Abstract
The in vitro activities of linezolid were determined alone and in combination with vancomycin, ciprofloxacin, gentamicin, fusidic acid, or rifampin against five methicillin-susceptible Staphylococcus aureus (MSSA) and five methicillin-resistant S. aureus (MRSA) strains. Similar responses were obtained against MSSA and MRSA. When combined with fusidic acid, gentamicin, or rifampin, linezolid prevented selection of resistant mutants but showed no synergy. When linezolid was combined with vancomycin and ciprofloxacin, a slight antagonism was observed. While the combination with linezolid may reduce the emergence of mutants resistant to the associated drugs, the absence of synergy, especially in the case of vancomycin and ciprofloxacin, does not argue in favor of such combinations.
Linezolid is an oxazolidinone which belongs to a new class of synthetic antimicrobial agents chemically unrelated to any commercially available agent (4). Its precise mechanism of action is unknown, but it is presumed to interfere with an early step in the protein synthesis process (4, 15). Its spectrum includes medically important gram-positive bacteria such as methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA) (7, 8, 16). The increasing prevalence of MRSA associated with decreased susceptibility to vancomycin for some of these strains has become a major therapeutic challenge. Therefore, linezolid may be an alternative treatment for infections caused by multiresistant S. aureus (2). In previous studies, linezolid was shown to be a bacteriostatic agent (8, 9, 14). In this study, we investigated the potential bactericidal effect of linezolid in combination with antibiotics which are generally used with oxacillin or vancomycin against MSSA or MRSA: ciprofloxacin, a fluoroquinolone which targets the DNA topoisomerases; fusidic acid and gentamicin, which inhibit different steps of protein synthesis; and rifampin, which inhibits RNA polymerase.
All S. aureus strains (10 strains) except ATCC 25923 were clinical isolates obtained from Saint-Joseph Hospital (Paris, France). Five strains were MSSA, and five were MRSA (see Table 1). The following antibiotics were provided by the respective manufacturers: linezolid, Pharmacia & Upjohn Co. (Kalamazoo, Mich.); vancomycin, Eli Lilly & Co. (Indianapolis, Ind.); gentamicin, fusidic acid, and rifampin, Sigma (Saint Quentin, France). The MICs were determined on Muller-Hinton agar plates (Bio-Rad, Marne la Coquette, France) by a standard method (11) with a Steers-type replicator device that delivered ca. 104 bacteria per spot. MICs were read after 18 h at 37°C. The standard time-kill method (3) was used to study the combined effect of linezolid and the different antibiotics tested. Muller-Hinton broth (MHB) cultures grown overnight were diluted to 1/100 in fresh MHB. After 1 h of incubation at 37°C, to yield an initial cell density of about 2 × 106 CFU/ml, antibiotics were added at concentrations equal to four- and eightfold their MICs for the different strains tested (see Table 1). Plating and CFU counts from different dilutions were done in duplicate at 0, 6, and 24 or 48 h using a Spiral plater and a CASBA 4 system (Interscience, Saint Nom, France). Synergy or antagonism was defined as an increase or decrease of at least 100-fold compared to the effect of the single most active agent at 24 or 48 h.
TABLE 1.
In vitro activities of antimicrobial agents against S. aureus strains
| Strain | Oxa- cillina | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| Line- zolid | Cipro- floxacin | Rifam- pin | Genta- micin | Vanco- mycin | Fusidic acid | ||
| ATCC 25923 | S | 1 | 0.5 | 0.015 | 0.12 | 1 | 0.12 |
| 8258 | S | 1 | 0.5 | 0.007 | 2b | 1 | 0.06 |
| 8249 | S | 1 | 0.5 | 0.007 | 0.12 | 1 | 0.06 |
| 8251 | S | 1 | 0.5 | 0.015 | 0.12 | 1 | 0.12 |
| 10389 | S | 1 | 0.5 | 0.015 | 0.25 | 1 | 0.12 |
| 10413 | R | 1 | >8b | 0.015 | 0.25 | 1 | 0.12 |
| 10774 | R | 1 | 0.5 | 0.015 | 0.12 | 1 | 4b |
| 10467 | R | 1 | >8b | 0.015 | 0.25 | 1 | 0.12 |
| 10391 | R | 1 | 0.5 | 0.015 | 0.25 | 1 | >4b |
| 10397 | R | 1 | 1 | 0.015 | >4b | 1 | 0.12 |
The strains were susceptible (S) or resistant (R) to oxacillin using NCCLS breakpoints.
This antibiotic was not tested in combination with linezolid in this strain.
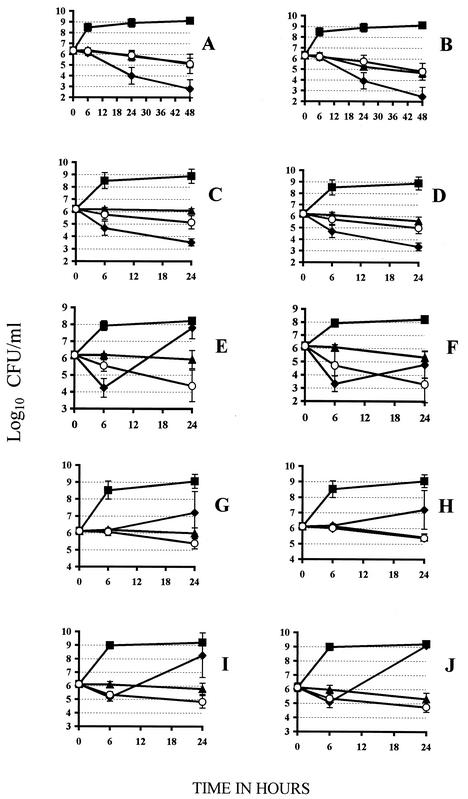
The MICs of the different antibiotics for the 10 strains studied are presented in Table 1. For all 10 strains studied, the linezolid MIC was 1 μg/ml, and at least eight strains were susceptible to the other antibiotics. In time-kill experiments, only susceptible strains were studied for each antimicrobial agent. Preliminary experiments using 2- to 32-fold the MIC of linezolid showed almost no difference (Fig. 1 and data not shown) in viable counts which never decreased more than 1 log10 unit at 24 h. This was in agreement with the previously reported bacteriostatic effect of linezolid on S. aureus (8, 9, 14). Linezolid was tested either alone or in combination at four- and eightfold its MIC. Vancomycin, ciprofloxacin, fusidic acid, gentamicin, and rifampin were also tested at four- and eightfold their respective MICs. Each point in the time-kill curves displayed in Fig. 1 is the mean and standard deviation for all time points obtained for the different S. aureus strains tested. Since there were no real differences in the overall effects of the different compounds tested against MSSA and MRSA strains, the results are presented together.
FIG. 1.
Time-kill curves of S. aureus strains exposed to antimicrobial agents. ▴, linezolid alone; ○, linezolid combined with a second antimicrobial agent (vancomycin, ciprofloxacin, gentamicin, fusidic acid, or rifampin); ⧫, second agent alone; ▪, control (no antimicrobial agent added). (A) Vancomycin (four times the MIC) and linezolid (four times the MIC), (B) vancomycin (eight times the MIC) and linezolid (eight times the MIC), (C) ciprofloxacin (four times the MIC) and linezolid (four times the MIC), (D) ciprofloxacin (eight times the MIC) and linezolid (eight times the MIC), (E) gentamicin (four times the MIC) and linezolid (four times the MIC), (F) gentamicin (eight times the MIC) and linezolid (eight times the MIC), (G) fusidic acid (four times the MIC) and linezolid (four times the MIC), (H) fusidic acid (eight times the MIC) and linezolid (eight times the MIC), (I) rifampin (four times the MIC) and linezolid (four times the MIC), and (J) rifampin (eight times the MIC) and linezolid (eight times the MIC). (A, B, I, and J) Five MSSA strains and five MRSA strains tested, (C, D, G, and H) five MSSA strains and three MRSA strains tested, and (E and F) four MSSA strains and four MRSA strains tested.
When tested alone, vancomycin at 24 and 48 h (Fig. 1A and B) and ciprofloxacin at 24 h (Fig. 1C and D) showed the most significant bactericidal effect with 2 to 4 log10 unit decreases in viable counts. In the presence of four- or eightfold the MIC of linezolid, a trend for antagonism was observed, with a reduced bactericidal effect between 1.6 log10 CFU for ciprofloxacin to 2.3 log10 CFU for vancomycin. When gentamicin (Fig. 1E and F), fusidic acid (Fig. 1G and H), and rifampin (Fig. 1I and J) were tested alone at four- and eightfold the MIC, a regrowth was observed after 24 h for 7 of 8, 5 of 8, and 9 of 10 strains, respectively. In all cases, it was explained by the selection of resistant mutants (data not shown). In the presence of linezolid at 6 h, there was no increase in bactericidal effect compared to that of fusidic acid or rifampin alone and even a slight antagonism (1.5 log10 CFU) with gentamicin. At 24 h, the presence of linezolid in the combination prevented the emergence of mutants.
Very few studies have reported the effects of combinations of linezolid with other antimicrobial agents. In one study (10), linezolid was tested at one-quarter its MIC with vancomycin or rifampin and the combination had no synergistic or antagonistic effect. In another study (6), linezolid reduced the bactericidal effect of fosfomycin against a MRSA strain by about 1.5 log10 units. In our study, the different combinations used at four or eight times their MICs against five MSSA and five MRSA strains showed no synergistic or antagonistic effect with fusidic acid, rifampin, and gentamicin, and the presence of linezolid, as expected for a compound which induces a low frequency of mutation (13), prevented the selection of mutants resistant to these associated compounds. For the most bactericidal antibiotics, vancomycin and ciprofloxacin, and in contrast to the results of a previous study with vancomycin (10) done under different conditions, a slight antagonism was found. Experimental models using linezolid have shown contradictory results. In a rat model of experimental MSSA osteomyelitis, linezolid was ineffective (12) but showed some effect in an MRSA rabbit endocarditis model (1). Compared to vancomycin, linezolid was less active in a murine soft tissue infection model against MSSA, but its level of activity in a systemic infection model against MRSA was similar to that of vancomycin (5). In the latter model, the 50% effective dose of gentamicin remained unchanged when it was combined with linezolid. However, when combined with linezolid, the 50% effective dose of rifampin increased 10-fold, but the combination was significantly more active than linezolid alone.
Linezolid is a compound which may be useful in the treatment of staphylococcal infections and particularly those caused by MRSA. From our in vitro results, one would not expect a synergistic effect of linezolid with vancomycin, ciprofloxacin, gentamicin, fusidic acid, or rifampin, but at least linezolid could prevent the emergence of mutants resistant to very efficient antistaphylococcal compounds such as rifampin and fusidic acid. Animal models will be required to discern if the slight antagonism observed with vancomycin and ciprofloxacin exists in vivo.
Acknowledgments
This work was supported by a grant from Pharmacia & Upjohn Co.
REFERENCES
- 1.Dailey, C., C. L. Dileto-Fang, L. V. Buchanan, M. P. Oramas-Shirey, D. H. Batts, C. W. Ford, and J. K. Gibson. 2001. Efficacy of linezolid in treatment of experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly, J. S., G. M. Eliopoulos, E. Reiszeiner, and R. C. Moellering, Jr. 1988. Mechanism of action and in vitro studies of DuP 105 and DuP 721, new oxazolidinone antibacterials. J. Antimicrob. Chemother. 21:721-730. [DOI] [PubMed] [Google Scholar]
- 3.Eliopoulos, G. M., and C. Moellering, Jr. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. Williams & Wilkins, Baltimore, Md.
- 4.Eustice, D. C., P. A. Feldman, I. Zajac, and A. M. Slee. 1988. Mechanism of action of DuP 721: inhibition of an early event during initiation of protein synthesis. Antimicrob. Agents Chemother. 32:1218-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford, C. W., J. C. Hamel, D. M. Wilson, J. K. Moerman, D. Staper, R. J. Yancey, Jr., D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vivo activities of U-100592 and U-100766, novel oxazolidinone antimicrobial agents, against experimental bacterial infections. Antimicrob. Agents Chemother. 40:1508-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grif, K., M. P. Dierich, K. Pfaller, P. A. Miglioli, and F. Allerberger. 2001. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. Antimicrob. Agents Chemother. 48:209-217. [DOI] [PubMed] [Google Scholar]
- 7.Jones, R. N., D. M. Johnson, and M. E. Erwin. 1996. In vitro antimicrobial activities and spectra of U-100592 and U-100766, two novel fluorinated oxazolidinones. Antimicrob. Agents Chemother. 40:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen, J. H., M. L. McElmeel, and C. W. Trippy. 1997. In vitro activities of the oxazolidinone antibiotics U-100592 and U-100766 against Staphylococcus aureus and coagulase-negative Staphylococcus species. Antimicrob. Agents Chemother. 41:465-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaatz, G. W., and S. M. Seo. 1996. In vitro activities of oxazolidinone compounds U100592 and U100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 40:799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulazimoglu, L., S. D. Drenning, and V. L. Yu. 1996. In vitro activities of two novel oxazolidinones (U100592 and U100766), a new fluoroquinolone (trovafloxacin), and dalfopristin-quinupristin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 40:2428-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. NCCLS approved standard. NCCLS document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Patel, R., K. E. Piper, M. S. Rouse, and J. M. Steckelberg. 2000. Linezolid therapy of Staphylococcus aureus experimental osteomyelitis. Antimicrob. Agents Chemother. 44:3438-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prystowsky, J., F. Siddiqui, J. Chosay, D. L. Shinabarger, J. Millichap, L. R. Peterson, and G. A. Noskin. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 45:2154-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rybak, M. J., D. M. Cappeletty, T. Moldovan, J. R. Aeschlimann, and G. W. Kaatz. 1998. Comparative in vitro activities and postantibiotic effects of the oxazolidinone compounds eperezolid (PNU-100592) and linezolid (PNU-100766) versus vancomycin against Staphylococcus aureus, coagulase-negative staphylococci, Enterococcus faecalis, and Enterococcus faecium. Antimicrob. Agents Chemother. 42:721-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinabarger, D. L., K. R. Marotti, R. W. Murray, A. H. Lin, E. P. Melchior, S. M. Swaney, D. S. Dunyak, W. F. Demyan, and J. M. Buysse. 1997. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob. Agents Chemother. 41:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zurenko, G. E., B. H. Yagi, R. D. Schaadt, J. W. Allison, J. O. Kilburn, S. E. Glickman, D. K. Hutchinson, M. R. Barbachyn, and S. J. Brickner. 1996. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 40:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]