers. OLE-cCDV also proved to be more active than cCDV in the ex vivo model. The EC90 and EC99 values of OLE-cCDV, 0.10 μg/ml and 0.33 μg/ml, respectively, were 10-fold lower than those of the parent compound. ODE-HPMPA appeared to be the most potent prodrug of HPMPA and inhibited virus production up to a concentration of 0.04 μg/ml (Fig. 3). The EC90 of this derivative was 700-fold lower than that of HPMPA, which was 0.57 μg/ml. Although ODBG-CDV exhibited the weakest activity in HEL fibroblasts, it was one of the CDV prodrugs with the strongest activities in PHK cell monolayers. This strong activity was confirmed on both morphology and virus yield in the ex vivo model. These data prove the importance of using a model that bears the most resemblance to the natural environment of the virus.
FIG. 2.
Activities of CDV, ODBG-CDV, and OLE-CDV against VV in organotypic epithelial raft cultures of PHK cells.
FIG. 3.
Activities of HPMPA, ODE-HPMPA, and HDP-HPMPA against VV in organotypic epithelial raft cultures of PHK cells.
It has already been reported that alkoxyalkyl derivatives containing an ethanediol linker (ODE, OLE) show the highest activities against orthopoxvirus replication in human foreskin fibroblasts (13). Similar effects were observed when activity against viral replication in HEL cells was evaluated. In PHK cells, derivatives that contain an ethanediol or glycerol linker (ODBG) showed the highest antiviral activities. These analogues also appeared to be the most toxic. The presence of a double bond had no influence on antiviral activity.
In summary, we have demonstrated the activities of several alkoxyalkyl esters of CDV, cCDV, and HPMPA against orthopox virus replication in vitro on HEL fibroblast and PHK monolayers and ex vivo in differentiated keratinocytes. Due to the epithelial tropism of the poxviruses, these epithelial in vitro and ex vivo systems are appropriate as a prelude for their exploration in an in vivo model. ODE-HPMPA emerged as the most selective molecule against the replication of VV and CPV. Due to the higher levels of activity of these derivatives in comparison to those of their parent compounds and their increased oral bioavailabilities, the results described here are promising for the development of drugs that could eventually be used for treatment in a potential poxvirus outbreak.
Acknowledgments
This work was supported in part by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (grant G.0267.04); by the Belgian Geconcerteerde Onderzoeksacties (GOA) (2005/19); by grant AI 062540-01 from NIH, Bethesda, MD (to I.L., G.A., and R.S.); and by grants AI 066499 and AI 064615 from the National Institute of Allergy and Infectious Disease, NIH, Bethesda, MD (to K.Y.H.).
We thank Christiane Callebaut for editorial help and Anita Camps, Steven Carmans, and Lies Van den Heurck for excellent technical assistance.
REFERENCES
- 1.Andrei, G., R. Snoeck, M. Vandeputte, and E. De Clercq. 1997. Activities of various compounds against murine and primate polyomaviruses. Antimicrob. Agents Chemother. 41:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrei, G., J. van den Oord, E. De Wolf-Peeters, E. De Clercq, and R. Snoeck. 2005. Organotypic epithelial raft cultures as a model for evaluating antiviral compounds against alpha-herpesviruses. Antimicrob. Agents Chemother. 49:4671-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berche, P. 2001. The threat of smallpox and bioterrorism. Trends Microbiol. 9:15-18. [DOI] [PubMed] [Google Scholar]
- 5.Breman, J. G., and I. Arita. 1980. The confirmation and maintenance of smallpox eradication. N. Engl. J. Med. 303:1263-1273. [DOI] [PubMed] [Google Scholar]
- 6.Buller, R. M., G. Owens, J. Schriewer, L. Melman, J. R. Beadle, and K. Y. Hostetler. 2004. Efficacy of oral active ether lipid analogs of cidofovir in a lethal mousepox model. Virology 318:474-481. [DOI] [PubMed] [Google Scholar]
- 7.Ciesla, S. L., J. Trahan, W. B. Wan, J. R. Beadle, K. A. Aldern, G. R. Painter, and K. Y. Hostetler. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antivir. Res. 59:163-171. [DOI] [PubMed] [Google Scholar]
- 8.Dal Pozzo, F., G. Andrei, A. Holy, J. van den Oord, E. Scagliarini, E. De Clercq, and R. Snoeck. 2005. Activity of acyclic nucleoside phosphonates against orf virus in human and ovine cell monolayers and organotypic ovine raft cultures. Antimicrob. Agents Chemother. 49:4843-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq, E. 2002. Cidofovir in the treatment of poxvirus infections. Antivir. Res. 55:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Clercq, E., and J. Neyts. 2004. Therapeutic potential of nucleoside/nucleotide analogues against poxvirus infections. Rev. Med. Virol. 14:289-300. [DOI] [PubMed] [Google Scholar]
- 11.Di Giulio, D. B., and P. B. Eckburg. 2004. Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 4:15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartline, C. B., K. M. Gustin, W. B. Wan, S. L. Ciesla, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2005. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J. Infect. Dis. 191:396-399. [DOI] [PubMed] [Google Scholar]
- 13.Keith, K. A., W. B. Wan, S. L. Ciesla, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2004. Inhibitory activity of alkoxyalkyl and alkyl esters of cidofovir and cyclic cidofovir against orthopoxvirus replication in vitro. Antimicrob. Agents Chemother. 48:1869-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kern, E. R. 2003. In vitro activity of potential anti-poxvirus agents. Antivir. Res. 57:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quenelle, D. C., D. J. Collins, and E. R. Kern. 2003. Efficacy of multiple- or single-dose cidofovir against vaccinia and cowpox virus infections in mice. Antimicrob. Agents Chemother. 47:3275-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quenelle, D. C., D. J. Collins, W. B. Wan, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2004. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snoeck, R., A. Holy, C. Dewolf-Peeters, O. J. Van Den, E. De Clercq, and G. Andrei. 2002. Antivaccinia activities of acyclic nucleoside phosphonate derivatives in epithelial cells and organotypic cultures. Antimicrob. Agents Chemother. 46:3356-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]