Abstract
The clinical strain Escherichia coli TO799 was resistant to penicillin-clavulanate combinations and ceftazidime and was not reproducibly detected as an extended-spectrum β-lactamase (ESBL) according to the standards of the Clinical Laboratory Standards Institute (CLSI; formerly NCCLS) and the national guidelines of the French Society for Microbiology (Comité de l'Antibiogramme de la Société Française de Microbiologie). A novel β-lactamase, designated TEM-125, was responsible for this phenotype. TEM-125 harbors a complex association of mutations previously described in the ESBL TEM-12 and in the inhibitor-resistant β-lactamase TEM-39. TEM-125 is the first complex mutant TEM to present hydrolytic activity against ceftazidime (kcat, 3.7 s−1) together with a high level of resistance to clavulanate (50% inhibitory concentration, 13.6 μM). The discovery of such an ESBL, which is difficult to detect by the usual ESBL detection methods, confirms the emergence of a complex mutant TEM subgroup and highlights the need to evaluate detection methods so as to avoid possible therapeutic failures.
Among Enterobacteriaceae, the most prevalent mechanism of acquired resistance to β-lactams is the production of β-lactamases such as the penicillinases TEM-1 and SHV-1, which hydrolyze penicillins and narrow-spectrum cephalosporins. In order to thwart these β-lactamases, two types of β-lactams were developed: β-lactam antibiotics resistant to the hydrolysis, such as expanded-spectrum cephalosporins (ceftazidime), and inhibitors of TEM and SHV penicillinases (clavulanic acid and tazobactam). However, the intensive use of these molecules was quickly followed by an evolution of TEM- and SHV-type β-lactamases.
The first TEM-type extended-spectrum β-lactamases (ESBLs) were characterized in 1987 (28). They differ from the SHV and TEM penicillinases by a few amino acid substitutions which confer hydrolytic activity against expanded-spectrum cephalosporins. These ESBLs are susceptible to β-lactamase inhibitors. Clinical laboratories are urged by the CLSI (formerly NCCLS) (8) and the Comité de l'Antibiogramme de la Société Française de Microbiologie (CA-SFM) (9) to use the association of hydrolytic activity against expanded-spectrum cephalosporins and susceptibility to inhibitors as a specific means of detecting this type of enzyme.
As the use of expanded-spectrum cephalosporins led to the selection of ESBLs, the clinical use of penicillin-β-lactamase inhibitor combinations led from 1990 onwards to the selection of point mutants of TEM penicillinases resistant to inhibitors (3). However, the strains producing these enzymes, designated inhibitor-resistant TEMs (IRTs), are generally susceptible to cephalosporins (5).
A few enzymes that combine ESBL and IRT mutations have recently emerged among the TEM β-lactamases. These new enzymes, designated complex mutant TEMs (CMTs), have been identified in different species of Enterobacteriaceae such as Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Enterobacter aerogenes (10, 19, 22, 24, 27). Despite this combination of substitutions, none of them combines significant hydrolytic activity against expanded-spectrum cephalosporins and a high level of resistance to inhibitors.
We report here an E. coli strain that combined high levels of resistance to both ceftazidime and penicillin-clavulanic acid combinations. The strain produced a new CMT-type β-lactamase difficult to detect as an ESBL because of its high level of resistance to clavulanate.
MATERIALS AND METHODS
Bacterial isolates and plasmids.
The strains used in this study were E. coli TO799, E. coli CF0102 producing TEM-39 (12), E. coli CF334 producing TEM-12 (6), E. coli CF001 producing the penicillinase TEM-1 (12), and E. coli CF1271 overproducing an AmpC cephalosporinase used as a negative control for ESBL detection tests (Table 1). E. coli DH5α (Novagen, Darmstadt, Germany) and E. coli BL21(DE3) (Novagen) were used for cloning experiments (25) and E. coli C600 for mating-out assays. Plasmid pBK-CMV (Stratagene, Amsterdam, The Netherlands) was used for the initial cloning experiments and a modified pET9a plasmid (18) for the overexpression of the β-lactamase-encoding genes.
TABLE 1.
Clinical strains and plasmids used in the study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli clinical strains | ||
| TO799 | Clinical strain harboring natural pTO799 containing blaTEM-125 (Toulouse, France, 2001) | This study |
| CF334 | Clinical strain harboring natural plasmid containing blaTEM-12 | 6 |
| CF0102 | Clinical strain harboring natural plasmid pCF0051 containing blaTEM-39 | 12 |
| CF001 | Clinical strain harboring natural plasmid pCF001 containing blaTEM-1 | 12 |
| CF1271 | Clinical strain overproducing an AmpC cephalosporinase | This study |
| Recombinant plasmids | ||
| pBK-TEM-125 | Recombinant plasmid of pBK-CMV containing the gene blaTEM-125 | This study |
| pBK-TEM-12 | Recombinant plasmid of pBK-CMV containing the gene blaTEM-12 | This study |
| pBK-TEM-39 | Recombinant plasmid of pBK-CMV containing the gene blaTEM-39 | This study |
| pBK-TEM-1 | Recombinant plasmid of pBK-CMV containing the gene blaTEM-1 | This study |
| pET-TEM-125 | Recombinant plasmid of pET9a containing the gene blaTEM-125 | This study |
| pET-TEM-12 | Recombinant plasmid of pET9a containing the gene blaTEM-12 | This study |
| pET-TEM-39 | Recombinant plasmid of pET9a containing the gene blaTEM-39 | This study |
| pET-TEM-1 | Recombinant plasmid of pET9a containing the gene blaTEM-1 | This study |
Susceptibility to β-lactams.
Antibiotic-containing disks were used for antibiotic susceptibility testing by the disk diffusion assay (Sanofi Diagnostics Pasteur, Marnes la Coquette, France). MICs were determined by a microdilution method on Mueller-Hinton agar (Sanofi Diagnostics Pasteur, Marnes la Coquette, France) with an inoculum of 104 CFU per spot and were interpreted according to the CLSI guidelines (8). The antibiotics were provided as powders by GlaxoSmithKline, Wyeth Laboratories, Eli Lilly, Roussel-Uclaf, Bristol-Myers Squibb, and Merck Sharp and Dohme-Chibret.
Detection of ESBL production.
The double-disk diffusion test, also called the synergy test, was performed as recommended by the CA-SFM (9, 13). Antibiotic disks, containing ceftazidime (30 μg), cefotaxime (30 μg), or aztreonam (30 μg), were placed on a plate, 30 mm (center to center) from an amoxicilline-clavulanate (20-μg/10-μg) disk. After overnight incubation at 37°C, an extension of the edge of an antimicrobial inhibition zone toward the disk containing clavulanate indicated synergy. Modified synergy tests were also performed with a 20-mm center-to-center distance.
As recommended by the CLSI for ESBL confirmatory tests, the MICs of cefotaxime and ceftazidime alone and combined with 4 μg/ml clavulanate were determined by broth microdilution assay. A ≥3-fold concentration decrease in either antimicrobial in combination with clavulanate compared with the same antimicrobial tested alone confirms production of an ESBL (8). The CLSI disk diffusion confirmatory test was performed by comparing the inhibition zone diameters given by 30 μg cefotaxime versus 30 μg cefotaxime plus 10 μg clavulanate and 30 μg ceftazidime versus 30 μg ceftazidime plus 10 μg clavulanate. A ≥5-mm increase between the zone diameters of cephalosporin disks and their respective cephalosporin-clavulanate disks confirms ESBL production (8).
Isoelectric focusing.
Isoelectric focusing of β-lactamases was performed with polyacrylamide gels containing ampholines with a pH range of 3.5 to 10.0, as previously described (4), with TEM-39 (pI 5.2), TEM-12 (pI 5.25), TEM-1 (pI 5.4), and TEM-2 (pI 5.6) as standards.
Mating-out experiment.
Direct transfers of plasmids coding for resistance genes were performed by mating donor strains with in vitro-obtained rifampin-resistant mutants of E. coli C600 as recipient strain at 37°C on solid Mueller-Hinton medium (25). Transconjugants were selected on agar containing rifampin (300 μg/ml) and ceftazidime (0.5 μg/ml).
Cloning experiments.
Recombinant DNA manipulation and transformations were performed as described by Sambrook et al. (25). T4 DNA ligase and proofreading Taq polymerase were purchased from Appligène (Oncor, Illkirch, France). The TEM-encoding genes were amplified by PCR with two pairs of primers. The PCR products obtained with primers TEM-A (5′ TAAAATTCTTGAAGACG 3′) and TEM-B2 (5′ TCTGACAGTTACCAATGC 3′) were cloned into the SmaI (Roche Diagnostics, Meylan, France) restriction site of the pBK-CMV plasmid. The TEM-encoding genes were also amplified with the primers NdeI-TEM-A (5′ GGAATTCCATATGAGTATTCAACATTTCCG 3′) and NotI-TEM-B (5′ ATAGTTTAGCGGCCGCTTAATGCTTAATCAGTGAG 3′), which included restriction sites for the enzymes NdeI and NotI (Roche Diagnostics), respectively. The PCR products were digested by these two enzymes and were ligated into the corresponding restriction sites of a modified pET9a plasmid (Stratagene). The plasmids derived from pBK-CMV and pET9a (Table 1) were transformed into E. coli strains DH5α and BL21(DE3), respectively. E. coli transformants were selected on Mueller-Hinton agar supplemented with 30 μg kanamycin and 0.5 μg ceftazidime.
Sequencing of DNA amplified by PCR.
Direct sequencing was performed on PCR products which were obtained from the transconjugant E. coli C600 and the transformant E. coli DH5α. These PCR products were sequenced by the dideoxy chain termination method on both strands with an Applied Biosystems sequencer (ABI 377) (26). The nucleotide and deduced protein sequences were analyzed using software available at the website of the National Center of Biotechnology Information (http://www.ncbi.nlm.nih.gov). The ClustalW program (http://www.infobiogen.fr) was used for the alignment of amino acid sequences (30).
Overexpression and purification of β-lactamases.
TEM-producing E. coli BL21(DE3) clones were used to overproduce the TEM-type β-lactamases, as previously described (7). The strains were cultured in 2x YT broth (Qbiogene, Irvine, California) containing kanamycin (20 μg/ml) and 0.1 mM isopropyl-β-d-thiogalactopyranoside (Sigma Chemical Co., St. Louis, Mo.). Bacteria were disrupted by sonication. TEM purification was carried out as previously described (24) by ion-exchange chromatography with a Q Sepharose column (Amersham Pharmacia Biotech, Orsay, France) and gel filtration chromatography with a Superose 12 column (Amersham Pharmacia Biotech), using a fast protein liquid chromatography system. The total protein concentration was estimated by the Bio-Rad protein assay (Bio-Rad, Richmond, Calif.), with bovine serum albumin (Sigma Chemical Co.) used as a standard. The level of purity was estimated at >97% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4, 16).
Determination of β-lactamase kinetic parameters kcat and Km and IC50.
The Michaelis constant (Km) and catalytic activity (kcat) were determined with purified extracts using a computerized microacidimetric method (15). The 50% inhibitory concentrations (IC50s) were determined for clavulanic acid and tazobactam as previously described (4) with 100 μM benzylpenicillin as reporter substrate, 10 nM of enzyme, and 10 min of incubation.
Nucleotide sequence accession number.
The nucleotide sequence of the blaTEM-125 gene has been assigned the accession number AY628176 in the GenBank nucleotide sequence database.
RESULTS
Clinical strain.
E. coli TO799 was isolated from the urinary tract of a patient admitted to an intensive care unit at the teaching hospital of Toulouse, France. The antibiogram, which was performed by the disk diffusion assay, revealed a high level of resistance to amoxicillin and ticarcillin alone or in combination with clavulanic acid, piperacillin, and ceftazidime. The strain exhibited inhibition diameters within the susceptible range for the piperacillin-tazobactam combination, cephalothin, cefuroxime, cefoxitin, cefotaxime, ceftriaxone, aztreonam, and imipenem. It was also resistant to gentamicin, netilmycin, kanamycin, tobramycin, sulfonamides, nalidixic acid, ofloxacin, and ciprofloxacin.
ESBL detection.
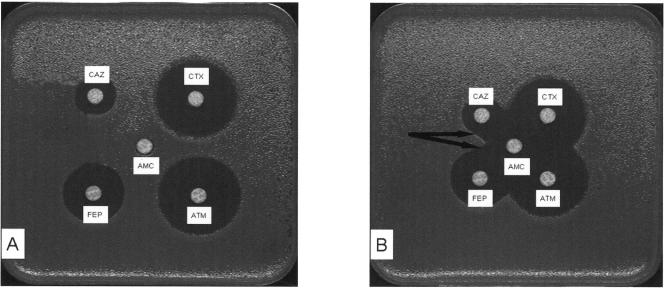
Both the double-disk diffusion test and the CLSI disk diffusion confirmatory test were negative for strain TO799 (Fig. 1). CLSI MIC testing was first negative and then positive at the third test. A modified double-disk synergy test, using a 20-mm center-to-center distance instead of 30 mm, was positive because the ceftazidime inhibition zone was enlarged (Fig. 1).
FIG. 1.
Comparison of the synergy tests performed with a 30-mm interdisk distance following the CA-SFM recommendations (9) and with a 20-mm interdisk distance. Interdisk distances were 30 mm (A) and 20 mm (B), respectively, for the TEM-125-producing E. coli. CAZ, ceftazidime; CTX, cefotaxime; ATM, aztreonam; FEP, cefepime; AMC, amoxicillin-clavulanate combination. The black arrows indicate synergy.
Isoelectric focusing, transfer, and cloning experiments.
E. coli TO799 produced two β-lactamases with pIs of 5.3 and 5.4. These pIs were compatible with TEM derivative enzymes. Mating-out experiments provided an E. coli transconjugant that was resistant to penicillins and cephalothin but susceptible to penicillin-clavulanic acid combinations and expanded-spectrum cephalosporins. This transconjugant produced only a β-lactamase with a pI of 5.4. Cloning experiments using TEM-A and TEM-B2 primers were performed to obtain an E. coli DH5α clone producing the other β-lactamase with a pI of 5.3. This clone, designated E. coli(pBK-TEM-125), produced only a β-lactamase with a pI of 5.3 and exhibited a resistance phenotype similar to that of E. coli TO799.
PCR experiments and DNA sequencing.
The blaTEM nucleic acid sequences revealed a TEM-1-encoding gene corresponding to the β-lactamase with a pI of 5.4 and a new blaTEM-type gene called blaTEM-125 corresponding to the β-lactamase with a pI of 5.3 (Table 2). In its promoter region, blaTEM-125 presented a deletion between positions 22 and 157 and a G162T substitution according to the Sutcliffe numbering system (29). In the coding region, this gene harbored five silent mutations (A346G, C436T, G469A, T682C, and G925A), and six other mutations resulted in five amino acid substitutions: T248C causing substitution Phe16Leu in the peptide signal region, A407C and G409A causing substitution Met69Leu, C692A causing substitution Arg164Ser, T695G causing substitution Trp165Arg, and A1022G causing substitution Asn276Asp. The novel resulting enzyme, designated TEM-125, combined the mutations of the inhibitor-resistant TEM TEM-39 (IRT-10) (Met69Leu, Trp165Arg, and Asn276Asp) and that of the ESBL TEM-12 (Arg164Ser).
TABLE 2.
Amino acid substitutions in TEM-125, TEM-12, and TEM-39 compared with TEM-1
β-Lactam MICs.
The clinical strain TO799 and the corresponding E. coli DH5α clone demonstrated high levels of resistance to amoxicillin, ticarcillin, and piperacillin (1,024 to >2,048 μg/ml) and were also resistant to ceftazidime (32 μg/ml) (Table 3). The MICs of aztreonam, cefepime, and cefpirome were in the susceptible range (0.5 to 1 μg/ml) but were higher than those observed for E. coli DH5α, which did not produce any TEM enzyme (<0.06 to 0.12 μg/ml, respectively). In contrast, the MICs of cephalothin (4 to 16 μg/ml), cefoxitin (4 to 8 μg/ml), cefuroxime (4 to 16 μg/ml), cefotaxime (0.06 to 0.12 μg/ml), and imipenem (0.25 μg/ml) were closely related to those observed for E. coli DH5α. Clavulanic acid did not restore complete susceptibility to amoxicillin, ticarcillin, and ceftazidime (MICs, >1,024, 1,024, and 8 μg/ml, respectively), whereas tazobactam restored susceptibility to piperacillin (MIC, 4 μg/ml).
TABLE 3.
MICs of β-lactam antibiotics for E. coli TO799 and recombinants E. coli DH5α(pBK-TEM-125), E. coli DH5α(pBK-TEM-12), E. coli DH5α(pBK-TEM-39), E. coli DH5α(pBK-TEM-1), and E. coli DH5α(pBK-CMV)
| β-Lactama | MIC (μg/ml) for E. coli strain:
|
|||||
|---|---|---|---|---|---|---|
| TO799 | DH5α(pBK-TEM-125) | DH5α(pBK-TEM-12) | DH5α(pBK-TEM-39) | DH5α(pBK-TEM-1) | DH5α(pBK-CMV) | |
| Amoxicillin | >2,048 | >2,048 | >2,048 | >2,048 | >2,048 | 4 |
| Amoxicillin-CLA | 1,024 | >1,024 | 64 | >1,024 | 16 | 4 |
| Ticarcillin | >2,048 | >2,048 | >2,048 | >2,048 | >2,048 | 2 |
| Ticarcillin-CLA | 256 | 1,024 | 32 | >1,024 | 32 | 2 |
| Piperacillin | 64 | 256 | 256 | >1,024 | 512 | 2 |
| Piperacillin-TZB | 4 | 4 | 2 | 256 | 2 | 2 |
| Cephalothin | 16 | 4 | 8 | 8 | 4 | 4 |
| Cefuroxime | 16 | 4 | 8 | 4 | 4 | 4 |
| Cefoxitin | 8 | 4 | 4 | 4 | 4 | 4 |
| Cefotaxime | 0.06 | 0.06 | 0.12 | 0.06 | 0.06 | 0.06 |
| Cefotaxime-CLA | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| Ceftazidime | 16 | 32 | 32 | 0.12 | 0.12 | 0.12 |
| Ceftazidime-CLA | 4 | 8 | 0.5 | 0.12 | 0.12 | 0.12 |
| Aztreonam | 0.5 | 0.5 | 4 | 0.06 | 0.12 | 0.12 |
| Aztreonam-CLA | 0.25 | 0.25 | 0.12 | 0.12 | 0.12 | 0.12 |
| Cefepime | 0.5 | 0.5 | 1 | 0.06 | 0.06 | <0.06 |
| Cefpirome | 1 | 1 | 0.5 | 0.06 | 0.06 | <0.06 |
| Imipenem | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
CLA, clavulanic acid at 2 μg/ml; TZB, tazobactam at 4 μg/ml.
We used the E. coli DH5α clones, which produce enzyme TEM-125 and its parental enzymes, ESBL TEM-12 and IRT TEM-39, and share the same genetic background, in order to compare the three related TEM-encoding genes. The resistance profile of the TEM-125-producing clone was identical to that of the TEM-12-producing clone for β-lactams alone, except for aztreonam (MICs, 0.5 versus 4 μg/ml). The MICs of penicillin-clavulanate combinations were significantly higher for the TEM-125-producing clone than for the TEM-12-producing clone (1,024 to >1,024 versus 32 to 64 μg/ml, respectively) but almost identical to those of the TEM-39-producing clone (≥1,024 μg/ml). However, the TEM-125-producing clone had a lower MIC for piperacillin-tazobactam than the TEM-39-producing clone (4 versus 256 μg/ml).
Enzymatic parameters.
The hydrolytic activities (kcat) of TEM-125 were 3- to 40-fold lower for penicillins than those of the penicillinase TEM-1 and the IRT-type enzyme TEM-39 (Table 4). These values were more closely related to those of ESBL TEM-12. Penicillin Km values, which increase when affinity decreases, were closely similar for TEM-125 and TEM-1 (21.2 to 89 versus 15 to 55 μM) but higher than those of TEM-12 (7 to 15 μM) and lower than those of TEM-39 (233 to 443 μM). Overall, the catalytic efficiency (kcat/Km) of TEM-125 against penicillins was 2-fold to 40-fold lower than that of TEM-1, TEM-12, and TEM-39.
TABLE 4.
Kinetic parameters of β-lactamases TEM-125, TEM-12, TEM-39, and TEM-1
| β-Lactam | TEM-125
|
TEM-12
|
TEM-39
|
TEM-1
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| kcat (s−1) | Km (μM) | kcat/Km (μM−1, s−1) | kcat (s−1) | Km (μM) | kcat/Km (μM−1, s−1) | kcat (s−1) | Km (μM) | kcat/Km (μM−1, s−1) | kcat (s−1) | Km (μM) | kcat/Km (μM−1, s−1) | |
| Benzylpenicillin | 136.5 ± 10 | 30 ± 4 | 4.6 | 80 ± 7 | 7 ± 0.5 | 11 | 3,570 ± 250 | 233 ± 30 | 15.3 | 1,500 ± 120 | 34 ± 4 | 44 |
| Amoxicillin | 68 ± 5 | 21.2 ± 1 | 3.2 | 60 ± 5 | 7.5 ± 1 | 8 | 2,500 ± 200 | 334 ± 35 | 7.5 | 1,125 ± 150 | 15 ± 2 | 77 |
| Ticarcillin | 9.6 ± 1 | 65 ± 4 | 0.1 | 19 ± 1 | 12 ± 1 | 1.6 | 260 ± 24 | 443 ± 35 | 0.6 | 135 ± 10 | 36 ± 4 | 4 |
| Piperacillin | 314 ± 32 | 89 ± 9 | 3.5 | 89 ± 8 | 15 ± 1 | 6 | 3,320 ± 350 | 253 ± 21 | 13.1 | 1,250 ± 70 | 55 ± 6 | 23 |
| Cephalothin | 4.6 ± 0.5 | 387 ± 30 | 0.012 | 46 ± 2 | 327 ± 33 | 0.02 | 7.1 ± 1 | 311 ± 25 | 0.02 | 165 ± 15 | 242 ± 12 | 0.7 |
| Cefuroxime | 0.6 ± 0.1 | 180 ± 12 | 0.003 | 1.6 ± 0.4 | 81 ± 9 | 0.1 | <0.1 | NDa | <0.1 | ND | ||
| Aztreonam | 0.7 ± 0.1 | 450 ± 42 | 0.0015 | 2 ± 0.4 | 247 ± 21 | 0.008 | <0.1 | ND | <0.1 | ND | ||
| Ceftazidime | 3.7 ± 0.5 | 318 ± 30 | 0.012 | 11.1 ± 1 | 254 ± 24 | 0.04 | <0.1 | ND | <0.1 | ND | ||
| Cefotaxime | 0.8 ± 0.2 | 254 ± 23 | 0.003 | 10.6 ± 1 | 320 ± 31 | 0.03 | <0.1 | ND | <0.1 | ND | ||
ND, not determined.
The hydrolytic activity of TEM-125 against cephalothin was lower than that of TEM-1, TEM-12, and TEM-39 (kcat values, 4.6 versus 7.1 to 165 s−1, respectively), whereas Km values of the four enzymes were closely related for this substrate (242 to 387 μM).
The hydrolytic activities of TEM-125 and ESBL TEM-12 for ceftazidime were closely related (kcat, 3.7 versus 11.1 s−1) as were Km for this substrate (318 versus 254 μM). The hydrolytic efficiency of TEM-125 was only threefold lower than that of TEM-12 for this substrate (kcat/Km, 0.012 and 0.04 μM−1 · s−1, respectively). kcat values for cefuroxime, aztreonam, and cefotaxime were 3-fold to 10-fold lower than those of TEM-12. Km values of TEM-125 and TEM-12 were closely related for these three substrates (180 versus 81, 450 versus 247, and 254 versus 320 μM for cefuroxime, aztreonam, and cefotaxime, respectively). As expected, TEM-1 and TEM-39 did not exhibit any significant activity against expanded-spectrum cephalosporins (<0.1 s−1).
Finally, the IC50 of clavulanic acid for TEM-125 was 600-fold higher than for TEM-12 and 160-fold higher than for TEM-1 but 6-fold lower than for TEM-39 (IC50s, 13.6, 0.02, 0.08, and 90 μM, respectively). The IC50 of tazobactam for TEM-125 was higher than for TEM-1 and TEM-12 but was almost 10-fold lower than for TEM-39 (IC50s, 0.27, 0.06, 0.13, and 2 μM, respectively).
DISCUSSION
The clinical strain E. coli TO799 produced a new TEM-type ESBL, designated TEM-125. This enzyme combined the amino acid substitutions of ESBL TEM-12 and of IRT TEM-39. It belongs therefore to the CMT subgroup of ESBLs. TEM-125 is the sixth member of this subgroup and the first CMT-type enzyme which combined a high level of resistance to clavulanate (IC50, 13.6 μM) and significant hydrolytic activity against ceftazidime (kcat, 3.6 s−1).
TEM-125 resistance to clavulanic acid was due to its combination of three substitutions: Met69Leu, Trp165Arg, and Asn276Asp. Substitutions at positions 69 and 276 are responsible for a low increase in resistance to inhibitors (5). The presence of an arginine instead of a tryptophan at position 165 moderately decreases the activity of clavulanic acid (21). Individually these IRT-type substitutions are responsible for only a slight enhancement of inhibitor resistance, but their combination confers a high level of resistance to clavulanic acid, as observed in TEM-39 (5). Among the previously described CMT-type enzymes, TEM-89 also exhibits a high level of resistance to clavulanate (IC50, 90 μM) (19). However, TEM-89 should not be considered an ESBL because of its Ser130Gly substitution, which greatly alters the activity against cephalosporins, as observed in the SHV-10 enzyme (23). The other ESBL-type CMTs previously reported were more susceptible to clavulanate than TEM-125 (IC50, 13.6 versus 0.15 to 1 μM).
TEM-125 harbors the Arg164Ser substitution, which confers improved catalytic efficiency against oxyimino β-lactams such as ceftazidime and aztreonam (14). The activity of TEM-125 against ceftazidime was low and closely related to that of the parental ESBL TEM-12 (kcat, 3.7 versus 11.1 s−1). The resistance of TEM-125-producing E. coli TO799 to ceftazidime is probably enhanced by the presence of the strong promoter, which has been described by Arlet et al. in blaTEM-20 (2). The presence of both the 22-157 deletion and the G162T substitution in the promoter zone of blaTEM-125 allows the combination of the −35 sequence of promoter Pa (17) with the −10 sequence of promoter P4 (17). These sequences were both close to the consensus sequences of the σ70 promoter region, which could explain the strength of this rare promoter (11). Overall, the TEM-125-encoding gene seems to constitute an efficient combination of mutations, conferring both extended-spectrum and inhibitor-resistant activities.
The use of expanded-spectrum cephalosporins led to therapeutic failure against ESBL-producing bacteria despite their exhibiting MICs in the susceptible range (20). The specific detection of ESBL is therefore a major concern for clinical microbiologists.
The CMT-type β-lactamase TEM-125 was difficult to identify as an ESBL because of its high level of resistance to clavulanate. The tests proposed by CLSI were not positive reproductively, and the CA-SFM double-disk synergy test was negative. However, a modified double-disk synergy test using an interdisk distance of 20 mm instead of 30 mm was slightly positive with ceftazidime and cefepime. This modification of the interdisk distance seems to make the double-disk synergy test more sensitive against CMT-producing strains.
By using the recommendations of CLSI or CA-SFM (8, 9), antibiotic options are reduced against the TEM-125-producing isolate reported in this study. Despite their in vitro activities, the clinical use of cephamycins should be limited because of the rapid selection of porin-resistant mutants (20). TEM-125 was not as susceptible to the inhibition activity of tazobactam as the common penicillinase TEM-1 or its parental ESBL TEM-12. The efficiency of the piperacillin-tazobactam combination is probably altered, despite the MIC remaining in the susceptible range. This alteration may be enhanced in vivo by the pharmacokinetic-pharmacodynamic parameters of piperacillin-tazobactam (20). Carbapenems seem therefore to be the best choices among β-lactams, especially for the treatment of serious infection.
In conclusion, we describe here an E. coli strain producing an ESBL that was highly resistant to clavulanic acid and which was difficult to detect when following the usual ESBL detection recommendations. This phenotype is due to the novel enzyme TEM-125, which harbored the substitutions of the ESBL TEM-12 and the IRT TEM-39. Our findings highlight the emergence of this new TEM-type ESBL subgroup and the need for continuous evaluation of ESBL detection methods.
Acknowledgments
We thank Marlene Jan, Rolande Perroux, and Dominique Rubio for technical assistance and Sophie Quevillon-Cheruel for providing us the modified pET9a plasmid.
This work was supported in part by a grant from Ministère de l′Education Nationale, de l'Enseignement Supérieur et de la Recherche, Paris, France, and a grant from the Centre Hospitalier Régional Universitaire de Clermont-Ferrand, France, and the Ministère de la Santé, de la Famille et des Personnes Handicapées, France (Projet Hospitalier de Recherche Clinique).
Footnotes
This work is dedicated to the memory of Catherine Chanal.
REFERENCES
- 1.Ambler, R. P., A. F. Coulson, J. M. Frere, J. M. Ghuysen, B. Joris, M. Forsman, R. C. Levesque, G. Tiraby, and S. G. Waley. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlet, G., S. Goussard, P. Courvalin, and A. Philippon. 1999. Sequences of the genes for the TEM-20, TEM-21, TEM-22, and TEM-29 extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 43:969-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belaaouaj, A., C. Lapoumeroulie, M. M. Canica, G. Vedel, P. Nevot, R. Krishnamoorthy, and G. Paul. 1994. Nucleotide sequences of the genes coding for the TEM-like β-lactamases IRT-1 and IRT-2 (formerly called TRI-1 and TRI-2). FEMS Microbiol. Lett. 120:75-80. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet, R., J. L. Sampaio, C. Chanal, D. Sirot, C. De Champs, J. L. Viallard, R. Labia, and J. Sirot. 2000. A novel class A extended-spectrum β-lactamase (BES-1) in Serratia marcescens isolated in Brazil. Antimicrob. Agents Chemother. 44:3061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaibi, E. B., D. Sirot, G. Paul, and R. Labia. 1999. Inhibitor-resistant TEM β-lactamases: phenotypic, genetic and biochemical characteristics. J. Antimicrob. Chemother. 43:447-458. [DOI] [PubMed] [Google Scholar]
- 6.Chanal, C., D. Sirot, H. Malaure, M. C. Poupart, and J. Sirot. 1994. Sequences of CAZ-3 and CTX-2 extended-spectrum β-lactamase genes. Antimicrob. Agents Chemother. 38:2452-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Y., J. Delmas, J. Sirot, B. Shoichet, and R. Bonnet. 2005. Atomic resolution structures of CTX-M β-lactamases: extended spectrum activities from increased mobility and decreased stability. J. Mol. Biol. 348:349-362. [DOI] [PubMed] [Google Scholar]
- 8.Clinical Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing, 15th informational supplement (M100-S15). Clinical Laboratory Standards Institute, Wayne, Pa.
- 9.Comité de l'Antibiogramme de la Société Française de Microbiologie. 2005. Communiqué 2005. Comité de l'Antibiogramme de la Société Française de Microbiologie, Paris, France.
- 10.Fiett, J., A. Palucha, B. Miaczynska, M. Stankiewicz, H. Przondo-Mordarska, W. Hryniewicz, and M. Gniadkowski. 2000. A novel complex mutant β-lactamase, TEM-68, identified in a Klebsiella pneumoniae isolate from an outbreak of extended-spectrum β-lactamase-producing klebsiellae. Antimicrob. Agents Chemother. 44:1499-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawley, D. K., and W. R. McClure. 1983. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 11:2237-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henquell, C., C. Chanal, D. Sirot, R. Labia, and J. Sirot. 1995. Molecular characterization of nine different types of mutants among 107 inhibitor-resistant TEM β-lactamases from clinical isolates of Escherichia coli. Antimicrob. Agents Chemother. 39:427-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 14.Knox, J. R. 1995. Extended-spectrum and inhibitor-resistant TEM-type β-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob. Agents Chemother. 39:2593-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labia, R., J. Andrillon, and F. Le Goffic. 1973. Computerized microacidimetric determination of β-lactamase Michaelis-Menten constants. FEBS Lett. 33:42-44. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Lartigue, M. F., V. Leflon-Guibout, L. Poirel, P. Nordmann, and M. H. Nicolas-Chanoine. 2002. Promoters P3, Pa/Pb, P4, and P5 upstream from blaTEM genes and their relationship to β-lactam resistance. Antimicrob. Agents Chemother. 46:4035-4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leulliot, N., S. Quevillon-Cheruel, I. Sorel, M. Graille, P. Meyer, D. Liger, K. Blondeau, J. Janin, and H. van Tilbeurgh. 2004. Crystal structure of yeast allantoicase reveals a repeated jelly roll motif. J. Biol. Chem. 279:23447-23452. [DOI] [PubMed] [Google Scholar]
- 19.Neuwirth, C., S. Madec, E. Siebor, A. Pechinot, J. M. Duez, M. Pruneaux, M. Fouchereau-Peron, A. Kazmierczak, and R. Labia. 2001. TEM-89 β-lactamase produced by a Proteus mirabilis clinical isolate: new complex mutant (CMT 3) with mutations in both TEM-59 (IRT-17) and TEM-3. Antimicrob. Agents Chemother. 45:3591-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petit, A. J., and R. Labia. 1991. Substitution du tryptophane-165 par une arginine dans la β-lactamase TEM-1. C. R. Acad. Sci. Paris Ser. II 312:993-997. [Google Scholar]
- 22.Poirel, L., H. Mammeri, and P. Nordmann. 2004. TEM-121, a novel complex mutant of TEM-type β-lactamase from Enterobacter aerogenes. Antimicrob. Agents Chemother. 48:4528-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prinarakis, E. E., V. Miriagou, E. Tzelepi, M. Gazouli, and L. S. Tzouvelekis. 1997. Emergence of an inhibitor-resistant β-lactamase (SHV-10) derived from an SHV-5 variant. Antimicrob. Agents Chemother. 41:838-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robin, F., J. Delmas, C. Chanal, D. Sirot, J. Sirot, and R. Bonnet. 2005. TEM-109 (CMT-5), a natural complex mutant of TEM-1 β-lactamase combining the amino acid substitutions of TEM-6 and TEM-33 (IRT-5). Antimicrob. Agents Chemother. 49:4443-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 1, p. 1659. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 26.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirot, D., C. Recule, E. B. Chaibi, L. Bret, J. Croize, C. Chanal-Claris, R. Labia, and J. Sirot. 1997. A complex mutant of TEM-1 β-lactamase with mutations encountered in both IRT-4 and extended-spectrum TEM-15, produced by an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 41:1322-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirot, D., J. Sirot, R. Labia, A. Morand, P. Courvalin, A. Darfeuille-Michaud, R. Perroux, and R. Cluzel. 1987. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel β-lactamase. J. Antimicrob. Chemother. 20:323-334. [DOI] [PubMed] [Google Scholar]
- 29.Sutcliffe, J. G. 1978. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc. Natl. Acad. Sci. USA 75:3737-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]