Abstract
We investigated the effect of short-term changes in temperature on alternative (Alt) and cytochrome (Cyt) pathway respiration, both in intact tissues and isolated mitochondria of 14-d-old cotyledons of soybean (Glycine max L. cv Stevens). We also established the extent to which temperature alters the interaction between the oxidizing pathways and the level of ubiquinone (UQ) reduction (UQr/UQt). No difference was found between the temperature coefficient of respiration (Q10; proportional change per 10°C) of Alt and Cyt pathway respiration in cotyledon slices (Q10 = 1.92 and 1.86, respectively). In isolated mitochondria, the Q10 of the fully activated Alt pathway (Q10 = 2.24–2.61) was always equal to, or higher than, that of Cyt c oxidase (COX) alone (Q10 = 2.08) and the complete Cyt pathway (Q10 = 2.40–2.55). This was true regardless of substrate or whether ADP was present. There was little difference in the Q10 of the Cyt pathway with or without ADP; however, the Q10 of COX was substantially lower in the presence of an uncoupler (Q10 = 1.61) than its absence (Q10 = 2.08). The kinetics of Alt and Cyt pathway activity in relation to UQr/UQt were not affected by temperature. For a given UQr/UQt value, the proportion of maximum flux taking place was similar at all temperatures for both pathways (±ADP). However, the Q10 of the Alt and the Cyt pathways (+ADP) increased with increasing UQr/UQt. We conclude that the Alt pathway is not less temperature sensitive than the Cyt pathway or COX per se and that changes in the degree of control exerted by individual steps in the respiratory apparatus could result in changes in the Q10 of mitochondrial O2 uptake.
Plant mitochondria possess a branched electron transport chain that contains two pathways: the cytochrome (Cyt) pathway (terminating at Cyt c oxidase; COX) and the alternative (Alt) pathway. Both the pathways obtain their electrons from reduced ubiquinone (UQr). The Alt pathway consists of a single-subunit quinol oxidase (Alt oxidase; AOX) which is activated by pyruvate (Millar et al., 1993, 1996); this increases the reactivity of the Alt pathway toward UQr (Umbach et al., 1994). In the presence of pyruvate, substantially higher rates of Alt pathway activity are observed at any given UQr/UQt value (e.g. Millar et al., 1998a). In addition, the AOX exists as a dimer and contains a regulatory sulfhydryl-disulfide system; the sulfhydryl linkages between paired subunits must be reduced for maximal activity (Umbach and Siedow, 1993). As a result, additions of the reducing agent dithiothreitol (DTT) increase Alt pathway activity in some tissues (Umbach and Siedow, 1993). Electron transport from UQr to O2 via the Cyt pathway results in the movement of protons across the inner mitochondrial membrane, thereby building a proton-motive force that drives ATP synthesis. Flux via the Cyt pathway is rapid so long as there is an abundant supply of ADP; whenever the turnover of ATP to ADP is limited, flux via the Cyt pathway will become adenylate restricted. In contrast, no protons are translocated when electrons pass directly from UQr to O2 via the Alt pathway. The Alt pathway is thus not subject to adenylate control per se. However, high rates of Alt pathway activity reduce the amount of ATP produced per unit O2 consumed (Millar et al., 1998a).
Several positive roles for the Alt pathway in plant metabolism have been suggested. It may allow carbon flux through the TCA cycle when ADP supply limits Cyt pathway activity, thereby providing carbon skeletons for other cellular processes (Lambers and Steingrover, 1978). Moreover, the Alt pathway may protect against harmful reactive O2 generation when the UQ pool is highly reduced (Purvis and Shewfelt, 1993; Wagner and Krab, 1995), as may occur when Cyt pathway activity is limited either by adenylates or by exposure to inhibitors such as nitric oxide (Millar and Day, 1996, 1997). Several authors have also proposed that the Alt pathway may maintain flux through the mitochondrial electron transport system in the cold (Kiener and Bramlage, 1981; Smakman and Hofstra, 1982; McNulty and Cummins, 1987; Rychter et al., 1988; Stewart et al., 1990a, 1990b; Vanlerberghe and Mcintosh, 1992; Purvis and Shewfelt, 1993; Ribas Carbo et al., 2000) and in doing so reduce the production of reaction O2 species (Purvis et al., 1995). This ability to maintain flux in the cold could be due to the Alt pathway being less temperature sensitive than the Cyt pathway after short-term changes in temperature [i.e. a lower temperature coefficient of respiration (Q10; proportional change in respiration per 10°C change in temperature) for the Alt pathway than the Cyt pathway; Kiener and Bramlage, 1981; McNulty and Cummins, 1987; Stewart et al., 1990a] and/or to the de novo synthesis of AOX protein after long-term exposure to the cold (Stewart et al., 1990a, 1990b; Vanlerberghe and Mcintosh, 1992; Gonzàlez Meler et al., 1999; Ribas Carbo et al., 2000). Whereas increases in AOX protein content invariably occur when plants are exposed to low temperatures, not all studies have reported lower Q10 values for the Alt pathway. Weger and Guy (1991) reported that the Alt pathway of Picea glauca roots is highly sensitive to changes in temperature. Moreover, a recent study using the 18O fractionation method found that the Q10 of the Alt pathway is not lower than the Q10 of the Cyt pathway in mung bean (Vigna radiata) leaves and hypocotyls or soybean (Glycine max L. Merr. cv Stevens) cotyledons (Gonzàlez Meler et al., 1999). Another study found that cold-induced increases in AOX protein and activity are not related to chilling tolerance in maize (Ribas Carbo et al., 2000). Clearly, the temperature sensitivity of the Alt pathway and its role in chilling tolerance has not been fully elucidated.
Many of the past studies that investigated the temperature sensitivity of the Alt and Cyt pathways used respiratory inhibitors to estimate Alt pathway activity at different temperatures (e.g. Kiener and Bramlage, 1981; Smakman and Hofstra, 1982; McNulty and Cummins, 1987; Weger and Guy, 1991). However, the use of respiratory inhibitors to measure flux via the Alt and Cyt pathways is now considered unreliable (Day et al., 1996). As a result, past estimates of the Q10 (proportional change in respiration per 10°C) of the Alt pathway in intact tissues (except Gonzàlez Meler et al., 1999) may be incorrect. Moreover, the results of studies on the temperature sensitivity of respiration in isolated mitochondria (e.g. Kiener and Bramlage, 1981; Lin and Markhart, 1990; Stewart et al., 1990a) need to be treated with caution, as activators of the Alt pathway (pyruvate and DTT) were not present in the assay media.
Although Q10 values of respiration are commonly around 2.0, values as low as 1.4 and as high as 4.0 have been reported (Azcón-Bieto, 1992; Atkin et al., 2000a). Respiratory Q10 values differ among species (e.g. Larigauderie and Körner, 1995) and are influenced by the metabolic state of the tissue and the growth environment (Atkin et al., 2000a, 2000b, 2000c). For example, Q10 values are higher in plants with high concentrations of soluble carbohydrates (Wager, 1941; Breeze and Elston, 1978; Berry and Raison, 1981; Azcón-Bieto and Osmond, 1983) and in tissues where the demand for energy is increased when tissues are coping with environmental stress (Atkin et al., 2000c). Moreover, leaf respiration is also more temperature sensitive in darkness than in the light (Atkin et al., 2000b). The cause of this variability in respiratory Q10 values has not been established. Nevertheless, it seems likely that variations in Q10 values depend on the availability of respiratory substrate, degree of adenylate control, and/or the temperature sensitivity of respiratory enzymes per se (Atkin et al., 2000a). Variations in the flux via the Alt and Cyt pathways, as well as the degree of adenylate control may thus play an important role in determining the overall temperature sensitivity of plant respiration.
Our study investigates the effect of short-term changes in temperature on respiration in intact tissues and mitochondria isolated from 14-d-old soybean cotyledons. There were two components to our study. First, we determined whether the temperature sensitivity of the Alt and Cyt pathways differs in intact tissues and isolated mitochondria when the AOX is fully activated. To avoid the complicating effects of temperature on dehydrogenase activity and/or other components of the mitochondrial electron transport chain, we also determined the Q10 value of the Alt pathway and COX per se (i.e. independent of limitations imposed by dehydrogenase activity) in isolated mitochondria. Second, we established the extent to which the UQr/UQt dependence of Alt pathway and Cyt pathway activity is affected by changes in temperature.
RESULTS
Temperature Sensitivity of Respiration
The effect of temperature on respiration in cotyledon slices is shown in Table I. Cotyledon respiration was temperature sensitive, both in the absence and presence of inhibitors (KCN or salicylhydroxamic acid [SHAM]). When calculated over the entire 10°C to 25°C range, uninhibited respiration, respiration in the presence of KCN, and respiration in the presence of SHAM exhibited Q10 values of 1.98, 1.86, and 1.92, respectively (calculated from the slope of log10 respiration versus temperature plots). There was therefore little difference in the temperature sensitivity of the KCN-resistant, Alt pathway and SHAM-resistant, Cyt pathway. The lack of inhibition by KCN (Table I) suggests that the capacity of the Alt pathway was equal to or greater than the control respiration rates. Residual respiration (i.e. respiration in the presence of SHAM and KCN) was temperature insensitive (Table I). Although the cause of the high residual rates (Table I) is unclear, it is unlikely that they are mitochondria in nature. Soybean cotyledons contain substantial amounts of lipid that can be degraded by beta-oxidation; there is an oxidation step that takes place in the glyoxysomes in which FADH2 is oxidized directly by O2 to produce H2O2, which is broken down by catalase. Respiratory inhibitors do not inhibit this process. Inhibitor-insensitive O2-dependent peroxidation of fatty acids by lipoxygenase may also contribute to the high residual rate.
Table I.
Effect of temperature on respiration rates in sliced soybean cotyledons (±se, n = 4)
| Temperature | Uninhibited Respiration | KCN Resistance | SHAM Resistance | Residual |
|---|---|---|---|---|
| °C | nmol O2 g−1 dry mass s−1 | |||
| 10.0 | 7.9 ± 0.7 | 8.1 ± 1.4 | 4.1 ± 0.7 | 3.5 ± 0.4 |
| 15.0 | 14.3 ± 0.7 | 15.3 ± 3.3 | 8.9 ± 0.7 | 5.6 ± 0.8 |
| 20.0 | 19.0 ± 1.8 | 19.9 ± 1.6 | 9.6 ± 0.7 | 4.2 ± 0.6 |
| 25.0 | 22.6 ± 1.4 | 20.9 ± 2.7 | 11.8 ± 0.9 | 5.1 ± 0.6 |
Respiration measured in the absence of inhibitors and in the presence of 1 mm KCN, 30 mm SHAM, or both KCN plus SHAM (residual).
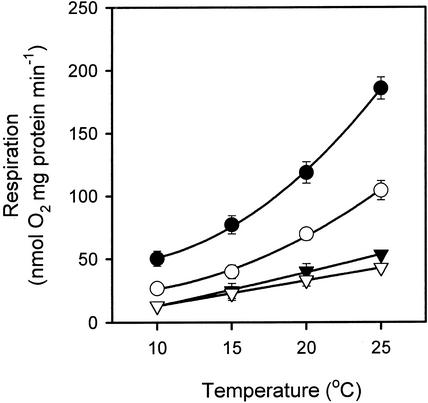
Figure 1 shows the effect of temperature on respiration in isolated mitochondria. Using succinate as a respiratory substrate, O2 consumption via the Alt pathway (in the presence of DTT, ±5 mm pyruvate) was substantially lower than that of respiration via the Cyt pathway, both in the presence or absence of ADP (Fig. 1). All three parameters [Alt pathway and Cyt pathway (± ADP) respiration] were temperature sensitive across the 10°C to 25°C range (Fig. 1). Stimulation of Alt pathway activity by pyruvate increased with increasing temperature.
Figure 1.
Effect of temperature on O2 consumption of soybean cotyledon mitochondria oxidizing succinate. Symbols represent O2 uptake via the following: Cyt pathway in the presence of ADP (●), Cyt pathway without ADP (○), Alt pathway activity in the presence of DTT and pyruvate (▾), and Alt pathway activity in the presence of DTT but without pyruvate (▿). Values represent the mean of three replicate mitochondrial isolations (±se). Pyruvate increases the reactivity of the Alt pathway toward UQr (Umbach et al., 1994), whereas the reducing agent DTT reduces the sulfhydryl linkages between paired subunits of the AOX; these must be reduced for maximal activity (Umbach and Siedow, 1993). Addition of DTT and pyruvate therefore ensured that maximal AOX activity was assayed. To calculate Q10 values, respiration rates were also plotted on a log10 scale versus temperature (not shown), with the slopes used to calculate the Q10 values. The r2 values of the slopes ranged from 0.84 to 0.94.
The fact that Cyt pathway activity was higher than Alt pathway activity in isolated mitochondria (Fig. 1) contrasts with the higher rates of KCN-resistant respiration than SHAM-resistant respiration in intact tissues (Table I). The most likely explanation for this discrepancy was that respiration was ADP- and substrate-limited in the intact tissues but not in isolated mitochondria (where saturating levels of ADP and substrate were supplied). Limitations in ADP supply in intact tissues will have a greater detrimental effect on Cyt pathway activity than Alt pathway activity, due to electron flux from UQ to O2 via the Cyt pathway being coupled to proton translocation and ATP synthesis. No protons are translocated when electrons are transported from UQ to O2 via the AOX. Evidence of substrate limitation in intact tissues come from the high rates of respiration in the presence of KCN (Table I). Whereas KCN inhibits electron transport via the Cyt pathway in both intact tissues and isolated mitochondria, this inhibition does not always result in reductions in O2 consumption in intact tissues. This is because KCN severely reduces the synthesis of ATP with the result that cellular ATP to ADP ratios decline. Low ATP to ADP ratios can stimulate the rate of glycolysis and, hence, the rates of substrate supply to the mitochondria (Lambers et al., 1996). Overall respiratory flux can therefore be higher in intact tissues in the presence of KCN than in its absence whenever the Alt pathway capacity is high. Because KCN-resistant respiration and uninhibited respiration rates were similar (Table I), it seems likely that O2 uptake in intact tissues was limited by the provision of substrate to the respiratory system. In contrast to KCN, SHAM does not decrease the ATP to ADP ratio or increase the rate of substrate supply in intact tissues. We, therefore, interpret the relatively low rates of SHAM-resistant respiration rates (Table I) to mean that the Cyt pathway was substrate (and ADP) limited in intact tissues.
To determine the temperature sensitivity (i.e. the Q10) of each parameter shown in Figure 1, we plotted the respiration rates on a log10 scale against temperature (not shown). In all cases, the resultant plots were linear. A single Q10 value therefore applied to the entire 10°C to 25°C range for each pathway in the isolated mitochondria. The Q10 of the Alt pathway was greater in the presence of pyruvate than in its absence (Table II). Although the fully activated Alt pathway was slightly more temperature sensitive than Cyt pathway respiration in the presence of ADP, there was little difference between the Q10 value of the fully activated Alt pathway and that exhibited by Cyt pathway respiration in the absence of ADP (Table II).
Table II.
Temperature sensitivity of respiration in isolated mitochondria in soybean cotyledons
| Respiratory Pathway | Substrates and Assay Components | Q10 Value | Source Data |
|---|---|---|---|
| Alt pathway | Suc + DTT + Pyr | 2.61 | Fig. 1 |
| Suc + DTT − Pyr | 2.25 | Fig. 1 | |
| UQ1H2+ DTT + Pyr | 2.24 | Fig. 2 | |
| Cyt Pathway | Suc + ADP | 2.40 | Fig. 1 |
| Suc − ADP | 2.55 | Fig. 1 | |
| COX | TMPD-ascorbate | 2.08 | Fig. 2 |
| TMPD-ascorbate + CCCP | 1.61 | Fig. 2 |
Q10 values were determined using the slope of log10 respiration versus temperature plots using the source data indicated. See text and figure legends for further details.
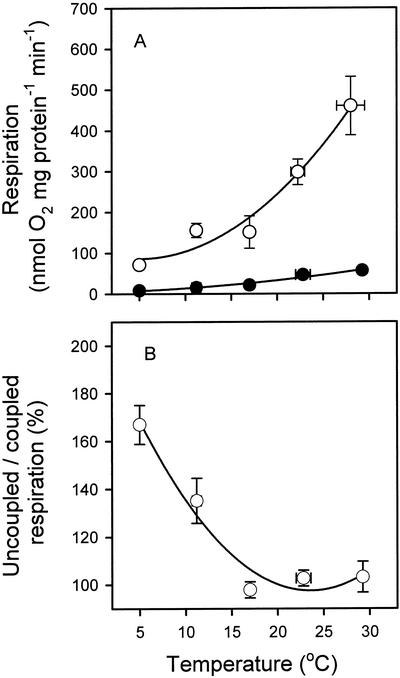
To further assess the temperature sensitivity of the Alt pathway and COX independent of limitations caused by dehydrogenase activity, we determined the effect of temperature on Alt pathway and COX activity using nonenzymatic electron donors (ubiquinol-1 [UQ1H2] and 2,3,5,6-tetramethyl-p-pheny-lenediamine dihydrochloride [TMPD]-ascorbate, respectively). Alt pathway assays were conducted in the presence of DTT and pyruvate. At 25°C, rates of UQ1H2-dependent O2 consumption via the Alt pathway (Fig. 2A) were similar to those exhibited by succinate-dependent O2 consumption via the Alt pathway when pyruvate was present (Fig. 1). However, TMPD-ascorbate resulted in far higher rates of O2 consumption via COX (Fig. 2A) compared with when succinate was used a respiratory substrate (Fig. 1). The Q10 value of the Alt pathway (in the presence of pyruvate) appeared to be lower with UQ1H2 as an electron source (Table II) than with succinate (Table II). Similarly, the Q10 was lower for TMPD-ascorbate-dependent COX activity (Table II) than that for succinate-dependent O2 consumption via the Cyt pathway in the presence of ADP (Table II). The stimulatory effect of carbonyl cyanide-m-chlorophenylhydrazone (CCCP) on COX activity (using TMPD-ascorbate as an electron donor) decreased with increasing temperature (Fig. 2B). As a result, the Q10 of uncoupled respiration was substantially lower than that of the coupled state (Table II). Taken together, these results demonstrate that in isolated mitochondria the Alt pathway is not less temperature sensitive than the Cyt pathway, regardless of the source of the respiratory substrate (i.e. succinate for both pathways or TMPD-ascorbate for COX and UQ1H2 for AOX).
Figure 2.
Respiration of soybean cotyledon mitochondria at several temperatures, using artificial electron donors. Rates of respiration via COX (○) and AOX (●). COX activity was determined in the presence of octyl gallate (OG) and using TMPD-ascorbate as an electron donor. Alt pathway activity was assessed in the presence of antimycin A and myxothiazol, and using UQ1H2 as an electron donor. To calculate Q10 values, respiration rates were also plotted on a log10 scale versus temperature (not shown), with the slopes used to calculate the Q10 values. The r2 values for the slopes of the log10 respiration versus temperature plots (used to calculate Q10 values) were 0.89 and 0.79 for the Alt pathway and COX, respectively. B, The effect of uncoupler (CCCP) on COX activity, expressed as a proportion of the values shown in A. Values represent the mean of three to five replicate mitochondrial isolations (±se).
UQ Redox Poise and Temperature
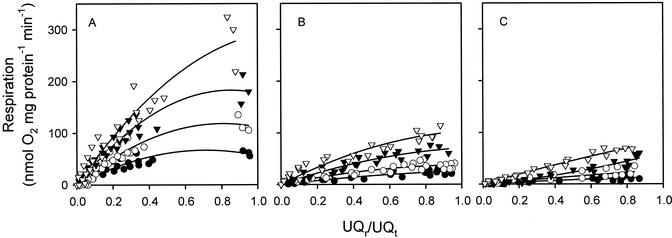
The experimental results above provide estimates of the Q10 of the Alt pathway, the full Cyt pathway and COX alone, either at UQr/UQt values that differ (Fig. 1) or near-saturating UQ reduction levels (Fig. 2A). To assess whether the Q10 values of the Cyt pathway and Alt pathway (in the presence of pyruvate) depend on the UQr/UQt value, we titrated succinate dehydrogenase with malonate (Fig. 3). Regardless of UQr/UQt value, low temperatures reduced Cyt pathway activity (both in the presence and absence of ADP) and Alt pathway activity (Fig. 3). However, for any given parameter, the UQr to UQt ratio was relatively similar at each temperature when respiration was measured in the absence of malonate (i.e. at maximum O2 uptake rate), being 0.35 to 0.5 for state 3, 0.8 to 0.95 for state 4, and 0.8 to 0.9 for the Alt pathway. Cyt pathway respiration rates were always higher in the presence of ADP than in its absence, regardless of the UQr/UQt value.
Figure 3.
The dependence of respiration rate on quinone redox state in soybean cotyledon mitochondria oxidizing succinate. Measurements were made at four temperatures: 25°C (▿), 20°C (▾), 15°C (○), and 10°C (●). A, Cyt pathway respiration in the presence of ADP. B, Cyt pathway respiration in the absence of ADP. C, Alt pathway respiration. In all three figures, data were obtained via malonate titration of succinate oxidation; in the absence of malonate and using succinate as a substrate, Cyt pathway state 3 UQr/UQt values were approximately 0.35 to 0.45 (A). To establish Cyt pathway state 3 rates at near saturating UQr/UQt values (i.e. to construct Fig. 5), we extended the regression lines shown in A using rates of O2 consumption and UQr/UQt values in the presence of NADH (i.e. the values shown where UQr/UQt was 0.85–0.95 in A). Cyt pathway activity was determined in the presence of OG. Alt pathway activity was assessed in the presence of myxothiazol and pyruvate but in the absence of DTT. Results of three mitochondrial isolations are shown (each value is the mean of two replicates from the same mitochondrial isolation).
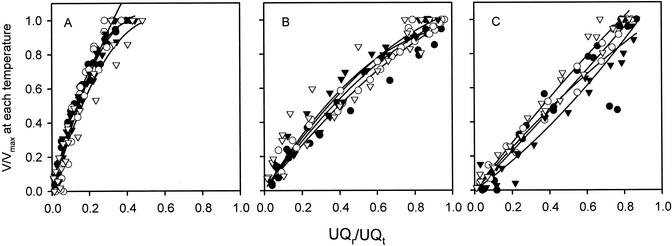
To assess whether temperature altered the overall relationship between respiration rates and UQr/UQt values, we expressed the data shown in Figure 3 as a ratio of the maximum rates (using succinate as a substrate) for each temperature (i.e. V/Vmax; Fig. 4). Figure 4 shows that changes in temperature had little affect on the relative rates of respiration obtained at each temperature. This suggests that the ability of each pathway to compete for a given amount of reduced UQ remains unaltered by changes in temperature.
Figure 4.
Dependence of V/Vmax on quinone redox state in soybean cotyledon mitochondria oxidizing succinate. Measurements were made at several temperatures: 25°C (▿), 20°C (▾), 15°C (○), and 10°C (●). In each case Vmax is taken as the maximum respiration rate at each temperature using succinate as a respiratory substrate. A, Cyt pathway respiration in the presence of ADP. B, Cyt pathway respiration in the absence of ADP. C, Alt pathway respiration. See Figure 3 legend for further details.
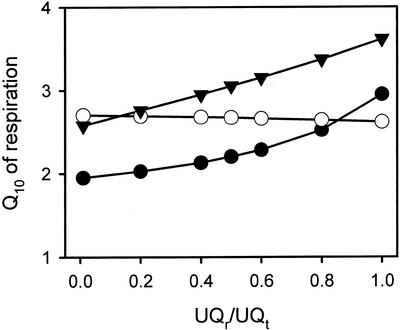
To assess whether the Q10 of the Alt pathway and/or Cyt pathway varies with UQr/UQt value, we fitted regression equations to the data shown in Figure 3 to predict respiration rates at set UQr/UQt values and temperature. These regression equations where then used to determine the temperature sensitivity of respiration at a set UQr/UQt value. Figure 5 suggests that the Q10 values for the Cyt pathway and the Alt pathway vary with UQr/UQt. The temperature sensitivity of the Cyt pathway in the absence of ADP was constant at all levels of Q reduction. In contrast, both the Alt pathway and the Cyt pathway in the presence of ADP appeared to become more temperature sensitive at high levels of UQ reduction. The calculated Alt pathway and Cyt pathway (+ADP) Q10 values remained UQr/UQt-dependent even when calculated using the highest and lowest 95% confidence intervals for the data shown in Figure 3 (data not shown). We are therefore confident that despite variability among the respiration rates of separate mitochondrial preparations (particularly at 25°C; Fig. 3), the Q10 did increase with increasing UQr/UQt. One result of this is that whereas the Cyt pathway respiration in the absence of ADP was more temperature sensitive than Cyt pathway respiration in the presence of ADP at low UQr/UQt values, there was little difference in the Q10 values in the presence and absence of ADP at high UQr/UQt (Fig. 5). It is important that the Q10 of the Alt pathway remained higher than that of the Cyt pathway in the presence of ADP at all UQr/UQt values (Fig. 5). The Q10 of the Alt pathway was also higher than that of the Cyt pathway in the absence of ADP for all except the lowest levels of UQ reduction (Fig. 5).
Figure 5.
Dependence of the temperature sensitivity (Q10) of respiration on quinone redox state in soybean cotyledon mitochondria oxidizing succinate. Symbols represent Q10 values for the following: Cyt pathway in the presence of ADP (●), Cyt pathway without ADP (○), and Alt pathway activity in the presence of pyruvate but in the absence of DTT (▾).
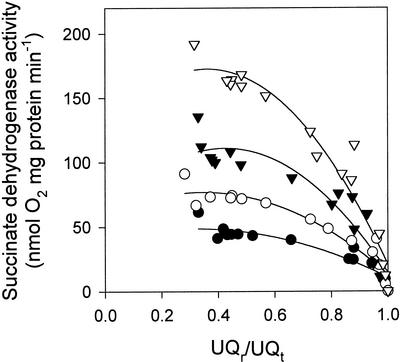
Data obtained in the absence of malonate represent the intersection between the kinetics of the oxidizing pathways (i.e. Cyt pathway ± ADP or Alt pathway) and the reducing pathway (succinate dehydrogenase) (Fig. 3). For any given temperature, a curve fitted through these intersections represents the activity of succinate dehydrogenase at different UQr/UQt values. When these points were plotted separately, it was clear that succinate dehydrogenase activity decreased with decreasing temperature (Fig. 6). To further illustrate this, we titrated the Cyt pathway (in the presence of ADP) with KCN at the following temperatures: 25°C, 20°C, 15°C, and 10°C. When these data were combined with the uninhibited Cyt pathway (±ADP) data, the kinetics of succinate dehydrogenase in the face of increasing UQr/UQt values at each temperature could be observed (Fig. 6). When coupled to oxidation via the Cyt pathway, succinate dehydrogenase activity was clearly temperature dependent at all UQr/UQt levels, decreasing by approximately 50% for each 10°C decline in temperature (i.e. the Q10 of succinate dehydrogenase was approximately two).
Figure 6.
Dependence of succinate dehydrogenase activity on quinone redox state in soybean cotyledon mitochondria operating via the Cyt pathway. Measurements were made at four temperatures: 25°C (▿), 20°C (▾), 15°C (○), and 10°C (●). Measurements were made in the presence of OG. Data shown include O2 consumption rates in the presence and absence of ADP, always in the absence of malonate (i.e. from Fig. 3) for three mitochondrial isolations, plus a KCN titration of Cyt pathway respiration (+ADP) for a single mitochondrial isolation.
DISCUSSION
Temperature Sensitivity of the Alt and Cyt Pathways
It has been suggested that the Alt pathway plays a role in protecting plant cells against the generation of high levels of superoxide and H2O2 in cold-stressed tissues (Purvis and Shewfelt, 1993). Support for this suggestion comes from the fact that long-term exposure to low temperatures increases AOX protein levels in tobacco leaves (Vanlerberghe and Mcintosh, 1992) and mung bean hypocotyls and leaves (Gonzàlez Meler et al., 1999). Moreover, the relative partitioning of electrons via the Alt pathway is increased after long-term cold acclimation in some species (Gonzàlez Meler et al., 1999). However, despite the clear role increases in Alt pathway activity may play during acclimation to long-term changes in temperature, our results suggest that during short-term exposure to low temperature the Alt pathway is not able to reduce the risk of oxidative damage. Rather than being less temperature sensitive than the Cyt pathway (see Kiener and Bramlage, 1981; Smakman and Hofstra, 1982; McNulty and Cummins, 1987; Stewart et al., 1990a), the Q10 of the Alt pathway was either similar to or greater than that exhibited by the Cyt pathway (Table II). Other studies have also reported that the short-term Q10 of the Alt pathway is not less than that of the Cyt pathway (Weger and Guy, 1991), including a recent study that used the 18O-discrimination method (Gonzàlez Meler et al., 1999). It seems likely, therefore, that the Alt pathway does not ameliorate the detrimental effects of short-term exposure to low temperature.
Why was the Alt pathway more temperature sensitive in our study than in previous studies? One explanation is that the Q10 for the Alt pathway reported by Stewart et al. (1990a) was an underestimate of the actual Q10, as neither pyruvate or DTT were present in their reaction media. In our study pyruvate stimulated Alt pathway activity by approximately 25% at 25°C but had little effect at 10°C (Fig. 1). As a result, the Q10 of the Alt pathway was higher in the presence of pyruvate than in its absence. We did not directly assess the effect of DTT on the Q10 of the Alt pathway. However, comparison of rates in the presence of DTT (Fig. 1) and those in its absence (Fig. 3), both in the presence of pyruvate, indicate that DTT had little effect on the Q10 of the Alt pathway in mitochondria isolated from our 14-d-old soybean cotyledons. Whereas this suggests that the AOX was fully reduced in our soybean cotyledon mitochondria, it is still important to include DTT in mitochondrial assay media whenever maximum AOX activity is being assessed as the reduced state of AOX is likely to vary among species, tissues and/or environmental treatments.
Distribution of Control within the Respiratory Apparatus and the Q10
Our study suggests that changes in the degree of control exerted by individual steps in the respiratory apparatus could result in changes in the Q10 of mitochondrial O2 uptake. For example, the Q10 of the COX was substantially lower in the presence of an uncoupler (i.e. when control over electron flow to O2 resides in the electron transport chain itself and/or the entry of reduced TMPD into the membrane) than in its absence (i.e. when electron flow is limited to a large extent by phosphorylation; Table II). Similarly, the Q10 of the Alt and Cyt pathways (+ADP) appear to increase with increasing UQr/UQt (Fig. 5). At low UQr/UQt, a greater proportion of control over electron flow is likely to be exerted by the UQ reducing pathways (e.g. succinate dehydrogenase) than by the UQ oxidizing pathways (e.g. Alt and Cyt pathways). In contrast, control over electron flow would shift toward the UQ oxidizing pathways at high UQr/UQt values.
In contrast to the apparent UQr/UQt dependence of the Q10 values exhibited by the Alt pathway and Cyt pathway respiration in the presence of ADP, Q10 values of Cyt pathway activity in the absence of ADP were clearly UQr/UQt-independent (Fig. 5). In the absence of ADP, the control exerted by the components of the Cyt path is near zero, with control being mostly exerted by the leak of protons across the inner mitochondrial membrane (Diolez et al., 1993). In contrast, control is distributed among several steps in the presence of ADP, including the dehydrogenase, proton leak, the ATPase, CIII, Cyt c, and COX (Douce and Neuburger, 1989). The overriding importance of the proton leak in controlling flux via the Cyt pathway in the absence of ADP suggests the Q10 value of Cyt pathway respiration in the absence of ADP reflects primarily the Q10 value of the proton leak. Moreover, the lack of change in the Q10 of the Cyt pathway respiration in the absence of ADP suggests that the temperature sensitivity of the proton leak is independent of UQr/UQt.
Our study highlights the importance of ensuring that the UQr/UQt is similar for the Alt and Cyt pathways when assessing their temperature sensitivity in isolated mitochondria. If we had only measured the Q10 of the two pathways using succinate or other non-saturating substrates such as malate, then the UQr/UQt value we would have overestimated the difference in Q10 values exhibited by the Alt pathway and Cyt pathway in the presence of ADP. In the absence of inhibitors, both pathways would be competing for electrons from the UQ pool, with a common UQr/UQt value being available for both terminal oxidase pathways. It is thus misleading to only compare the Q10 of the Alt and Cyt pathways without ensuring that the comparison is made at a common UQr/UQt value.
In addition to not altering the ability of each pathway to compete for a given amount of reduced UQ, changes in temperature also appear to have not altered the balance between the activity of the reducing and oxidizing pathways that determine the level of UQ reduction in mitochondria provided with succinate as a substrate. Decreases in temperature reduced flux via the UQ reducing (succinate dehydrogenase; Fig. 6) and oxidizing pathways (Fig. 3) to a similar extent. As a result, UQr/UQt remained unaltered in the absence of malonate for each pathway, regardless of temperature (Fig. 3). If temperature had had a greater impact on either the reducing or oxidizing pathways, then UQr/UQt values in the absence of malonate would not have been the same at each temperature.
Our conclusion that the Q10 of the Alt and Cyt (+ADP) pathways is UQr/UQt-dependent was supported by the fact that the Q10 remained UQr/UQt-dependent when the analysis was repeated using 95% confidence interval values for the data shown in Figure 3 (see “Materials and Methods” and “Results”). One reason for why the Q10 increases with increasing UQ reduction might be that high UQr/UQt activates the oxidizing pathways and that the degree of activation is greatest at high temperatures. Indeed, the AOX is activated by high UQr/UQt values (Hoefnagel and Wiskich, 1998). However, it is not known if this degree of activation increases with increasing temperature. Moreover, the extent to which the Cyt pathway (+ADP) is activated by high UQr/UQt at different temperatures is not known.
Effect of Temperature on the Regulation of Respiratory Flux
Respiratory flux can be controlled by the availability of substrate supply, the degree to which respiration is regulated by adenylates and/or the maximum capacity of respiratory enzymes (Atkin et al., 2000a). Changes in temperature may alter the degree to which each factor regulates the overall rate of O2 consumption. At moderate temperatures (e.g. 25°C), limitations in the amount of active AOX may limit O2 consumption by the Alt pathway. However, in plant tissues with high Cyt pathway capacity O2 consumption is unlikely to be strongly limited by enzymatic capacity, when measured at 25°C. Rather, respiratory flux at moderate temperatures is controlled largely via adenylate regulation of respiratory enzymes in glycolysis (phosphofructokinase and pyruvate kinase) and the mitochondrial electron transport chain (Day and Lambers, 1983). Changes in substrate supply also play a role in some situations (Lambers et al., 1996). Wiskich and Dry (1985) found that the activity of isolated mitochondria typically exceeds actual measured rates of intact tissues. Similarly, several studies where the activity of enzymes of glycolysis, the TCA cycle and the electron transport chain have been altered in transgenic plants have led to, at best, only modest changes in respiratory rates, when measured at moderate temperatures (e.g. Gottlob-McHugh et al., 1992; Millar et al., 1998b). Moreover, whereas glycolytic flux is regulated by the activity of two key enzymes, phosphofructokinase and pyruvate kinase, changes in the rate of respiration likely occur primarily via adenylate (i.e. ATP to ADP ratio) regulation of these enzymes with little control being exerted by the enzymatic capacity per se (Thomas et al., 1997). Respiratory flux is also likely to be regulated in part via changes in the activation state of pyruvate dehydrogenase complex (PDC). PDC is strongly regulated, with its activity being determined by the concentration of substrates, products, NADH and ATP, and other factors (Moore et al., 1993). The actual amount of PDC protein is unlikely to control respiratory flux at moderate temperatures. To our knowledge, no study has investigated the extent to which capacities of enzymes control respiratory flux at low temperatures. Nevertheless we can get some insight by measuring the Q10 values of intact tissues and those of isolated mitochondria provided with saturating substrate and abundant ADP (i.e. not adenylate or substrate limited). If the Q10 value of substrate/ADP-saturated, enzyme-limited systems is substantially greater than that of intact tissues, then exposure to low temperatures may result in a transition from control of respiration by extra-mitochondrial reactions (e.g. by the ratio of ATP to ADP) to control exerted by the capacity of respiratory enzymes. Q10 values are greater in substrate-saturated, enzyme-limited isolated mitochondria than in substrate-unsaturated, intact shoot segments at measuring temperatures less than 10°C to 15°C in wheat and rye but are similar at higher temperatures (Pomeroy and Andrews, 1975). This suggests that respiration might be partly controlled by the capacity of mitochondrial processes at low temperatures.
CONCLUSIONS
Our measurements demonstrate that the temperature sensitivity of the Alt pathway is not less than that of COX alone, or the complete Cyt pathway. Moreover, we have shown that the kinetics of Alt pathway and Cyt path activity in relation to the UQ pool are temperature independent and that the Q10 of Alt pathway and the Cyt pathway (in the presence of ADP) increase with increasing levels of Q reduction. Our results also demonstrate that the Q10 of O2 consumption can be highly variable, depending on the level of reduction of the UQ pool, degree of adenylate control of the Cyt pathway and the activation state of the Alt pathway. This may partly explain why Q10 values of plant respiration vary so markedly in nature.
MATERIALS AND METHODS
Plant Culture and Reagents
All work was conducted using 14-d-old cotyledons harvested from soybean (Glycine max L. Merr. cv Stevens) seedlings propagated in trays of vermiculite. For the isolated mitochondria studies, plants were grown under glasshouse conditions. In vivo respiration measurements were conducted using controlled-environment grown seedlings (constant 25°C, 14-h day, 60% relative humidity, 300 μmol photons m−2 s−1). Percoll was purchased from Pharmacia Biochemicals Inc. (Uppsala), whereas UQ1 was prepared by Dr A.D. Ward (University of Adelaide, Australia). All other chemicals were purchased from Sigma (St. Louis).
In Vivo Measurements
In vivo measurements of dark respiration of the cotyledons were determined using a Rank Brothers Clark-type O2 electrode (Cambridge, UK). KCN and SHAM were used to inhibit COX and Alt pathway, respectively. In preliminary studies very high rates of respiration remained in the presence of KCN and SHAM, regardless of the concentrations of KCN or SHAM used, or the solvent in which SHAM was dissolved (including dimethyl sulfoxide). We therefore decided to slice the cotyledons into 2 mm thick slices using a sharp razor blade to minimize residual respiration. One millimolar KCN (1.0 m stock in 20 mm HEPES, pH 5.8) and 30 mm SHAM (from a 1.0 m stock in methoxyethanol) were used to inhibit the Cyt and Alt pathways, respectively. Although substantial residual respiration remained in the presence of both inhibitors (see Table I), the absolute rates of residual respiration were less than one-half that compared with unsliced cotyledons (data not shown). During slicing, cotyledons were submerged in a buffer (2 mm CaCl2, 10 mm HEPES, and 10 mm MES, pH 7.2). Slices were left in the buffer and darkness for 30 min to overcome transient postillumination and wounding effects (Azcón-Bieto et al., 1983). O2 consumption was then measured in darkness with the sliced cotyledons submerged in a fresh volume of the same buffer.
Mitochondrial Assays and Temperature Treatments
Mitochondria were prepared according to Day et al. (1985). O2 consumption was measured using a Rank Brothers electrode in a standard reaction medium (0.3 m Suc, 10 mm TES buffer, 5 mm KH2PO4, and 2 mm MgCl2, pH 7.2). O2 consumption was measured at several temperatures, typically in the 10°C to 25°C range unless otherwise stated. Air-saturated, temperature-equilibrated water was used to calibrate the electrode at each temperature. For each temperature treatment the pH of the standard reaction medium was adjusted to 7.2 using a pH meter calibrated using buffers at the same temperature. Unless otherwise stated, all Alt pathway activity assays included 5 mm pyruvate (Millar et al., 1993). DTT (5 mm) was also used to fully reduce the AOX, where indicated. The protein content of samples was estimated according to the method of Lowry et al. (1951).
Alt Pathway and COX Activity
To assess the temperature dependence of Alt pathway activity, independent of limitations imposed by dehydrogenase activity, 400 μm UQ1H2 (an analog of ubiquinol; Hoefnagel et al., 1997) was provided as electron sources in the standard reaction medium, in the presence of antimycin A (5 μm) and KCN (1 mm). Antimycin A (5 μm) was added to inhibit transhydrogenase activity from UQ1H2 to UQ10 (Hoefnagel and Wiskich, 1998). The temperature dependency of COX activity, independent of dehydrogenase activity, was determined using TMPD in the presence of ascorbic acid as an electron donor. The reaction medium contained 5 mm TMPD and 20 mm ascorbic acid. KCN (1 mm) was added to subtract residual activity at the end of each assay. The effect of the uncoupler CCCP on TMPD-ascorbate dependent COX activity was also assessed. These latter experiments were conducted in the presence of 2 μm OG, an inhibitor of the Alt pathway.
Malonate Titrations and UQ Reduction Measurements
Malonate (0.5–20 mm) was used to titrate succinate (10 mm) oxidation for Cyt (± ADP) and Alt pathway respiration according to Dry et al. (1989). At each stage of the malonate titration, we measured the redox state of UQ using glassy carbon and platinum electrodes (Bioanalytical Systems, West Lafayette, IN) and a non-cycling UQ Electrode Potentiostat (ARN Electronics, Adelaide, Australia) according to the method of Moore et al. (1988). Before adding succinate, mitochondria were pre-incubated in 1.0 mm UQ1 and 0.5 mm ATP for 3 min. OG (2 μm) and myxothiazol (6 μm) were used to inhibit Alt pathway and the Cyt pathway, respectively. Fully oxidized levels of UQ were taken as the voltametric reading before succinate was added. To obtain a fully reduced UQ reading, we added 2 mm NADH at the end of each experiment in the presence of OG and myxothiazol. The direct effects of NADH, OG, and myxothiazol on the UQ electrode were determined in the absence of mitochondria and subtracted from the final fully reduced UQ signal where necessary.
Calculations
The temperature sensitivity of respiration was assessed by calculating Q10 values (i.e. the proportional change in respiration per 10°C change in temperature). Q10 values were calculated using the equation Q10 = 10[(regression slope) · 10] where the regression slope is the slope of a log10 respiration rate versus temperature plot. In all our measurements, the regression slope was linear in the 5°C to 30°C range; consequently, a single Q10 value could be applied across this temperature range.
To assess the temperature sensitivity of respiration at any given UQ reduction level, we first fitted second order regression equations to the respiration-UQr/UQt curves generated from the malonate titrations (SigmaPlot Version 5, Jandel Scientific, San Rafael, CA). To obtain a respiration rate at a high UQr/UQt value for Cyt pathway in the presence of ADP, both the respiration rate and UQ reduction level were measured in the presence of NADH (plus OG, minus myxothiazol). Again, the direct effect of NADH on the UQ electrode was taken into account when determining the level of UQ reduction. For each temperature, respiration rates for Cyt pathway (±ADP) and the Alt pathway for five UQr/UQt values (0.01, 0.2, 0.4, 0.5, 0.6, 0.8, and 1.0) were then estimated from 2nd order regression equations fitted to the flux versus UQr/UQt. Log10 transformed values of respiration were then plotted against temperature, with the slopes then used to calculate the Q10 value at the seven UQr/UQt values. To assess the robustness of these calculated Q10 values, we repeated the above calculations using the highest and lowest 95% confidence interval values fitted to the respiration-UQr/UQt plots.
ACKNOWLEDGMENTS
The technical assistance of David Sherlock is gratefully acknowledged, as is the valuable advice given by Dr. Marcel Hoefnagel during the lead up to this study.
Footnotes
This work was supported by a Royal Society Research Grant and Australian Research Council Postdoctoral Fellowship (to O.K.A.) and by an Australian Research Council Grant (to J.T.W.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010326.
LITERATURE CITED
- Atkin OK, Edwards EJ, Loveys BR. Response of root respiration to changes in temperature and its relevance to global warming. New Phytol. 2000a;147:141–154. [Google Scholar]
- Atkin OK, Evans JR, Ball MC, Lambers H, Pons TL. Leaf respiration of snow gum in the light and dark: interactions between temperature and irradiance. Plant Physiol. 2000b;122:915–923. doi: 10.1104/pp.122.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin OK, Holly C, Ball MC. Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature: the importance of changes in the capacity and temperature sensitivity of respiration. Plant Cell Environ. 2000c;23:15–26. [Google Scholar]
- Azcón-Bieto J. Relationships between photosynthesis and respiration in the dark in plants. In: Barber J, Guerrero MG, Medrano H, editors. Trends in Photosynthesis Research. Andover, NH: Intercept Ltd; 1992. pp. 241–253. [Google Scholar]
- Azcón-Bieto J, Lambers H, Day DA (1983) Respiratory properties of developing bean and pea leaves. Aust J Plant Physiol 237–245
- Azcón-Bieto J, Osmond CB. Relationship between photosynthesis and respiration: the effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiol. 1983;71:574–581. doi: 10.1104/pp.71.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JA, Raison JK. Responses of macrophytes to temperature. In: Lange OL, Nobel PS, Osmond CB, Zeigler H, editors. Physiological Plant Ecology I. Responses to the Physical Environment. Berlin: Springer-Verlag; 1981. pp. 277–338. [Google Scholar]
- Breeze V, Elston J. Some effects of temperature and substrate content upon respiration and the carbon balance of field beans (Vicia faba L.) Ann Bot. 1978;42:863–876. [Google Scholar]
- Day DA, Krab K, Lambers H, Moore AL, Siedow JN, Wagner AM, Wiskich JT. The cyanide-resistant oxidase: to inhibit or not to inhibit, that is the question. Plant Physiol. 1996;110:1–2. doi: 10.1104/pp.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DA, Lambers H. The regulation of glycolysis and electron transport in roots. Physiol Plant. 1983;58:155–160. [Google Scholar]
- Day DA, Neuburger M, Douce R. Biochemical characterization of chlorophyll-free mitochondria from pea leaves. Aust J Plant Physiol. 1985;12:219–228. [Google Scholar]
- Diolez P, Kesseler A, Haraux F, Valerio M, Brinkmann K, Brand MD. Regulation of oxidative phosphorylation in plant mitochondria. Biochem Soc Trans. 1993;21:769–773. doi: 10.1042/bst0210769. [DOI] [PubMed] [Google Scholar]
- Douce R, Neuburger M. The uniqueness of plant mitochondria. Ann Rev Plant Physiol Mol Biol. 1989;40:371–414. [Google Scholar]
- Dry IB, Moore AL, Day DA, Wiskich JT. Regulation of alternative pathway activity in plant mitochondria: nonlinear relationship between electron flux and redox poise of the quinone pool. Arch Biochem Biophys. 1989;273:148–157. doi: 10.1016/0003-9861(89)90173-2. [DOI] [PubMed] [Google Scholar]
- Gonzàlez Meler MA, Ribas Carbo M, Giles L, Siedow JN. The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiol. 1999;120:765–772. doi: 10.1104/pp.120.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlob-McHugh SG, Sangwan RS, Vanlerberghe GC, Ko K, Turpin DH, Plaxton WC, Miki BL, Dennis DT. Normal growth of transgenic tobacco plants in the absence of cytosolic pyruvate kinase. Plant Physiol. 1992;100:820–825. doi: 10.1104/pp.100.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Rich PR, Zhang QS, Wiskich JT. Substrate kinetics of the plant mitochondrial alternative oxidase and the effects of pyruvate. Plant Physiol. 1997;115:1145–1153. doi: 10.1104/pp.115.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefnagel MHN, Wiskich JT. Activation of the plant alternative oxidase by high reduction levels of the Q-pool and pyruvate. Arch Biochem Biophys. 1998;355:262–270. doi: 10.1006/abbi.1998.0737. [DOI] [PubMed] [Google Scholar]
- Kiener CM, Bramlage WJ. Temperature effects on the activity of the alternative respiratory pathway in chill-sensitive Cucumis sativus. Plant Physiol. 1981;68:1474–1478. doi: 10.1104/pp.68.6.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Atkin OK, Scheurwater I. Respiratory patterns in roots in relation to their functioning. In: Waisel Y, Eshel A, Kafkaki K, editors. Plant Roots. The Hidden Half. New York: Marcel Dekker, Inc.; 1996. pp. 323–362. [Google Scholar]
- Lambers H, Steingrover E. Efficiency of root respiration of a flood-tolerant and a flood-intolerant Senecio species as affected by low oxygen tension. Physiol Plant. 1978;42:179–184. [Google Scholar]
- Larigauderie A, Körner C. Acclimation of leaf dark respiration to temperature in alpine and lowland plant species. Ann Bot. 1995;76:245–252. [Google Scholar]
- Lin T-Y, Markhart IIIAH. Temperature effects on mitochondrial respiration in Phaseolus acutifolius A. Gray and Phaseolus vulgaris L. Plant Physiol. 1990;94:54–58. doi: 10.1104/pp.94.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McNulty AM, Cummins WR. The relationship between respiration and temperature in leaves of the arctic plant Saxifraga cernua. Plant Cell Environ. 1987;10:319–325. [Google Scholar]
- Millar AH, Atkin OK, Menz RI, Henry B, Farquhar G, Day DA. Analysis of respiratory chain regulation in roots of soybean seedlings. Plant Physiol. 1998a;117:1083–1093. doi: 10.1104/pp.117.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Day DA. Nitric oxide inhibits the cytochrome oxidase but not the alternative oxidase of plant mitochondria. FEBS Lett. 1996;398:155–158. doi: 10.1016/s0014-5793(96)01230-6. [DOI] [PubMed] [Google Scholar]
- Millar AH, Day DA. Alternative solutions to radical problems. Trends Plant Sci. 1997;2:289–290. [Google Scholar]
- Millar AH, Hoefnagel MHN, Day DA, Wiskich JT. Specificity of the organic acid activation of alternative oxidase in plant mitochondria. Plant Physiol. 1996;111:613–618. doi: 10.1104/pp.111.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Saeed S, Jenner HL, Knorpp C, Leaver CJ, Hill SA. Control and regulation of the tribcarboxylic acid cycle in potato tubers. In: Moller IM, Gardeström P, Glimelius K, Glaser E, editors. Plant Mitochondria: From Gene to Function. Leiden, The Netherlands: Backhuys Publishers; 1998b. pp. 551–557. [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA. Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Lett. 1993;329:259–262. doi: 10.1016/0014-5793(93)80233-k. [DOI] [PubMed] [Google Scholar]
- Moore AL, Dry IB, Wiskich JT. Measurement of the redox state of the ubiquinone pool in plant-mitochondria. FEBS Lett. 1988;235:76–80. [Google Scholar]
- Moore AL, Gemel J, Randall DD. The regulation of pyruvate dehydrogenase activity in pea leaf mitochondria: the effect of respiration and oxidative phosphorylation. Plant Physiol. 1993;103:1431–1435. doi: 10.1104/pp.103.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy MK, Andrews CJ. Effect of temperature on respiration of mitochondria and shoot segments from cold-hardended and nonhardened wheat and rye seedlings. Plant Physiol. 1975;56:703–706. doi: 10.1104/pp.56.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues. Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Purvis AC, Shewfelt RL, Gegogeine JW. Superoxide production by mitochondria isolated from green bell pepper fruit. Physiol Plant. 1995;94:743–749. [Google Scholar]
- Ribas Carbo M, Aroca R, Gonzàlez Meler MA, Irigoyen JJ, Sanchezdiaz M. The electron partitioning between the cytochrome and alternative respiratory pathways during chilling recovery in two cultivars of maize differing in chilling sensitivity. Plant Physiol. 2000;122:199–204. doi: 10.1104/pp.122.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychter AM, Ciesla E, Kacperska A. Participation of the cyanide-resistant pathway in respiration of winter rape leaves as affected by plant cold acclimation. Physiol Plant. 1988;73:299–304. [Google Scholar]
- Smakman G, Hofstra RJJ. Energy metabolism of Plantago lanceolata, as affected by change in root temperature. Physiol Plant. 1982;56:33–37. [Google Scholar]
- Stewart CR, Martin BA, Reding L, Cerwick S. Respiration and alternative oxidase in corn seedlings during germination at different temperatures. Plant Physiol. 1990a;92:755–760. doi: 10.1104/pp.92.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Martin BA, Reding L, Cerwick S. Seedling growth, mitochondrial characteristics, and alternative respiratory capacity of corn genotypes differing in cold tolerance. Plant Physiol. 1990b;92:761–766. doi: 10.1104/pp.92.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Mooney PJF, Burrell MM, Fell DA. Finite change analysis of glycolytic intermediates in tuber tissue of lines of transgenic potato (Solanum tuberosum) overexpressing phosphofructokinase. Biochem J. 1997;322:111–117. doi: 10.1042/bj3220111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Siedow JN. Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiol. 1993;103:845–854. doi: 10.1104/pp.103.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach AL, Wiskich JT, Siedow JN. Regulation of alternative oxidase kinetics by pyruvate and intermolecular disulfide bond redox status in soybean seedling mitochondria. FEBS Lett. 1994;348:181–184. doi: 10.1016/0014-5793(94)00600-8. [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, Mcintosh L. Lower growth temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiol. 1992;100:115–119. doi: 10.1104/pp.100.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager HG. On the respiration and carbon assimilation rates of some arctic plants as related to temperature. New Phytol. 1941;40:1–19. [Google Scholar]
- Wagner AM, Krab K. The alternative respiration pathway in plants: role and regulation. Physiol Plant. 1995;95:318–325. [Google Scholar]
- Weger HG, Guy RD. Cytochrome and alternative pathway respiration in white spruce (Picea glauca) roots: effects of growth and measurement temperature. Physiol Plant. 1991;83:675–681. [Google Scholar]
- Wiskich JT, Dry IB. The tricarboxylic acid cycle in plant mitochondria: its operation and regulation. Encycl Plant Physiol. 1985;85:281–313. [Google Scholar]