Abstract
Bisbenzamidines, such as pentamidine isethionate, are aromatic dicationic compounds that are active against Pneumocystis and other microbes but are oftentimes toxic to the host. To identify potential anti-Pneumocystis agents, we synthesized bisbenzamidine derivatives in which the parent compound pentamidine was modified by a 1,4-piperazinediyl, alkanediamide, or 1,3-phenylenediamide moiety as the central linker. Several of the compounds were more active against P. carinii and less toxic than pentamidine in cytotoxicity assays. For this study, we evaluated nine bisbenzamidine derivatives representing a range of in vitro activities, from highly active to inactive, for the treatment of pneumocystosis in an immunosuppressed mouse model. Six of these in vitro-active compounds, 01, 02, 04, 06, 100, and 101, exhibited marked efficacies against infection at a dose of 10 mg/kg of body weight, and four compounds, 01, 04, 100, and 101, showed significant increases in survival versus that of untreated infected control mice. Compound 100 was highly efficacious against the infection at 20 mg/kg and 40 mg/kg, with >1,000-fold reductions in burden, and resulted in improved survival curves versus those for pentamidine-treated mice (at the same doses). All six bisbenzamidine compounds that exhibited high in vitro activity significantly decreased the infection in vivo; two compounds, 12 and 102, with marked to moderate in vitro activities had slight or no activity in vivo, while compound 31 was inactive in vitro and was also inactive in vivo. Thus, the selection of highly active compounds from in vitro cytotoxicity assays was predictive of activity in the mouse model of Pneumocystis pneumonia. We conclude that a number of these bisbenzamidine compounds, especially compound 100, may show promise as new anti-Pneumocystis drugs.
Pneumocystis is an opportunistic fungus that is a leading cause of fatal pneumonia in persons infected with human immunodeficiency virus and in other immunocompromised hosts (15, 23, 30). Drugs in clinical use to treat pneumocystosis are limited by problems of efficacy, frequent adverse effects, and emerging resistance (1, 19, 21, 22). Most development and testing of new compounds for anti-Pneumocystis activity have been conducted by using organisms derived from rodents (11, 31). Because a long-term in vitro propagation system for any species of Pneumocystis is unavailable, we developed a short-term cell-free system that assesses viability by measuring Pneumocystis ATP with a luciferase-luciferin bioluminescence system (7, 8). This system is semiautomated and permits the screening of hundreds of compounds per year. Promising agents are selected on the basis of anti-Pneumocystis activity and a lack of toxicity or low toxicity in one to three mammalian cell lines for further study in an immunosuppressed rodent model of Pneumocystis pneumonia (12, 43, 45). The relationship of this system to human pneumocystosis has become more complicated by the increasing evidence of genetic diversity and host specificity among Pneumocystis populations from different mammalian species (3, 18, 25, 32). Currently, the literature contains descriptions of the following four Pneumocystis species: Pneumocystis carinii and P. wakefieldiae from rats (9, 10, 16, 32) P. murina from mice (24), and P. jirovecii from humans (16, 32). The lack of availability of P. jirovecii organisms necessitates the use of organisms isolated from rodent sources. Despite genetic differences, drug responses in animal models have had a high correlation to activities in humans with Pneumocystis pneumonia and continue to be reliable predictors (11, 43).
Bisbenzamidines are aromatic dicationic compounds that have a broad range of activities against protozoa, fungi, mycobacteria, viruses, and tumors but are usually toxic to the host and not well absorbed (2, 6, 31). The only bisbenzamidine in clinical use is pentamidine isethionate, which is used to treat pneumocystosis, leishmaniasis, and trypanosomiasis. We and other investigators have studied the structure-activity relationships of bisbenzamidine derivatives and their efficacies in rodent models of Pneumocystis pneumonia (14, 17, 20, 34-36, 39, 42, 44). A series of compounds in which pentamidine was modified by a 1,4-piperazinediyl, alkanediamide, or phenylenediamide moiety as the central linker to modulate conformational flexibility was developed in our laboratories (12, 13, 28, 29, 37). Forty-six of these agents were evaluated in the ATP assay, and some were found to be more active than pentamidine against P. carinii, with minimal toxicity to mammalian cells (12, 37). For this study, we have extended these observations to examine the activities and toxicities of selected bisbenzamidine derivatives in an immunosuppressed mouse model of pneumocystosis.
MATERIALS AND METHODS
Compounds.
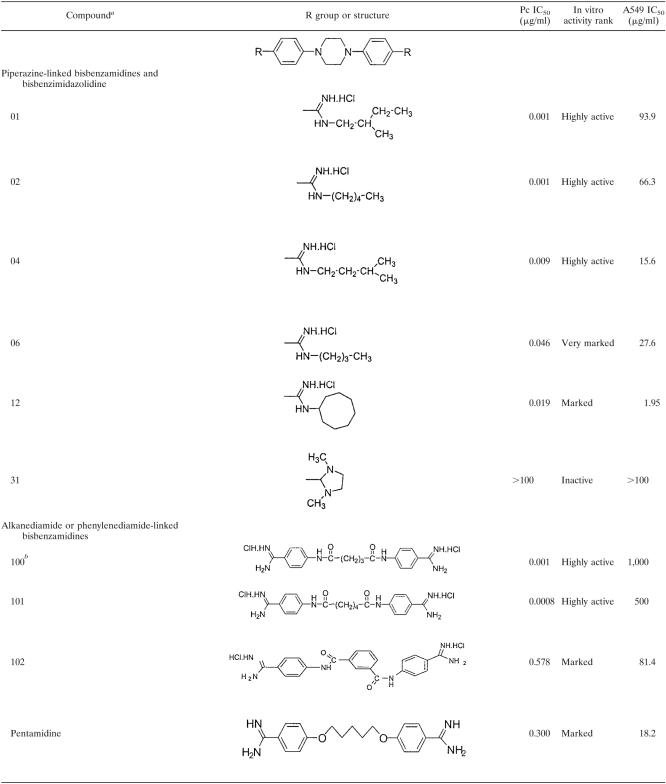
Bisbenzamidines were synthesized in the laboratory of Tien Huang, College of Pharmacy, Xavier University of Louisiana, according to previously described procedures (12, 13, 28, 29, 33, 37, 38). The structures and purity of the compounds were confirmed by spectroscopic methods (infrared and high-resolution proton nuclear magnetic resonance) and elemental analyses. The general formulas of the compounds are shown in Table 1. Pentamidine was obtained from American Pharmaceutical Partners, Inc. (Los Angeles, CA).
TABLE 1.
Structures, in vitro activities, and toxicities of bisbenzamidine and bisbenzimidazolidine compounds
Numbers correspond to compound numbers in reference 12.
Number unique to this study.
In vivo studies.
Evaluations of anti-Pneumocystis activities were conducted with an immunosuppressed mouse model of pneumocystosis, using mice infected with P. murina; the techniques used have been described in our earlier reports (11, 40, 41, 43, 45). All procedures were performed under IACUC-approved protocols at the University of Cincinnati. The immunosuppressed state was induced by administration of 4 μg/ml dexamethasone (Butler, Columbus, OH) in the drinking water. When the infection reached a moderate intensity (after 6 to 7 weeks), the mice were randomly divided into treatment and control groups of 8 to 12 animals each. Compounds to be tested and pentamidine were dissolved in 2% ethanol and were administered by intraperitoneal injection on a mg/kg-of-body-weight basis once daily for 5 days/week, at 1, 5, and 10 mg/kg for most compounds and 20 and 40 mg/kg for selected compounds. The drugs were administered for 3 weeks, during which the mice remained on the immunosuppressive regimen. Control animals receiving steroids (C/S) received no treatment. The data presented represent a total of three separate experiments.
Efficacy was based on a reduction in organism burden, as determined by microscopic quantification. All specimens were examined on a blinded basis (11, 39, 43, 45). The mice were required to receive treatment for at least 7 days to be included in the data analysis. The right lung was homogenized and stained with cresyl echt violet, which selectively stains the cyst form of P. murina. The lower limit of detection by this enumeration method is 1.75 × 104 (log10 4.24)/lung. The cyst counts were log transformed, and the group outcomes were compared by the Kruskal-Wallis test, followed by Dunn's multiple comparison test, using INSTAT v.3 (GraphPad Software for Science, San Diego, CA). Survival curves were based on the 21-day treatment period and were compared using GraphPad Prism v.4. Significance was accepted when the P value was <0.05.
Anti-Pneumocystis activity in the mouse model was scored as follows: very marked activity, ≥500-fold reduction in mean cyst count; marked activity, 100- to 499-fold reduction; moderate activity, 10- to 99-fold reduction; slight activity, 5- to 9-fold reduction; and inactive, <5-fold reduction. In vivo activities of the compounds were compared to their in vitro activities in the ATP assay, which had the following categories: highly active, 50% inhibitory concentration (IC50) of <0.010 μg/ml; very marked activity, IC50 of 0.011 to 0.099 μg/ml; marked activity, IC50 of 0.10 to 0.99 μg/ml; moderate activity, IC50 of 1.00 to 9.99 μg/ml; slight activity, IC50 of 10.0 to 49.9 μg/ml; and inactive, IC50 of ≥50 μg/ml.
In vivo toxicities of test compounds.
Evaluation of the toxicities of test compounds was performed in three ways and followed previously described procedures (17, 20). In the first type of evaluation, the dexamethasone-immunosuppressed mice receiving the compounds were monitored for acute reactions to the compounds immediately after injection by direct observation for 10 to 15 min. The overall health and general well-being of the mice were observed daily throughout the 21-day course of treatment. Weights were taken weekly. A twofold loss of weight compared to C/S controls was considered a toxic response. The second method of evaluation relied on histopathological analysis of livers, lungs, and pancreases removed from selected treated mice at necropsy. Hematoxylin- and eosin-stained sections were graded subjectively according to a previously published scale, from 0, indicating no deleterious effects compared to C/S mice, to 3+, indicating severe cellular damage (20).
The third method was to determine the maximum tolerated doses (MTDs) of pentamidine and the most promising candidate, compound 100, in non-dexamethasone-treated mice (male C3H/HeN mice of 5 weeks of age and 21.1 to 26.4 g; Charles River, Hollister, CA). These studies were performed under an NIH contract held by Chuck Litterest. All procedures were performed under IACUC-approved protocols. A three-phase experimental approach was used. In phase I, the pilot study, single intraperitoneal injections of the compounds were administered to one male mouse per group, at dose levels from 20 mg/kg to 500 mg/kg. Mortality, body weight, and clinical observations were made on days 1 and 8 (the end of the study). Phases II and III determined the MTDs of compound 100 and pentamidine following single administration at dose levels from 5 to 40 mg/kg, using three male mice per group. Besides the end points listed for the pilot study, clinical pathology, gross necropsy, and histopathology were performed on mice on day 4.
Blood was collected from the retro-orbital sinuses of mice under isoflurane anesthesia. Blood for clinical pathology evaluation was sent to Quality Clinical Laboratories (Mountain View, CA) for analysis. Standard chemistries and hematological assessments were conducted. Gross necropsy was performed on animals sacrificed on day 4 in phases II and III and on all animals that were found dead or were sacrificed due to moribund conditions. External examination of all body orifices and surfaces and examination of all cranial, thoracic, and abdominal organs were performed and recorded. Organs were weighed, and the heart, kidney, liver, lung, spleen, thymus, small intestine, and mesenteric lymph nodes were retained and fixed in phosphate-buffered 10% formalin for histopathological examination.
A one-way analysis of variance was performed on body weight and clinical pathology data and organ weight data (LABCAT software; Innovative Programming Associates, Inc., Princeton, NJ). Null hypothesis rejection was set at P values of <0.05.
RESULTS
The selection criteria for the compounds evaluated in these studies were based on their activities in our in vitro drug screening system and their lack of or low toxicities in one or more mammalian cell lines (12). For each compound, Table 1 shows the structure in relation to the parent compound, the in vitro efficacy against P. carinii, expressed as the IC50 (the concentration of compound required to reduce the ATP content of treated cells by 50% versus that of untreated cells), the in vitro activity rank, and the IC50 for the A549 cell line, a human lung cell line (toxicity). Compounds exhibiting a range of in vitro activities, from highly active to inactive, were chosen for this study to evaluate the in vitro-in vivo correlation.
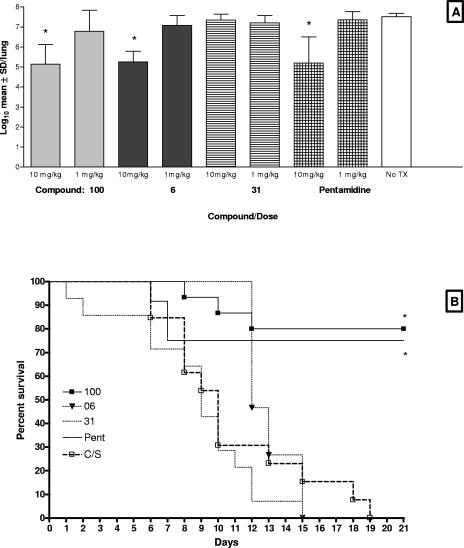
In the first study, the following four compounds were evaluated in a mouse model of Pneumocystis pneumonia: compound 100, a pentanediamide-linked bisbenzamidine, which showed the highest in vitro activity; compound 06, a piperazine-linked bisbenzamidine, with very marked in vitro activity; pentamidine, the prototype bisbenzamidine compound, with marked activity; and compound 31, a piperazine-linked bisbenzamidazolidine with no in vitro activity (Table 1). All agents were administered at 10 and 1 mg/kg. At 10 mg/kg, compounds 100 and 06 and pentamidine significantly reduced the organism burden relative to that for the untreated control group (P < 0.001 for all groups) (Fig. 1A). All three of these compounds were comparable in their efficacies in reducing the mean log10 P. murina cyst count, from 7.52/lung in the corticosteroid (C/S)-treated control group, by 234-, 204-, and 182-fold, respectively. As expected, compound 31 had no effect on the infection, and mice in these groups died at the same rate as the untreated mice (Fig. 1B). In contrast, both compound 100 and pentamidine significantly extended the survival of the mice in their respective groups versus those in the untreated control group (Fig. 1B). There was no significant difference in survival curves between the groups receiving 10 mg/kg pentamidine and 10 mg/kg compound 100. Toxicity was observed for the mice that were given 10 mg/kg compound 06, in spite of the reduction in organism burden. These mice experienced dramatic and rapid weight loss and tremors. They were sacrificed by day 15 after initiation of treatment, and necropsy revealed pale livers and kidneys. Compound 100 given in doses of 1 mg/kg and 0.1 mg/kg (data not shown) exhibited some anti-Pneumocystis activity, decreasing the cyst count 12- and 5-fold, respectively, yet these reductions did not reach statistical significance (Fig. 1A). Likewise, pentamidine and compound 06 were ineffective at 1 mg/kg, and no further experiments were conducted using that or lower concentrations.
FIG. 1.
In vivo efficacies of compounds 100, 06, and 31 and pentamidine at 1 and 10 mg/kg and survival curves for the 10-mg/kg dose. (A) P. murina cyst burdens determined by microscopic quantification were log transformed and expressed as means ± standard deviations (log10 cysts per lung) (y axis). All four compounds were evaluated at 10 and 1 mg/kg and compared to the untreated controls (no Tx) (x axis). Asterisks denote statistical significance, with P values of <0.05. (B) Survival curves were based on the 21-day treatment period with 10 mg/kg and were analyzed using GraphPad Prism v.4. Asterisks denote curves that are statistically different from the untreated control curve (P < 0.05).
In study 2, we extended the investigation to include several other bisbenzamidines, including compound 101, a hexanediamide-linked bisbenzamidine; compounds 01, 02, and 04, which are piperazine-linked bisbenzamidines; and compound 12, a piperazine-linked bisbenzimidazolidine (Table 2). Compound 101, which was highly active in vitro (IC50, 0.0008 μg/ml), significantly reduced the P. murina cyst counts, from 7.23 ± 0.20/lung to 5.31 ± 0.69/lung (83-fold) and 6.01 ± 0.66/lung (17-fold), when given at doses of 10 mg/kg and 5 mg/kg, respectively. Compounds 01, 02, and 04, which exhibited the highest activities in vitro, all significantly reduced the organism burdens in mice at 10 mg/kg (Table 2). Only compound 01 showed a significant reduction (15-fold) at the 5-mg/kg dose. In contrast, compound 12, a piperazine-linked bisbenzimidazolidine which showed reduced in vitro activity (IC50, 0.195 μg/ml), did not significantly decrease the infection at either dosage. Mice receiving a 10-mg/kg dose of compounds 101, 01, and 04 had significantly increased survival (Table 2), while mice receiving 5 mg/kg of compounds 101 and 01, but not 04, also experienced longer survival times. In contrast, mice receiving both doses of compound 02 experienced lethargy immediately after receiving the drug and had symptoms similar to those described for mice receiving compound 06. Although mice receiving the 10-mg/kg dose recovered, they then developed rapid weight loss and tremors, which resulted in all animals being sacrificed by day 8 of treatment. Mice administered the 5-mg/kg dose recovered from their lethargy, did not develop other signs of toxicity, and were not sacrificed prematurely. The survival curve for mice given the 5-mg/kg dose of compound 02 was no different from that for untreated mice, suggesting that the infection, not the compound, was the cause of death (Table 2). However, mice receiving the 10-mg/kg dose of compound 02 had statistically shorter survival times than did untreated control mice, with treated animals surviving an average of about 7 days versus 13 days for the untreated mice. Since the treated animals had significantly reduced organism burdens, the premature death was likely caused by the toxic effects of compound 02. Compound 102, a 1,3-phenylenediamide-linked bisbenzamidine with reduced in vitro activity (IC50, 0.578 μg/ml), was ineffective against P. murina when administered at doses of 5 and 10 mg/kg.
TABLE 2.
Study 2 activities of bisbenzamidines against P. carinii
| Compound | Dose (mg/kg) | No. of cysts (log10 [mean ± SD]) | Fold reduction (vs C/S) | Significance of cyst no. vs C/S value (P < indicated value)a | Significance vs C/S survival (P value)a |
|---|---|---|---|---|---|
| 01 | 10 | 5.33 ± 0.75 | 80 | 0.001 | 0.04 |
| 5 | 6.09 ± 0.82 | 14 | NS | 0.002 | |
| 02 | 10 | 5.63 ± 1.50 | 40 | 0.05 | 0.0002b |
| 5 | 6.80 ± 0.80 | 3 | NS | NS | |
| 04 | 10 | 5.56 ± 0.93 | 47 | 0.01 | 0.0002 |
| 5 | 7.00 ± 0.53 | 2 | NS | NS | |
| 12 | 10 | 6.39 ± 0.72 | 7 | NS | NS |
| 5 | 6.63 ± 0.20 | 4 | NS | NS | |
| 101 | 10 | 5.31 ± 0.69 | 83 | 0.001 | 0.0001 |
| 5 | 6.01 ± 0.66 | 17 | 0.05 | 0.001 | |
| 102 | 10 | 7.56 ± 0.23 | NS | NS | |
| 5 | 7.06 ± 0.45 | 2 | NS | NS |
NS, not significant.
Decreased survival versus untreated C/S controls due to toxicity.
In the third and final study (Table 3), compound 100, the pentanediamide-linked bisbenzamidine with the highest in vitro activity and no apparent toxicity at the doses tested, was compared to pentamidine at dosage levels of 40 and 20 mg/kg to assess whether toxicity would occur at these increased levels. Also included in the study were 5-mg/kg and 10-mg/kg doses of compound 100, compound 01, and pentamidine to assess the reproducibility of the previous results. The 40-mg/kg dose of pentamidine was highly toxic to mice. Within a day after the first injection, 10 of the 12 mice had died. The remaining two mice were sacrificed the following day. Early deaths indicating toxicity were also apparent for the mice receiving 40 mg/kg of compound 100. After the first injection, three mice were found dead the following day, and one other mouse died after the fourth day of treatment. The remaining seven mice received the minimum length of treatment to be included in the analysis (7 days); four completed the full 21-day regimen. The mean log10 P. murina cyst count for the seven surviving mice was dramatically reduced, from 7.52 ± 0.35 for the untreated control mice to 4.48 ± 0.62, for a >1,000-fold reduction (P < 0.001) (Table 3). There was no difference in the survival curves for the mice treated with 40 mg/kg of compound 100 and the untreated controls, which was most likely due to the toxic effects of the compound, which were most apparent during the early days of dosing. At 20 mg/kg, both compound 100 and pentamidine were highly effective in reducing the mean log10 P. murina cyst count, by 1,660-fold and 851-fold, respectively (P < 0.001) (Table 3). Likewise, a significant increase in survival was observed with both treatments versus that for untreated control mice.
TABLE 3.
Study 3 activities of bisbenzamidines against P. carinii
| Compound | Dose (mg/kg) | No. of cysts (log10 [mean ± SD]) | Fold reduction (vs C/S) | Significance of cyst no. vs C/S value (P < indicated value)a | Significance vs C/S survival (P value)a |
|---|---|---|---|---|---|
| 100 | 40 | 4.48 ± 0.62 | 1,096 | 0.001 | NS |
| 20 | 4.30 ± 0.20 | 1,660 | 0.001 | 0.0005 | |
| 10 | 5.26 ± 1.01 | 182 | 0.01 | NS | |
| 5 | 6.09 ± 0.95 | 27 | NS | 0.0016 | |
| 01 | 10 | 5.50 ± 1.35 | 104 | 0.01 | NS |
| 5 | 6.36 ± 1.28 | 15 | NS | NS | |
| Pentamidine | 40b | 0.0001c | |||
| 20 | 4.59 ± 0.51 | 851 | 0.001 | 0.01 | |
| 10 | 5.44 ± 1.02 | 93 | 0.01 | 0.016 | |
| 5 | 5.62 ± 1.17 | 79 | 0.05 | NS |
NS, not significant.
Not scored due to toxicity.
Survival of treated mice was significantly decreased versus that of C/S control mice.
As in the previous studies, all three compounds significantly reduced the organism burden when administered at 10 mg/kg (Table 3), and the magnitudes of the reductions were similar to the results in the first study (Fig. 1). However, pentamidine was the only compound causing a significant decrease in organism burden at 5 mg/kg and increased survival times versus those for untreated mice (P = 0.016) at 10 mg/kg (Table 3). Interestingly, compound 100 was the only compound to show a significant increase in mouse survival at 5 mg/kg (P = 0.0016), despite the lack of significant efficacy at this concentration (Table 3). Eleven of 12 mice receiving compound 100 at 5 mg/kg survived the entire 21-day regimen, compared to 8 of 12 mice in the pentamidine group, 6 of 12 mice treated with compound 01, and 4 of 12 mice in the C/S group.
Acute toxicity studies with escalating doses from 5 to 500 mg/kg were conducted with compound 100 and pentamidine. Toxicities associated with compound 100 at doses of 50 mg/kg and above included severe hypoactivity, dyspnea, and general hypothermia. Pentamidine was toxic at doses of 20 mg/kg and higher, and mice in these groups experienced moderately ruffled fur, squinting, hunched posture, slight to severe hypoactivity, dyspnea, and weight loss. Doses of 50, 100, and 500 mg/kg of compound 100 and doses of 100 and 500 mg/kg of pentamidine were lethal in these acute studies.
At doses below 50 mg/kg, mice treated with pentamidine showed ruffled fur, while no toxicity was observed for compound 100 at these doses. More animals exhibited ruffled fur when pentamidine was administered in 20% dimethyl sulfoxide than when it was administered in sterile saline, suggesting that dimethyl sulfoxide may potentiate the pentamidine-associated adverse effects.
None of the observed hematology or clinical chemistry values for either drug were considered to be of toxicologic significance. This was consistent with the lack of effects on organ weights and the absence of histopathologic findings for mice treated with dexamethasone and compound 100 or pentamidine. The MTD of compound 100 was estimated to be 40 mg/kg and that for pentamidine in these studies was approximately 50 mg/kg.
DISCUSSION
Although the bisbenzamidines have been available for many years, problems such as toxicity have resulted in only one compound, pentamidine, being licensed for clinical use (2, 4, 5, 26, 34). Yet since these agents are active against a variety of microorganisms and because much is known about their structure-activity relationships, investigators have remained interested in developing new drugs that overcome the limitations of their predecessors. In previous reports, we found promising antiprotozoal and anti-Pneumocystis activities of bisbenzamidines by using a 1,4-piperazinediyl skeleton as a rigid linker (12, 13, 28, 33, 37). We then synthesized a library of piperazine-linked bisbenzamidine derivatives and showed that many of these drugs were highly potent and had minimal toxicities in vitro (12). In addition, we recently discovered that bisbenzamidines linked with an alkanediamide linker were highly potent against rat P. carinii in vitro and displayed minimal toxicities in several human tumor cell lines (37).
The major difference between the compounds reported in the present study and compounds reported earlier by others (4, 14, 17, 20, 34-36) is the type of linker joining the two benzamidine groups. Previous investigators used a wide range of both flexible and restricted linkers, with 2,5-furan being the most promising. The linkers used herein (1,4-piperazinediyl, alkanediamide, and 1,3-phenylenediamide) are uniquely different from these linkers. We have demonstrated the importance of the nature (electronic and conformational effects) of the central linker in influencing the biological properties of this class of compounds (13, 28, 33, 37).
In the present study, we tested several of these compounds in our mouse model of pneumocystosis to determine if in vitro activity could be translated into in vivo activity. Of the piperazine-linked bisbenzamidines, compounds 01, 02, 04, and 06 exhibited highly active (IC50s of <0.01 mg/μl) or very marked (IC50s of <0.099 mg/μl) activity in vitro. In agreement with their in vitro activities, these agents exhibited marked (>100-fold reduction) or moderate (>10-fold reduction) anti-Pneumocystis activity when administered at a dose of 10 mg/kg in our immunosuppressed mouse model of pneumocystosis. However, notable toxicity was associated with compounds 02 and 06, which was not predicted at the in vitro level. The in vivo data suggest that piperazine-linked bisbenzamidines with straight alkyl chains of four or five carbons substituted at one of the amidinium nitrogens are linked with toxicity (compounds 02 and 06), whereas those compounds with branched alkyl chains of five carbons (compounds 01 and 04) or a bulky cyclic ring (compound 12) were not toxic at the tested doses. In vivo testing of additional analogs will be needed to confirm this structure-activity relationship. Compound 31, a piperazine-linked bisbenzimidazolidine, exhibited no anti-Pneumocystis activity in vitro and was also ineffective in vivo. Compound 12 was predicted to have at least modest efficacy in vivo because it achieved a reduced but marked activity in vitro. Although it reduced the P. murina cyst burden sevenfold, the effect was not statistically significant.
Of the alkane or phenylenediamide-linked bisbenzamidines, compounds 100 and 101 were highly active in the ATP assay, with little apparent toxicity (9). In vivo, compound 100 exhibited marked anti-Pneumocystis activity at 10 mg/kg and very marked activity at 20 mg/kg and 40 mg/kg. Compound 101 showed moderate activity at 5-mg/kg and 10-mg/kg doses. In contrast, compound 102, which had marked activity in vitro, was ineffective in vivo. The reasons for this discrepancy are unclear. None of these compounds were toxic to the treated mice, in agreement with the in vitro data. Compound 100 consistently provided robust therapeutic efficacy and was better tolerated at higher doses than pentamidine in the P. murina-infected mouse model. Future studies will include combinations with other compounds at reduced doses and in orally available preparations.
In light of the in vivo toxicity resulting from two of the piperazine-linked bisbenzamidines and the apparent inconsistency of in vivo activities for compounds showing reduced in vitro activities, it would seem prudent to modify our in vitro screening criteria to select compounds that have P. carinii IC50/A549 IC50 ratios of ≤0.00001 with only the highest anti-P. carinii activities in vitro.
The data obtained from our in vitro and in vivo studies establish a basis for future analyses of structure-activity relationships of the bisbenzamidines and their mechanism of action. Despite many years of use, how these drugs exert their antimicrobial effects remains controversial. Targets of interest have included topoisomerase I (14, 31), topoisomerase II (4), group I introns (27, 46), transporters (6), and mitochondria (6, 7, 12, 13). Using the described in vitro-in vivo approach, it should be possible to develop highly potent drugs with minimal toxicity to the host.
Acknowledgments
This work was supported by Public Health Service contract NO1 AI25467 from the National Institute of Allergy and Infectious Diseases (to M.T.C. and P.D.W.), by PHS grant R01 HL64570 (to P.D.W.), by the Medical Research Service, Department of Veterans Affairs (M.T.C. and P.D.W.), and by grant 2S06GM08008 from the National Institutes of General Medical Sciences (to T.L.H.).
REFERENCES
- 1.Armstrong, W., S. Meshnick, and P. Kazanjian. 2000. Pneumocystis carinii mutations associated with sulfa and sulfone prophylaxis failures in immunocompromised patients. Microbes Infect. 2:61-67. [DOI] [PubMed] [Google Scholar]
- 2.Assan, R., C. Perronne, D. Assan, L. Chotard, C. Mayaud, S. Matheron, and D. Zucman. 1995. Pentamidine-induced derangements of glucose homeostasis. Determinant roles of renal failure and drug accumulation. A study of 128 patients. Diabetes Care 18:47-55. [DOI] [PubMed] [Google Scholar]
- 3.Beard, C. B., P. Roux, G. Nevez, P. M. Hauser, J. A. Kovacs, T. R. Unnasch, and B. Lundgren. 2004. Strain typing methods and molecular epidemiology of Pneumocystis pneumonia. Emerg. Infect. Dis. 10:1729-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell, C. A., C. C. Dykstra, N. A. Naiman, M. Cory, T. A. Fairley, and R. R. Tidwell. 1993. Structure-activity studies of dicationically substituted bis-benzamidazoles against Giardia lamblia: correlation of antigiardial activity with DNA-binding affinity and giardial topoisomerase II inhibition. Antimicrob. Agents Chemother. 37:2668-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchard, P., I. Caubarriere, D. Ganeval, P. Even, and R. Assan. 1978. Pentamidine, hypoglycemia, diabetes mellitus. J. Annu. Diabetol. Hotel Dieu 1978:273-279. [PubMed] [Google Scholar]
- 6.Bray, P. G., M. P. Barrett, S. A. Ward, and H. P. de Koning. 2003. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 19:232-239. [DOI] [PubMed] [Google Scholar]
- 7.Chen, F., and M. T. Cushion. 1994. Use of an ATP bioluminescence assay to evaluate viability of Pneumocystis carinii from rats. J. Clin. Microbiol. 32:2791-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cushion, M. T., F. Chen, and N. Kloepfer. 1997. A cytotoxicity assay for evaluation of candidate anti-Pneumocystis carinii agents. Antimicrob. Agents Chemother. 41:379-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushion, M. T., S. P. Keely, and J. R. Stringer. 2005. Validation of the name Pneumocystis wakefieldiae. Mycologia 97:268. [PubMed] [Google Scholar]
- 10.Cushion, M. T., and J. R. Stringer. 2004. Molecular and phenotypic description of Pneumocystis wakefieldiae sp. nov., a new species in rats. Mycologia 96:429-438. [PubMed] [Google Scholar]
- 11.Cushion, M. T., and P. D. Walzer. 2005. Development of candidate anti-Pneumocystis drugs: in vitro and in vivo approaches, p. 641-694. In P. D. Walzer and M. T. Cushion (ed.), Pneumocystis pneumonia. Marcel-Dekker, New York, N.Y.
- 12.Cushion, M. T., P. D. Walzer, M. S. Collins, S. Rebholz, J. J. Vanden Eynde, A. Mayence, and T. L. Huang. 2004. Highly active anti-Pneumocystis carinii compounds in a library of novel piperazine-linked bisbenzamidines and related compounds. Antimicrob. Agents Chemother. 48:4209-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donkor, I. O., T. L. Huang, B. Tao, D. Rattendi, S. Lane, M. Vargas, B. Goldenberg, and C. J. Bacchi. 2003. Trypanocidal activity of conformationally restricted pentamidine congeners. J. Med. Chem. 46:1041-1048. [DOI] [PubMed] [Google Scholar]
- 14.Dykstra, C. C., and R. R. Tidwell. 1991. Inhibition of topoisomerases from Pneumocystis carinii by aromatic dicationic molecules. J. Protozool. 38:78S-81S. [PubMed] [Google Scholar]
- 15.Fisk, D. T., S. Meshnick, and P. H. Kazanjian. 2003. Pneumocystis carinii pneumonia in patients in the developing world who have acquired immunodeficiency syndrome. Clin. Infect. Dis. 36:70-78. [DOI] [PubMed] [Google Scholar]
- 16.Frenkel, J. K. 1976. Pneumocystis jiroveci n. sp. from man: morphology, physiology, and immunology in relation to pathology. Natl. Cancer Inst. Monogr. 43:13-30. [PubMed] [Google Scholar]
- 17.Hall, J. E., J. E. Kerrigan, K. Ramachandran, B. C. Bender, J. P. Stanko, S. K. Jones, D. A. Patrick, and R. R. Tidwell. 1998. Anti-Pneumocystis activities of aromatic diamidoxime prodrugs. Antimicrob. Agents Chemother. 42:666-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser, P. M., D. S. Blanc, J. Bille, and P. Francioli. 1998. Typing methods to approach Pneumocystis carinii genetic heterogeneity. FEMS Immunol. Med. Microbiol. 22:27-35. [DOI] [PubMed] [Google Scholar]
- 19.Huang, L., K. Crothers, C. Atzori, T. Benfield, R. Miller, M. Rabodonirina, and J. Helweg-Larsen. 2004. Dihydropteroate synthase gene mutations in Pneumocystis and sulfa resistance. Emerg. Infect. Dis. 10:1721-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, S. K., J. E. Hall, M. A. Allen, S. D. Morrison, K. A. Ohemeng, V. V. Reddy, J. D. Geratz, and R. R. Tidwell. 1990. Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob. Agents Chemother. 34:1026-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazanjian, P., W. Armstrong, P. A. Hossler, C. H. Lee, L. Huang, C. B. Beard, J. Carter, L. Crane, J. Duchin, W. Burman, J. Richardson, and S. R. Meshnick. 2001. Pneumocystis carinii cytochrome b mutations are associated with atovaquone exposure in patients with AIDS. J. Infect. Dis. 183:819-822. [DOI] [PubMed] [Google Scholar]
- 22.Kazanjian, P., A. B. Locke, P. A. Hossler, B. R. Lane, M. S. Bartlett, J. W. Smith, M. Cannon, and S. R. Meshnick. 1998. Pneumocystis carinii mutations associated with sulfa and sulfone prophylaxis failures in AIDS patients. AIDS 12:873-878. [DOI] [PubMed] [Google Scholar]
- 23.Kazanjian, P. H., D. Fisk, W. Armstrong, Q. Shulin, H. Liwei, Z. Ke, and S. Meshnick. 2004. Increase in prevalence of Pneumocystis carinii mutations in patients with AIDS and P. carinii pneumonia, in the United States and China. J. Infect. Dis. 189:1684-1687. [DOI] [PubMed] [Google Scholar]
- 24.Keely, S. P., J. M. Fischer, M. T. Cushion, and J. R. Stringer. 2004. Phylogenetic identification of Pneumocystis murina sp. nov., a new species in laboratory mice. Microbiology 150:1153-1165. [DOI] [PubMed] [Google Scholar]
- 25.Keely, S. P., J. M. Fischer, and J. R. Stringer. 2003. Evolution and speciation of Pneumocystis. J. Eukaryot. Microbiol. 50(Suppl.):624-626. [DOI] [PubMed] [Google Scholar]
- 26.Lachaal, M., and R. C. Venuto. 1989. Nephrotoxicity and hyperkalemia in patients with acquired immunodeficiency syndrome treated with pentamidine. Am. J. Med. 87:260-263. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Y., R. R. Tidwell, and M. J. Leibowitz. 1994. Inhibition of in vitro splicing of a group I intron of Pneumocystis carinii. J. Eukaryot. Microbiol. 41:31-38. [DOI] [PubMed] [Google Scholar]
- 28.Mayence, A., J. J. Vanden Eynde, F. M. Krogstad, D. J. Krogstad, M. T. Cushion, and T. L. Huang. 2004. Parallel solution-phase synthesis of conformationally restricted congeners of pentamidine and evaluation of their antiplasmodial activities. J. Med. Chem. 47:2700-2705. [DOI] [PubMed] [Google Scholar]
- 29.Mayence, A., J. J. Vanden Eynde, L. LeCour, Jr., L. A. Walker, B. L. Tekwani, and T. L. Huang. 2004. Piperazine-linked bisbenzamidines: a novel class of antileishmanial agents. Eur. J. Med. Chem. 39:547-553. [DOI] [PubMed] [Google Scholar]
- 30.Morris, A., J. D. Lundgren, H. Masur, P. D. Walzer, D. L. Hanson, T. Frederick, L. Huang, C. B. Beard, and J. E. Kaplan. 2004. Current epidemiology of Pneumocystis pneumonia. Emerg. Infect. Dis. 10:1713-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Queener, S. F. 1995. New drug developments for opportunistic infections in immunosuppressed patients: Pneumocystis carinii. J. Med. Chem. 38:4739-4759. [DOI] [PubMed] [Google Scholar]
- 32.Redhead, S. A., M. T. Cushion, J. K. Frenkel, and J. R. Stringer. 2006. Pneumocystis and Trypanosoma cruzi: nomenclature and typifications. J. Eukaryot. Microbiol. 53:2-11. [DOI] [PubMed] [Google Scholar]
- 33.Tao, B., T. L. Huang, Q. Zhang, L. Jackson, S. F. Queener, and I. O. Donkor. 1999. Synthesis and anti-Pneumocystis carinii activity of conformationally restricted analogues of pentamidine. Eur. J. Med. Chem. 34:531-538. [Google Scholar]
- 34.Tidwell, R. R., S. K. Jones, J. D. Geratz, K. A. Ohemeng, C. A. Bell, B. J. Berger, and J. E. Hall. 1990. Development of pentamidine analogues as new agents for the treatment of Pneumocystis carinii pneumonia. Ann. N. Y. Acad. Sci. 616:421-441. [DOI] [PubMed] [Google Scholar]
- 35.Tidwell, R. R., S. K. Jones, J. D. Geratz, K. A. Ohemeng, M. Cory, and J. E. Hall. 1990. Analogues of 1,5-bis(4-amidinophenoxy)pentane (pentamidine) in the treatment of experimental Pneumocystis carinii pneumonia. J. Med. Chem. 33:1252-1257. [DOI] [PubMed] [Google Scholar]
- 36.Tidwell, R. R., S. G. Kilgore, K. A. Ohemeng, J. D. Geratz, and J. E. Hall. 1989. Treatment of experimental Pneumocystis carinii pneumonia with analogs of pentamidine. J. Protozool. 36:74S-76S. [DOI] [PubMed] [Google Scholar]
- 37.Vanden Eynde, J. J., A. Mayence, T. L. Huang, M. S. Collins, S. Rebholz, P. D. Walzer, and M. T. Cushion. 2004. Novel bisbenzamidines as potential drug candidates for the treatment of Pneumocystis carinii pneumonia. Bioorg. Med. Chem. Lett. 14:4545-4548. [DOI] [PubMed] [Google Scholar]
- 38.Vanden Eynde, J. J., A. Mayence, L. LeCour, Jr., and T. L. Huang. 2003. Synthesis, antituberculosis activity, and DNA binding affinity of a highly diverse library of 1,4-diarylpiperazines. Med. Chem. Res. 12:401-414.
- 39.Walzer, P. D. 1994. Development of new anti-Pneumocystis carinii drugs: cumulative experience at a single institution, p. 511-544. In P. D. Walzer (ed.), Pneumocystis carinii pneumonia. Marcel-Dekker, Inc., New York, N.Y.
- 40.Walzer, P. D., and A. Ashbaugh. 2002. Use of terbinafine in mouse and rat models of Pneumocystis carinii pneumonia. Antimicrob. Agents Chemother. 46:514-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walzer, P. D., A. Ashbaugh, M. Collins, and M. T. Cushion. 2001. In vitro and in vivo effects of quinupristin-dalfopristin against Pneumocystis carinii. Antimicrob. Agents Chemother. 45:3234-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walzer, P. D., J. Foy, J. Runck, P. Steele, M. White, R. S. Klein, B. A. Otter, and R. J. Sundberg. 1994. Guanylhydrazones in therapy of Pneumocystis carinii pneumonia in immunosuppressed rats. Antimicrob. Agents Chemother. 38:2572-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walzer, P. D., J. Foy, P. Steele, and M. White. 1992. Treatment of experimental pneumocystosis: review of 7 years of experience and development of a new system for classifying antimicrobial drugs. Antimicrob. Agents Chemother. 36:1943-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walzer, P. D., C. K. Kim, J. Foy, M. J. Linke, and M. T. Cushion. 1988. Cationic antitrypanosomal and other antimicrobial agents in the therapy of experimental Pneumocystis carinii pneumonia. Antimicrob. Agents Chemother. 32:896-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walzer, P. D., J. Runck, P. Steele, M. White, M. J. Linke, and C. L. Sidman. 1997. Immunodeficient and immunosuppressed mice as models to test anti-Pneumocystis carinii drugs. Antimicrob. Agents Chemother. 41:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, Y., Z. Li, D. S. Pilch, and M. J. Leibowitz. 2002. Pentamidine inhibits catalytic activity group I intron Ca.LSU by altering RNA folding. Nucleic Acids Res. 30:2961-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]