Abstract
To study the antimalarial action of chloroquine, normal mouse erythrocytes were used as surrogates for erythrocytoid bodies. These bodies form in the endosomes of intraerythrocytic malaria parasites as they feed on their host and consist of erythrocyte cytoplasm enclosed in a vestige of the erythrocyte membrane. In suspensions of normal erythrocytes or lysates (equivalent to 5 μl of erythrocytes per ml in each case), hemoglobin underwent denaturation when it was incubated at 38°C in 150 mM sodium acetate (pH 5). It is reasonable to assume that the same phenomenon occurs in acidic endosomes. Addition of 100 μM chloroquine to the incubation mixture caused the rate of hemoglobin denaturation to double to 40 nanomoles per hour per ml of packed erythrocytes. This effect required the presence of erythrocyte stroma and was inhibited by reducing the temperature to 24°C or increasing the pH to 6. We propose that the primary antimalarial action of chloroquine is to bind to ferriprotoporphyrin IX (FP) and remove it from oxidized hemoglobin, thus producing toxic FP-chloroquine complexes and an excess of denatured globin. Furthermore, we suggest that these substances inhibit endosomal maturation and thereby cause hemoglobin accumulation in immature endosomes and masking of the lipids needed for FP dimerization. The term “masking” is used to signify that unsaturated lipids are present in parasitized erythrocytes but are specifically unavailable to promote FP dimerization.
Chloroquine kills malaria parasites by interfering with ferriprotoporphyrin IX (FP) detoxification and causing it to accumulate to lethal levels (10). FP is produced when the parasites denature or degrade hemoglobin. It is detoxified by dimerization to β-hematin, which is a process that is promoted by unsaturated lipids (10). Chloroquine treatment interferes with detoxification by causing unsaturated lipids in parasitized erythrocytes to be unavailable for the promotion of FP dimerization (13). We have previously labeled this phenomenon “masking” and attributed it to an interaction of an unidentified substance with membrane lipids (13). It is probable that the masking substance is an intrinsic membrane protein and that the parasite hydrolyzes it to unmask the lipids needed to promote FP dimerization. Normal erythrocyte membranes provide an example of lipid masking. They contain lipids capable of promoting FP dimerization; but they do not dimerize FP because the lipids are unavailable to perform this function (12), although they may be available to serve other functions. When the lipids are separated from the membrane proteins, however, they promote FP dimerization (12).
Intraerythrocytic malaria parasites live in vacuoles derived from erythrocyte membranes (1, 6, 17, 19, 24), and the lipids used to promote FP dimerization are probably obtained from these membranes (14, 20) during the feeding process. Malaria parasites feed on the erythrocyte cytoplasm from within their vacuoles. In the process, they also ingest vacuolar membrane. Initially, this membrane lines the inside of young endosomes (22) and creates erythrocytoid bodies, which we define as inclusions of erythrocyte cytoplasm bounded by vestiges of the erythrocyte membrane. As the endosomes mature, they become acidic (7, 15, 26) and the extra membrane disappears (3, 22). It is well known that chloroquine affects this part of the feeding process by impairing endosomal maturation and causing hemoglobin-laden vesicles with double membranes to accumulate (8, 9, 11, 18, 21, 25, 27).
We now report that chloroquine accelerates hemoglobin denaturation in erythrocytes under acidic conditions. Based on this new observation and the similarity of erythrocytes and erythrocytoid bodies, we propose that the primary antimalarial action of chloroquine is to bind to and remove FP from oxidized hemoglobin, producing toxic FP-chloroquine complexes and an excess of denatured globin. Impaired endosomal maturation and masking of unsaturated lipids are probably caused by the toxic FP-chloroquine complexes and/or the excess of denatured globin.
MATERIALS AND METHODS
Young male Swiss mice weighing approximately 25 g each were purchased from Harlan-Teklad (Madison, WI), provided a commercial diet and water ad libitum, and handled in accordance with relevant federal guidelines and Saint Louis University policies. To obtain normal erythrocytes, the mice were exsanguinated while they were under ether anesthesia. Then they were killed by cervical dislocation while they were deeply anesthetized. The blood was anticoagulated with heparin (approximately 1 mg per ml) and centrifuged at 860 × g at room temperature for 5 min to sediment the erythrocytes. For some experiments, the blood was passed through a cellulose fiber column to remove leukocytes prior to centrifugation, but this extra step made no difference in the outcome of the experiments and was not done routinely. Whether the erythrocytes were depleted of leukocytes or not, the erythrocytes were washed twice by resuspension in approximately 10 volumes of a standard medium and centrifugation, after which they were resuspended to a hematocrit of approximately 25% in the standard medium. This medium contains 68 mM sodium chloride, 4.8 mM potassium chloride, and 1.2 mM magnesium sulfate and is buffered to pH 7.4 with 50 mM sodium phosphate. To permit the use of packed erythrocyte volume as a reference base, the actual hematocrits of the erythrocyte suspensions were measured by using a microhematocrit centrifuge.
Suspensions of intact erythrocytes, lysates prepared from these suspensions, and the erythrocyte cytoplasm and stroma prepared from the lysates were used in the present experiments. The lysates were prepared by freezing suspensions of intact erythrocytes in liquid nitrogen and thawing. Commonly, the frozen suspensions were stored at −70°C for several days before they were thawed for an experiment. The erythrocyte cytoplasm and stroma were prepared by centrifuging freshly thawed lysates at 100,000 × g for 1 h at 4°C. After the soluble cytoplasm was removed, the insoluble stroma was reconstituted to the original volume of lysate in 150 mM sodium acetate (pH 5), frozen in liquid nitrogen, and stored at −70°C until it was thawed for study. The cytoplasm was also frozen in liquid nitrogen and was stored at −70°C for future use. The length of storage at −70°C did not affect the outcome of experiments with any of the frozen preparations.
Aliquots of the various preparations were incubated under the conditions described in the legends to the figures to evaluate the effect of chloroquine. At the end of the incubation, the tubes were centrifuged at 3,400 × g for 5 min at room temperature to sediment the denatured hemoglobin, the precipitated FP, and other debris. Then the supernatants were removed and the amount of FP in the pellets was measured.
To measure FP, pellets derived from 50 μl of packed erythrocytes were mixed with 1 ml of 150 mM sodium acetate (pH 5), frozen in liquid nitrogen, thawed at room temperature, diluted to 10 ml with 150 mM sodium acetate (pH 5), mixed, and centrifuged at 3,400 × g for 5 min at room temperature to recover the pellets. These pellets were washed three more times by mixing them with 10 ml of 150 mM sodium acetate (pH 5) and centrifugation. This procedure was performed to remove undenatured hemoglobin and other soluble proteins that might be trapped in the pellets.
The washed pellets were suspended in 1 ml of 2.5% sodium dodecyl sulfate in the standard medium; sodium hydroxide was added to achieve a predetermined concentration of 0.25 mM or less, depending on the concentration needed to solubilize the FP; and the mixture was allowed to stand at room temperature for an hour. The absorbance of this solution at 700 nm was then subtracted from the absorbance at 401 nm to correct for turbidity, and the concentration of FP was calculated from the difference by using an extinction coefficient of 90.8 mM−1 for the absorbance of FP at 401 nm (2). The FP in these thoroughly washed pellets was considered to represent either the FP still present in denatured hemoglobin or the FP released from denatured hemoglobin. Therefore, four molecules of FP were considered to represent one molecule of denatured hemoglobin.
To determine the background value for denatured hemoglobin, lysate derived from 50 μl of packed erythrocytes was added to 10 ml of ice-cold 150 mM sodium acetate (pH 5) and immediately centrifuged at 3,400 × g for 5 min at room temperature. Then, the supernatant was removed and the pellet was mixed with 1 ml of 150 mM sodium acetate (pH 5), frozen in liquid nitrogen, thawed, washed, and used for FP measurement, all as described above. Background values were measured for each set of experiments and were used to make appropriate corrections to the data.
RESULTS
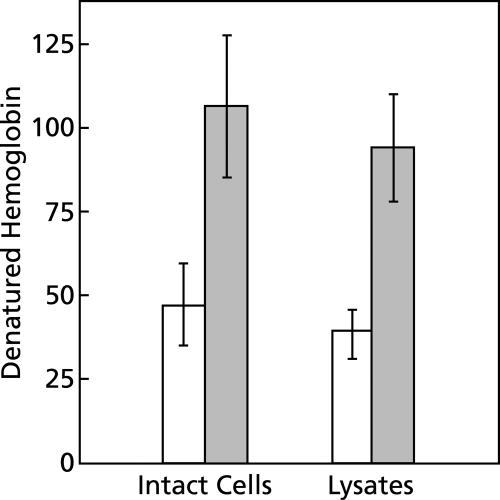
The data presented in Fig. 1 indicate that significant amounts of hemoglobin are denatured when preparations of normal erythrocytes are incubated under room air at body temperature and pH 5. Under these conditions, the erythrocytes lyse within 5 min, the lysates quickly turn brown, and a precipitate forms, as would be expected with the oxidative denaturation of hemoglobin and the release of FP. FP rapidly precipitates out of solution at pH 5.
FIG. 1.
Effect of chloroquine on hemoglobin denaturation. Suspensions of intact erythrocytes or lysates, each prepared from 50 μl of packed erythrocytes, were diluted to 10 ml with 150 mM sodium acetate (pH 5) and incubated for 1 h at 38°C under room air in the presence or the absence of chloroquine. Shaded bars indicate the presence of 500 μM chloroquine. Means ± standard deviations for five experiments are shown. For each preparation, the effect of chloroquine was significant (P < 0.001, t test). The differences between intact erythrocytes and lysates were not statistically significant. The data are expressed as the nanomoles of denatured hemoglobin minus the background per ml of packed erythrocytes per hour. The background value was 24 ± 13 nanomoles of denatured hemoglobin per ml of packed erythrocytes for this set of experiments.
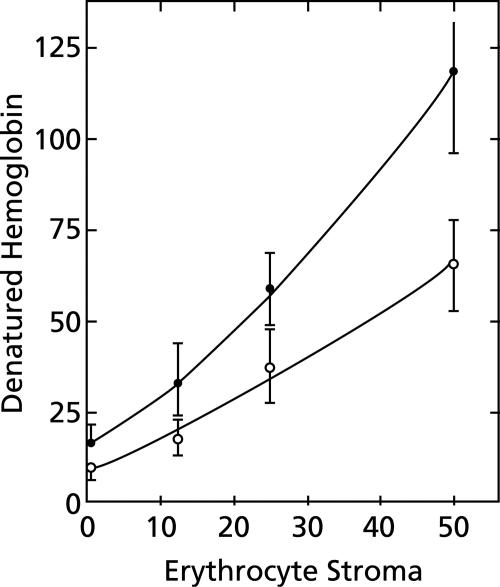
Although it is not necessary for erythrocytes to be intact for hemoglobin denaturation to occur (Fig. 1), stroma must be present (Fig. 2). In the absence of stroma, the erythrocyte cytoplasm did not turn brown or produce a precipitate of FP when it was incubated for an hour at 38°C and pH 5.
FIG. 2.
Effect of erythrocyte stroma on hemoglobin denaturation. Aliquots of erythrocyte cytoplasm, each derived from 50 μl of packed erythrocytes, and various amounts of erythrocyte stroma were diluted to 10 ml with 150 mM sodium acetate (pH 5) and incubated for 1 h at 38°C under room air. The ordinate shows the nanomoles of denatured hemoglobin minus the background per ml of packed erythrocytes per hour, and the abscissa shows the amounts of stroma derived from various volumes of erythrocytes (expressed in terms of μl of packed erythrocytes). Solid symbols indicate the presence of 100 μM chloroquine. Means ± standard deviations are shown for four experiments except for zero time in the presence of 100 μM chloroquine, when there were only three experiments.
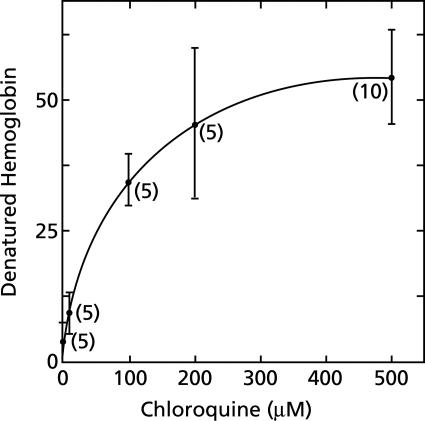
Chloroquine greatly increases hemoglobin denaturation in erythrocyte lysates (Fig. 1 to 3), and this effect is saturable. As little as 1 μM chloroquine has a measurable effect. Saturation is approached with 100 μM chloroquine and is reached with 500 μM chloroquine (Fig. 3). These concentrations are large compared to therapeutic extracellular concentrations and raise a concern about the relationship of acceleration of hemoglobin denaturation to the primary antimalarial action of chloroquine. Moderating this concern is the expectation that chloroquine would be extensively concentrated in acidic endosomes of malaria parasites (26). Removal of the stroma from erythrocyte lysates practically eliminated the effect of chloroquine (Fig. 2).
FIG. 3.
Relationship between chloroquine concentration and hemoglobin denaturation. Suspensions of lysates, each prepared from 50 μl of packed erythrocytes, were diluted to 10 ml with 150 mM sodium acetate (pH 5) and incubated for 1 h at 38°C under room air in the absence of chloroquine or in the presence of various concentrations of chloroquine. The nanomoles of denatured hemoglobin produced per ml of packed erythrocytes per hour in the presence of chloroquine minus the amount produced in the absence of chloroquine are shown. In the absence of chloroquine, 52 ± 12 nanomoles of denatured hemoglobin was produced per ml of packed erythrocytes per hour. Means ± standard deviations and the number of experiments performed at each concentration of chloroquine (in parentheses) are shown.
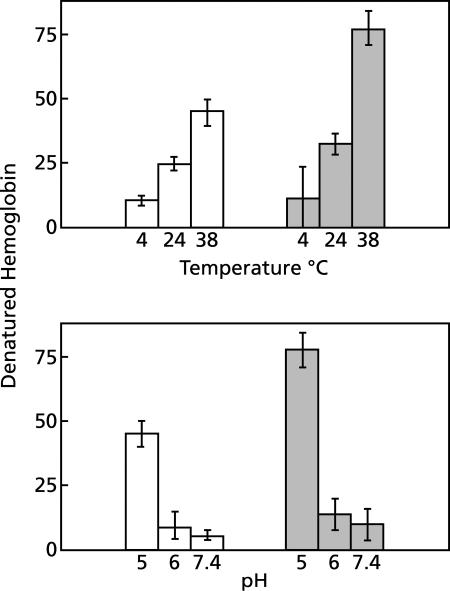
Although the quantitative effect is striking, the characteristics of hemoglobin denaturation were unaffected by chloroquine (Fig. 1 to 4). The conditions used routinely, pH 5 and 38°C, were chosen to simulate the conditions in maturing endosomes of the malaria parasite in vivo (7, 15, 26); and they allowed hemoglobin denaturation to proceed. Raising the pH to 6 or above or lowering the temperature to 24°C or below significantly reduced hemoglobin denaturation and nearly eliminated the effect of chloroquine (Fig. 4). We did not attempt to determine more precisely the pH and temperature optima for hemoglobin denaturation.
FIG. 4.
Effect of pH and temperature on hemoglobin denaturation. (Lower panel) Effect of pH. Suspensions of erythrocyte lysates, each prepared from 50 μl of packed erythrocytes, were diluted to 10 ml with 150 mM sodium acetate at pH 5 or 6 or with the standard medium at pH 7.4 and incubated for 1 h at 38°C under room air. Shaded bars indicate the presence of 100 μM chloroquine. The data are expressed as nanomoles of denatured hemoglobin minus the background per ml of packed erythrocytes per hour. Means ± standard deviations are shown for four experiments. (Upper panel) Effect of temperature. Suspensions of erythrocyte lysates, each prepared from 50 μl of packed erythrocytes, were diluted to 10 ml with 150 mM sodium acetate (pH 5) and incubated under room air for 1 h at the indicated temperatures. Shaded bars indicate the presence of 100 μM chloroquine. The data are expressed as nanomoles of denatured hemoglobin minus the background per ml of packed erythrocytes per hour. Means ± standard deviations for four experiments are shown.
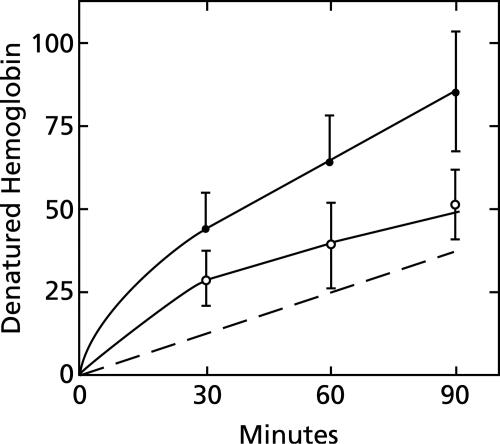
The time course of hemoglobin denaturation (Fig. 5) suggests that two processes are involved. For the first few minutes, a fast component is apparent both in the presence and in the absence of chloroquine. This component is completed within 30 min and is followed by a slower component, which is linear between 30 and 90 min. The dashed line in Fig. 5 shows the difference between incubations in the presence and the absence of 100 μM chloroquine and, thus, represents the response to chloroquine. It extrapolates approximately through zero, indicating that chloroquine has little or no effect on the putative fast component. By contrast, chloroquine accelerates the slower component to approximately 40 nanomoles of hemoglobin denatured per hour per ml of packed erythrocytes. This rate is twice that observed in the absence of chloroquine (Fig. 5).
FIG. 5.
Time course of the effect of chloroquine on hemoglobin denaturation. Suspensions of erythrocyte lysates, each prepared from 50 μl of packed erythrocytes, were diluted to 10 ml with 150 mM sodium acetate (pH 5) and incubated for various lengths of time at 38°C under room air in the presence or the absence of 100 μM chloroquine. The nanomoles of denatured hemoglobin minus the background per ml of packed erythrocytes are shown for each time interval. Solid symbols indicate the presence of 100 μM chloroquine. The dashed line shows the difference between incubations in the presence and the absence of chloroquine. Means ± standard deviations are shown for four experiments.
DISCUSSION
Since we observed that normal erythrocytes quickly lyse at pH 5 and 38°C, it is probable that erythrocytoid bodies lyse spontaneously and release erythrocyte stroma and cytoplasm in endosomes as the pH approaches 5. The endosomal pH in malaria parasites has not been measured, but by the time that hemoglobin appears in digestive vacuoles, the pH is approximately 5 (7, 15, 26). At this pH and in the presence of erythrocyte stroma, hemoglobin denaturation and FP release can occur without endosomal intervention (Fig. 1 to 5). It is possible that the proteolysis of hemoglobin also would occur in acidic endosomes of malaria parasites and increase the release of FP.
Presumably, membrane lipids in the stroma promote hemoglobin oxidation and denaturation. In support of this possibility, extensive oxidation of purified hemoglobin in the presence of purified lipids has been observed by other investigators (16, 23). By comparison, the amount of hemoglobin denaturation in our preparations was relatively small. It should be noted, however, that we studied erythrocytes and whole lysates, which would be expected to contain antioxidants and other modulators that would protect hemoglobin from oxidation.
The stroma is also required for chloroquine to accelerate hemoglobin denaturation (Fig. 2). We interpret this requirement to mean that chloroquine does not initiate hemoglobin denaturation. Instead, it apparently acts only after an initial oxidation step. Since oxidative denaturation of hemoglobin can alter the structure of hemoglobin sufficiently to cause precipitation, it is reasonable to suppose that it also increases the accessibility of chloroquine to FP that is bound to globin. We propose that chloroquine acts as an antimalarial drug primarily by binding with a high affinity to the FP and removing it from globin. In this way, toxic FP-chloroquine complexes and an excess of denatured globin could be produced in endosomes. Since FP-chloroquine complexes have membrane toxicity (5), we suggest that they inhibit endosomal maturation, which ultimately would result in a reduction in hemoglobin degradation and cause the formation of hemoglobin-laden vesicles with double membranes.
With regard to the masking of lipids, we have reported previously that unsaturated lipids in erythrocyte membranes, although they are capable of promoting FP dimerization when they are separated from protein, normally are masked and are unavailable for the promotion of FP dimerization (12). It is possible, therefore, that unsaturated lipids in the membranes of erythrocytoid bodies, which are derived from erythrocyte membranes, remain masked until the membranes are degraded. If so, inhibition of endosomal maturation due to the toxicity of FP-chloroquine complexes could allow unsaturated lipids to remain masked and unavailable to promote FP dimerization yet leave them available to promote hemoglobin oxidation and to serve other functions. As an alternative possibility, denatured globin may bind to unsaturated lipids, mask them, and inhibit endosomal maturation. In support of the latter possibility, heat denaturation of lysates of parasitized erythrocytes inhibits FP dimerization (4). Further work is needed to determine the merits of these two possible ways to explain chloroquine-induced masking of the unsaturated lipids needed for FP dimerization.
Acknowledgments
This work was supported in part by a grant from the Alsam Foundation and in part by the Department of Internal Medicine of the Saint Louis University School of Medicine.
REFERENCES
- 1.Aikawa, M., L. H. Miller, J. Johnson, and J. Rabbege. 1978. Erythrocyte entry by malarial parasites: a moving junction between erythrocyte and parasite. J. Cell Biol. 77:72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asakura, T., K. Minakata, K. Adachi, M. O. Russell, and E. Schwartz. 1977. Denatured hemoglobin in sickle erythrocytes. J. Clin. Investig. 59:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannister, L. H., J. M. Hopkins, R. E. Fowler, S. Krishna, and G. H. Mitchell. 2000. A brief illustrated guide to the ultrastructure of Plasmodium falciparum asexual blood stages. Parasitol. Today 16:427-433. [DOI] [PubMed] [Google Scholar]
- 4.Chou, A. C., and C. D. Fitch. 1992. Heme polymerase: modulation by chloroquine treatment of a rodent malaria. Life Sci. 51:2073-2078. [DOI] [PubMed] [Google Scholar]
- 5.Chou, A. C., and C. D. Fitch. 1980. Hemolysis of mouse erythrocytes by ferriprotoporphyrin IX and chloroquine. Chemotherapeutic implications. J. Clin. Investig. 66:856-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dluzewski, A. R., P. R. Fryer, S. Griffiths, R. J. M. Wilson, and W. B. Gratzer. 1989. Red cell membrane protein distribution during malarial invasion. J. Cell Sci. 92:691-699. [DOI] [PubMed] [Google Scholar]
- 7.Dzekunov, S. M., L. M. B. Ursos, and P. D. Roepe. 2000. Digestive vacuolar pH of intact intraerythrocytic P. falciparum either sensitive or resistant to chloroquine. Mol. Biochem. Parasitol. 110:107-124. [DOI] [PubMed] [Google Scholar]
- 8.Einheber, A., D. M. Palmer, and M. Aikawa. 1976. Plasmodium berghei: phase contrast and electron microscopical evidence that certain antimalarials can both inhibit and reverse pigment clumping caused by chloroquine. Exp. Parasitol. 40:52-61. [DOI] [PubMed] [Google Scholar]
- 9.Famin, O., and H. Ginsburg. 2002. Differential effects of 4-aminoquinoline-containing antimalarial drugs on hemoglobin digestion in Plasmodium falciparum-infected erythrocytes. Biochem. Pharmacol. 63:393-398. [DOI] [PubMed] [Google Scholar]
- 10.Fitch, C. D. 2004. Ferriprotoporphyrin IX, phospholipids, and the antimalarial actions of quinoline drugs. Life Sci. 74:1957-1972. [DOI] [PubMed] [Google Scholar]
- 11.Fitch, C. D., G.-Z. Cai, and Y.-F. Chen. 2003. Relationship of chloroquine-induced redistribution of a neutral aminopeptidase to hemoglobin accumulation in malarial parasites. Arch. Biochem. Biophys. 410:296-306. [DOI] [PubMed] [Google Scholar]
- 12.Fitch, C. D., G.-Z. Cai, Y.-F. Chen, and J. D. Shoemaker. 1999. Involvement of lipids in ferriprotoporphyrin IX polymerization in malaria. Biochim. Biophys. Acta 1454:31-37. [DOI] [PubMed] [Google Scholar]
- 13.Fitch, C. D., Y.-F. Chen, and G.-Z. Cai. 2003. Chloroquine-induced masking of a lipid that promotes ferriprotoporphyrin IX dimerization in malaria. J. Biol. Chem. 278:22596-22599. [DOI] [PubMed] [Google Scholar]
- 14.Hempelmann, E., C. Motta, R. Hughes, S. A. Ward, and P. G. Bray. 2003. Plasmodium falciparum: sacrificing membrane to grow crystals? Trends Parasitol. 19:23-26. [DOI] [PubMed] [Google Scholar]
- 15.Krogstad, D. J., P. H. Schlesinger, and I. Y. Gluzman. 1985. Antimalarials increase vesicle pH in Plasmodium falciparum. J. Cell Biol. 101:2302-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaBrake, C. C., and L. W.-M. Fung. 1992. Phospholipid vesicles promote human hemoglobin oxidation. J. Biol. Chem. 267:16703-16711. [PubMed] [Google Scholar]
- 17.Ladda, R. L. 1969. New insights into the fine structure of rodent malarial parasites. Military Med. 134(Special Issue):825-865. [PubMed] [Google Scholar]
- 18.Macomber, P. B., H. Sprinz, and A. J. Tousimis. 1967. Morphological effects of chloroquine on Plasmodium berghei in mice. Nature 214:937-939. [DOI] [PubMed] [Google Scholar]
- 19.McLaren, D. J., L. H. Bannister, P. I. Trigg, and G. A. Butcher. 1977. A freeze-fracture study on the parasite-erythrocyte interrelationship in Plasmodium knowlesi infections. Bull. W. H. O. 55:199-203. [PMC free article] [PubMed] [Google Scholar]
- 20.Orjih, A. U. 2001. On the mechanism of hemozoin production in malaria parasites: activated erythrocyte membranes promote β-hematin synthesis. Exp. Biol. Med. 226:746-752. [DOI] [PubMed] [Google Scholar]
- 21.Orjih, A. U., J. S. Ryerse, and C. D. Fitch. 1994. Hemoglobin catabolism and the killing of intraerythrocytic Plasmodium falciparum by chloroquine. Experientia 50:34-39. [DOI] [PubMed] [Google Scholar]
- 22.Slomianny, C., G. Prensier, and P. Charet. 1985. Ingestion of erythrocytic stroma by Plasmodium chabaudi trophozoites: untrastructural study by serial sectioning and 3-dimensional reconstruction. Parasitology 90:579-588. [DOI] [PubMed] [Google Scholar]
- 23.Szebeni, J., H. Hauser, C. D. Eskelson, R. R. Watson, and K. H. Winterhalter. 1988. Interaction of hemoglobin derivatives with liposomes. Membrane cholesterol protects against the changes of hemoglobin. Biochemistry 27:6425-6434. [DOI] [PubMed] [Google Scholar]
- 24.Ward, G. E., L. H. Miller, and J. A. Dvorak. 1993. The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J. Cell Sci. 106:237-248. [DOI] [PubMed] [Google Scholar]
- 25.Warhurst, D. C., and D. J. Hockley. 1967. Mode of action of chloroquine on Plasmodium berghei and P. cynomogi. Nature 214:935-936. [DOI] [PubMed] [Google Scholar]
- 26.Yayon, A., Z. I. Cabantchik, and H. Ginsburg. 1984. Identification of the acidic compartment of Plasmodium falciparum-infected human erythrocytes as the target of the antimalarial drug chloroquine. EMBO J. 3:2695-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarchin, S., M. Krugliak, and H. Ginsburg. 1986. Digestion of the host erythrocyte by malaria parasites is the primary target for quinoline-containing antimalarials. Biochem. Pharmacol. 35:2435-2442. [DOI] [PubMed] [Google Scholar]