Abstract
Chemotherapy of leishmaniasis is mainly based on antimonials. However, they are extremely toxic and cause serious side effects, and there is a worldwide increasing frequency of chemoresistance to antimonials. These issues emphasize the urgent need for affordable alternative drugs against leishmaniasis. Leishmania cysteine proteases are essential for parasite growth, differentiation, pathogenicity, and virulence and are thus attractive targets for combating leishmaniasis. Herein we demonstrate that the cysteine protease inhibitors aziridine-2,3-dicarboxylates 13b and 13e impaired promastigote growth at mid-micromolar concentrations and decreased the infection rate of peritoneal macrophages at concentrations 8- to 13-fold lower than those needed to inhibit parasite replication. Simultaneous treatment of infected cells with compound 13b and gamma interferon resulted in an even further reduction of the concentration needed for a significant decrease in macrophage infection rate. Notably, treatment with the compounds alone modulated the cytokine secretion of infected macrophages, with increased levels of interleukin-12 and tumor necrosis factor alpha. Furthermore, the decreased infection rate in the presence of compound 13b correlated with increased nitric oxide production by macrophages. Importantly, at the concentrations used herein, compounds 13b and 13e were not toxic against fibroblasts, macrophages, or dendritic cells. Together, these results suggest that the aziridine-2,3-dicarboxylates 13b and 13e are potential antileishmanial lead compounds with low toxicity against host cells and selective antiparasitic effects.
Chemotherapy against leishmaniasis is based mainly on antimony compounds, initially described in 1912 by Vianna (42) in Brazil as trivalent antimonials [Sb(III)]. These compounds exhibit high toxicity and a narrow therapeutic window, issues that led to the development of the pentavalent antimonium [Sb(V)] agents sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime), introduced around 1940 (31, 38). Pentavalent antimonium compounds exhibit a wider therapeutic window and thus became the drugs of choice against leishmaniasis. However, their toxicity causes serious side effects that often result in patients deserting the treatment. Furthermore, there is a worldwide increasing frequency of chemoresistance to antimonials (31). Second-line drugs, such as pentamidine and amphotericin B, display often serious liver and heart toxicities, require continuous clinical surveillance, and remain expensive for countries in which leishmaniasis is endemic. All these issues emphasize the urgent need for affordable alternative drugs against leishmaniasis (11).
One promising strategy to develop new leishmanicidal drugs has been to target the parasites' cysteine proteases (CPs) (25). CPs of Leishmania are essential for growth, differentiation, and pathogenicity (9, 24) and play crucial roles in host-parasite interactions (27, 28). The relative lack of redundancy of CPs in parasites compared to their mammalian hosts, as well as the unique functions fulfilled by parasite CPs (although they share structural homology with mammalian CPs), makes them attractive targets for the development of new strategies of antiparasitic chemotherapy (25, 28). Leishmania major expresses CPs of the clans CA, CD, CF, and PC(C), as well as one CP inhibitor (28). Most of the proteases belong to the clan CA, family C1 (papain-like enzymes), and are designated CPB (eight enzymes in L. major) or CPA (one enzyme in L. major), for cathepsin L-like proteases, or CPC (one enzyme in L. major), for cathepsin B-like proteases (28), the cathepsin L-like CPB enzymes being the most widely studied.
Some CPs play roles common to eukaryotes, such as lysosomal function, or specific ones, unique to the parasite (28). CP expression is higher in metacyclic stages and amastigotes than in promastigotes (4, 29, 37), and CP inhibition impairs intracellular Leishmania proliferation (34). In addition, Leishmania virulence in vivo requires multiple CPs (12, 28), and disruption of amastigote CP genes weakens Leishmania infection and pathogenesis (29).
Studies in mouse models of leishmaniasis demonstrated that the host defense against L. major infection depends on the interleukin-12 (IL-12)-driven expansion of T-helper 1 (Th1) cells, production of gamma interferon (IFN-γ) mediating macrophage activation, and release of nitric oxide (NO) (6, 15, 16, 41). Several parasite macromolecules are putative NO targets, but it has recently been revealed that NO-releasing compounds inhibit CPs of Plasmodium falciparum, Trypanosoma cruzi, and Leishmania infantum in a dose-dependent manner (18). The host CPs involved in antigen processing are not well defined. Of note, both lysosomal cathepsin L- and cathepsin B-like proteases are crucial for the immune response during Leishmania infection (23, 30, 45).
In the search for novel pharmacophores that may serve as antileishmanial lead compounds, we compared the ability of 38 aziridine-2,3-dicarboxylates, which contain either proteinogenic [Gly, (S)-Leu, (S)-Pro, (S)-Ala, and (S)-Phe] or nonproteinogenic [(R)-Leu, (R)-Pro, (R)-Ala, (R)-Phe, (S)-Azy, (R+S)-Azet, (R)-Pip, (S)-Pip, (R+S)-Nip, and Ini] amino acids and were developed as peptidomimetic CP inhibitors (43), to inhibit the growth of L. major promastigotes, J774.1 macrophages, and NIH 3T3 fibroblasts and to affect the survival of dendritic cells and peritoneal macrophages. In addition, we evaluated the efficacies of selected aziridine-2,3-dicarboxylates to decrease the infection rate of macrophages and to regulate their cytokine and NO production.
Importantly, the aziridine-2,3-dicarboxylates evaluated here belong to a group of irreversible CP inhibitors (39, 40) with high selectivity for cathepsin L-like parasite CPs (43). The trans-configured aziridine ring [designated Azi, either in (S,S) or in (R,R) configuration] of the scrutinized compounds reacts as an electrophilic building block alkylating the active-site cysteine residue of the target enzyme, while the peptidic/peptidomimetic sequences address the substrate-binding pockets. The results of the present study show that the CP inhibitors 13b and 13e impair the growth of L. major promastigotes and decrease the infection rate of macrophages. Moreover, the compounds modulated the cytokine secretion and stimulated NO production by infected macrophages.
MATERIALS AND METHODS
Aziridine-2,3-dicarboxylates.
The compounds (Table 1; Fig. 1A) were prepared as peptide and peptidomimetic derivatives of trans-aziridine-2,3-dicarboxylic acids (43). For in vitro studies, the compounds were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Taufkirchen, Germany) and further diluted in medium.
TABLE 1.
In vitro activity of aziridine-2,3-dicarboxylates against L. major promastigotes and J774.1 macrophagesb
| Compound | Structure | IC50 (μM)
|
Indexa | |
|---|---|---|---|---|
| L. major | J774.1 | |||
| 1c+d | Cbz-(R+S)-Azet-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 2a | Boc-(S)-Pro-(S,S)-Azi(OEt)2 | >100 | >100 | ND |
| 2c | Boc-(S)-Pro-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 3a | Boc-(S)-Pip-(S,S)-Azi(OEt)2 | >100 | >100 | ND |
| 3b | Boc-(R)-Pip-(S,S)-Azi(OEt)2 | >100 | >100 | ND |
| 3c | Boc-(S)-Pip-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 3d | Boc-(R)-Pip-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 4c | Cbz-(S)-Pip-(R,R)-Azi(OEt)2 | >100 | 33.57 ± 0.23 | <1.00 |
| 4d | Cbz-(R)-Pip-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 5a+b | Boc-(R+S)-Nip-(S,S)-Azi(OEt)2 | >100 | >100 | ND |
| 5c+d | Boc-(R+S)-Nip-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 6a | Boc-Ini-(S,S)-Azi(OEt)2 | >100 | >100 | ND |
| 6c | Boc-Ini-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 7c | Cbz-(S)-Leu-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 8a | Boc-(S)-Leu-(S)-Pro-(S,S)-Azi(OEt)2 | >100 | >100 | ND |
| 8c | Boc-(S)-Leu-(S)-Pro-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 9a+b | Boc-(S)-Leu-(R+S)-Nip-(S,S)-Azi(OEt)2 | >100 | >100 | ND |
| 9c+d | Boc-(S)-Leu-(R+S)-Nip-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 10a | Boc-(S)-Leu-Ini-(S,S)-Azi(OEt)2 | >100 | >100 | ND |
| 10c | Boc-(S)-Leu-Ini-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 11c+d | Boc-(S)-Leu-(R+S)-Azet-(R,R)-Azi(OEt)2 | >100 | >100 | ND |
| 12a+b | Boc-(S)-Leu-(R+S)-Azet-(S,S)-Azi(OBn)2 | 32.05 ± 0.35 | 41.05 ± 3.04 | >1.00 |
| 13a | Boc-(S)-Leu-(S)-Pro-(S,S)-Azi(OBn)2 | >100 | >125 | ND |
| 13b | Boc-(S)-Leu-(R)-Pro-(S,S)-Azi(OBn)2 | 39.59 ± 4.42 | >100 | >2.00 |
| 13c | Boc-(S)-Leu-(S)-Pro-(R,R)-Azi(OBn)2 | >100 | >125 | ND |
| 13e | Boc-(R)-Leu-(S)-Pro-(S,S)-Azi(OBn)2 | 35.36 ± 2.40 | >100 | >2.00 |
| 13f | Boc-(R)-Leu-(R)-Pro-(S,S)-Azi(OBn)2 | 72.00 | >100 | >1.00 |
| 14a | Boc-Gly-(S)-Pro-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 14b | Boc-Gly-(R)-Pro-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 16a+b | Boc-(S)-Leu-(R+S)-Nip-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 16e+f | Boc-(R)-Leu-(R+S)-Nip-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 17a+b | Boc-Gly-(R+S)-Nip-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 18a | Boc-(S)-Leu-(S)-Azy-(S,S)-Azi(OBn)2 | 66.89 ± 4.11 | 61.67 ± 27.00 | <1.00 |
| 18e | Boc-(R)-Leu-(S)-Azy-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 19a | Boc-(S)-Phe-(S)-Ala-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 19b | Boc-(S)-Phe-(R)-Ala-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 19e | Boc-(R)-Phe-(S)-Ala-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
| 19f | Boc-(R)-Phe-(R)-Ala-(S,S)-Azi(OBn)2 | >100 | >100 | ND |
IC50 towards J774.1 divided by IC50 towards L. major promastigotes. ND, not determined.
Where applicable, values include means ± standard errors of the means. Abbreviations: Ala, alanine; Azet, azetidine-2-carboxylic acid; Azi, aziridine-2,3-dicarboxylic acid; Azy, aziridine-2-carboxylic acid; Bn, benzyl; Boc, tert-butoxycarbonyl; Cbz, benzyloxy carbonyl; Et, ethyl; Gly, glycine; Ini, isonipecotic acid; Leu, leucine; Nip, nipecotic acid; Phe, phenylalanine; Pip, pipecolic acid; Pro, proline; R, any residue.
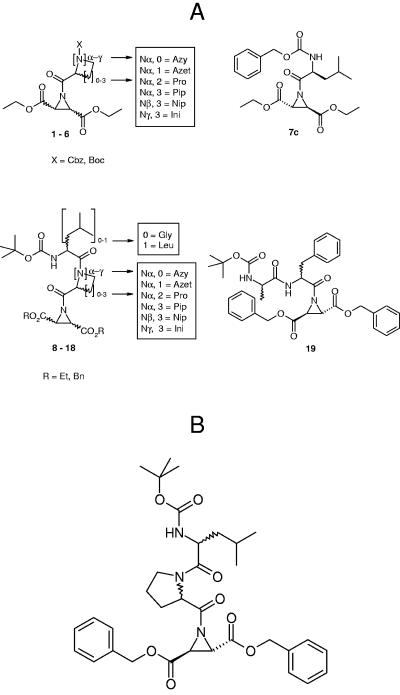
FIG. 1.
(A) Schematic representation of the structures of the investigated aziridine-2,3-dicarboxylates: compounds 1 to 6 are aziridinyl dipeptides of the general structure X-Caa-Azi(OEt)2 with Caa as cyclic amino acid, compound 7c is the aziridinyl dipeptide Cbz-Leu-Azi(OEt)2, compounds 8 to 18 are aziridinyl tripeptides of the general structure Boc-Leu(or Gly)-Caa-Azi(OR)2, and compound 19 is the aziridinyl tripeptide of the general structure Boc-Phe-Ala-Azi(OBn)2. For detailed structures and absolute stereoconfiguration of the compounds see Table 1. Abbreviations used: Azet, azetidine-2-carboxylic acid; Azy, aziridine-2-carboxylic acid; Bn, benzyl; Boc, tert-butoxycarbonyl; Cbz, benzyloxy carbonyl; Et, ethyl; Gly, glycine; Ini, isonipecotic acid; Leu, leucine; Nip, nipecotic acid; Pip, pipecolic acid; Pro, proline; R, any residue. (B) Structures of compounds 13b [Boc-(S)-Leu-(R)-Pro-(S,S)-Azi(OBn)2] and 13e [Boc-(R)-Leu-(S)-Pro-(S,S)-Azi(OBn)2].
Parasites.
The cloned virulent L. major isolate MHOM/IL/81/FE/BNI was maintained by passage in BALB/c mice. Promastigotes were grown in blood agar cultures at 26°C, 5% CO2, and 95% humidity. For the experiments described here, promastigotes were washed twice with phosphate-buffered saline (PBS) and suspended at 1 × 108 cells ml−1 in Click RPMI 1640 medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal calf serum (FCS; PAA Laboratories, Linz, Austria), 2 mM l-glutamine (Biochrom, Berlin, Germany), 10 mM HEPES buffer (pH 7.2) (Invitrogen), 100 μg ml−1 penicillin, 160 μg ml−1 gentamicin, 7.5% NaHCO3, and 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich) (complete medium).
Cells and cell lines.
The macrophage cell line J774.1 was maintained in complete medium. For the experimental procedures, cells were detached from the flasks with a rubber policeman, washed twice with PBS, and suspended at 2 × 106 cells ml−1 in complete medium. Peritoneal macrophages and bone marrow-derived dendritic cells (BMDCs) were obtained from naive BALB/c mice (6 to 8 weeks old) (Charles River Breeding Laboratories, Sulzfeld, Germany). Resident peritoneal exudate cells were obtained by peritoneal washing with 5 ml of ice-cold complete medium. The cells were subsequently incubated for 10 min with ice-cold TAC buffer (10 mM NH4Cl in PBS [pH 7.2]) to eliminate erythrocytes, centrifuged, and suspended at 2 × 106 cells ml−1 in complete medium. Macrophages constituted the major population of peritoneal exudate cells (80% expressed the macrophage surface marker F4/80, and no IL-4 and IFN-γ were detected in cell culture supernatants). BMDCs were generated from bone marrow progenitors as described previously (22, 35). Briefly, bone marrow cells were cultured in Click RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 10 mM HEPES buffer (pH 7.2), 60 μg ml−1 penicillin, and 20 μg ml−1 gentamicin, in the presence of 200 U ml−1 of granulocyte-macrophage colony-stimulating factor (PeproTech, London, United Kingdom). Cultures were fed with granulocyte-macrophage colony-stimulating factor on days 3, 6, and 8. At day 10, the nonadherent cells were collected and washed with PBS. Cells were suspended at 2 × 106 cells ml−1 in complete medium. These cells show typical dendritic-cell morphology with a myeloid dendritic-cell phenotype (35). The mouse embryo fibroblast cell line NIH 3T3 was grown to 80 to 90% confluence in Dulbecco's modified Eagle medium (DMEM; Invitrogen) supplemented with 10% FCS, 0.1 mM nonessential amino acids, 100 U ml−1 penicillin, and 100 U ml−1 streptomycin, on gelatin-coated (Sigma-Aldrich) (0.1% in PBS) cell culture dishes, at 37°C in 5% CO2 at 95% humidity. For the experimental procedures cells were detached from the flasks with a rubber policeman, washed with PBS, and suspended in DMEM at 2 × 106 cells ml−1.
Analysis of in vitro antiproliferative activity.
Promastigotes were seeded into 96-well plates in complete medium without phenol red (200 μl), in the absence or presence of increasing concentrations of aziridine-2,3-dicarboxylates. They were then incubated for 24 h at 26°C, 5% CO2, and 95% humidity. Following the addition of 20 μl of Alamar Blue (Trinova Biochem, Gieβen, Germany), the plates were incubated again and the optical densities (ODs) measured 24 and 48 h later with a Multiskan Ascent enzyme-linked immunosorbent assay (ELISA) reader (Thermo Electron Corporation, Dreieich, Germany) using a test wavelength of 540 nm and a reference wavelength of 630 nm (26). Absorbance in the absence of compounds was set as 100% of growth (26). J774.1 macrophages, peritoneal macrophages, and BMDCs were cultured in complete medium without phenol red, and fibroblasts in DMEM (200 μl) in the absence or presence of increasing concentrations of aziridine-2,3-dicarboxylates, for 24 h at 37°C, 5% CO2, and 95% humidity. Following the addition of 20 μl of Alamar Blue, the plates were incubated again and the ODs measured 24, 48, and 72 h later as described previously (1). Amphotericin B (Sigma-Aldrich) was used as a reference compound and positive control. The effects of cell density, incubation time, and the concentration of DMSO were examined in control experiments. The final concentration of DMSO in the medium never exceeded 1% (vol/vol) and had no effect on the proliferation of extracellular or intracellular parasites. For each experiment, each drug concentration was assayed in duplicate wells.
Analysis of macrophage infection rate.
Freshly isolated peritoneal macrophages (4 × 105 cells ml−1) were allowed to adhere in vitro for 2 h at 37°C, 5% CO2, and 95% air humidity. Nonadherent cells were removed by extensive washing with complete medium. Subsequently, adherent cells were infected for 24 h with stationary-phase L. major promastigotes at a parasite-to-macrophage ratio of 15 to 1, in a final volume of 0.5 ml of complete medium. After removal of extracellular parasites by thorough rinsing with fresh complete medium, cells were incubated further for 48 h in the absence of drugs or in the presence of increasing concentrations of compounds, with or without IFN-γ (100 U ml−1; BD Biosciences Pharmingen, Heidelberg, Germany). Amphotericin B was used as a reference compound. Intracellular parasites were quantified by staining with acridine orange (Sigma-Aldrich) and ethidium bromide (Sigma-Aldrich) and analyzed by fluorescence microscopy at 495 nm, as described previously (32).
Analysis of cytokine production.
Freshly isolated peritoneal macrophages (2 × 106 cells ml−1) were incubated in 24-well plates as described for the infection rate analysis. Subsequently, the cells were infected with L. major and treated with the compounds, in the absence or presence of IFN-γ (100 U ml−1) or lipopolysaccharide (LPS) (15 μg ml−1; Sigma-Aldrich), or with IFN-γ or LPS alone. Concentrations of 40 μM of compounds 13b and 13e and 1 μM of amphotericin B, close to the 50% inhibitory concentration (IC50) against L. major promastigotes, were used for this assay. After 24 h, the culture supernatants were collected and the levels of IL-1β, IL-6, IL-12, and tumor necrosis factor alpha (TNF-α) were quantified by sandwich ELISA using the following procedure. Flat-bottomed high-binding plates (Costar, Cambridge, MA) were coated by overnight incubation at 4°C with anticytokine monoclonal antibodies (BD Biosciences Pharmingen and R&D Systems, Wiesbaden, Germany) diluted in binding solution (0.1 M NaHCO3, pH 8.3, for IL-1β, IL-6, and IL-12; 0.1 M Na2HPO4, 0.1 M NaH2PO4, pH 6.0, for TNF-α), 50 μl per well. Sites for nonspecific binding of protein were blocked with 10% FCS in PBS and 0.05% Tween 20, 150 μl per well, for 4 h at room temperature (RT). The plates were then washed extensively with PBS-0.05% Tween 20. Samples or medium blanks were added (50 μl per well) and a standard curve was set up for each plate using dilutions of recombinant cytokines; plates were incubated again overnight at 4°C prior to adding the detection antibody dissolved in 1% FCS-0.05% Tween 20 in PBS (50 μl per well). After incubation for 1 h at RT, 50 μl of alkaline phosphatase-streptavidin complex (1:1,000) (Dako Cytomation, Glostrup, Denmark) was added to each well, and the plates were incubated at RT for 45 min. To detect the cytokines, the alkaline phosphatase substrate (Sigma-Aldrich) in 10% NH(CH2CH2OH)2 (Merck, Darmstadt, Germany) and 2 mM MgCl2 (Merck) at pH 9.8 were added to each well. The developing color in the wells was read at a test wavelength of 405 nm and a reference wavelength of 490 nm using the Multiskan Ascent ELISA reader. Data were calculated as nanograms per milliliter. Cytokines secreted by noninfected, untreated macrophages were normalized to 100% for further analysis of the results. The detection thresholds were 30.72 pg ml−1 for IL-1β, 39.0 pg ml−1 for IL-4, 27.24 pg ml−1 for IL-6, 72.0 pg ml−1 for IL-12, 222.0 pg ml−1 for IFN-γ, and 40.0 pg ml−1 for TNF-α.
Analysis of NO production.
Freshly isolated peritoneal macrophages (2 × 106 cells ml−1) were cultured in flat-bottomed microtiter wells as described for the infection rate analysis. The cells were washed 24 h later and incubated for a further 48 h with the compounds in the absence or presence of IFN-γ or LPS. Thereafter, the supernatants were collected and the concentration of nitrite (NO2−) was determined by addition of 100 μl of culture supernatant to 100 μl of Griess reagent (1% sulfanilamide, 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride, 2.5% phosphoric acid) (Sigma-Aldrich) and incubation for 15 min at RT (19). The absorbance of the reaction product at 540 nm was measured with the Multiskan Ascent ELISA reader using a test wavelength of 540 nm and a reference wavelength of 630 nm. The nitrite concentrations were determined using sodium nitrite as a standard. Data are expressed as micromolar concentrations of NO2−. Nitrite concentrations reflect the NO levels released by macrophages.
Statistical analysis.
Data on antiproliferative activity (from at least two experiments) were analyzed with Thermo Electron Ascent software (Thermo Electron Corporation) and Microsoft Excel. OD values at 48 h were used to calculate the drug concentrations that inhibit 50% cell growth or cell survival (IC50) via linear interpolation (21). Data on infection rate are expressed as mean values ± standard errors of the means of at least three experiments in which 300 macrophages were analyzed for each drug concentration. The program Graph-pad was used to fit the data to a nonlinear regression and to describe the concentration that decreases the infection rate to 50% (the 50% effective concentration [EC50]). Data on cytokine levels and on nitrite concentrations are expressed as mean values ± standard errors of the means of at least two experiments. Differences in IC50, EC50, cytokine levels, and NO2− concentrations of treated or untreated macrophages were tested for statistical significance by the unpaired Student's t test using the Graph-pad program.
RESULTS
Antiproliferative activity of aziridine-2,3-dicarboxylates against L. major promastigotes and the macrophage cell line J774.1.
The cytotoxic activity of 38 aziridine-2,3-dicarboxylates (Fig. 1A) against L. major promastigotes and the macrophage cell line J774.1 was tested in vitro. The majority of the compounds (Table 1) did not impair Leishmania proliferation; compound 4c did not affect Leishmania proliferation but inhibited the growth of the macrophage cell line J774.1 (IC50, 33.57 ± 0.23 μM). Treatment of L. major promastigotes with compounds 12a+b, 13b, 13e, and 18a resulted in a dose-dependent inhibition of parasite growth. The IC50 values were 32.05 ± 0.35, 39.59 ± 4.42, 35.36 ± 2.40, and 66.89 ± 4.11 μM, respectively. Compounds 12a+b (IC50, 41.05 ± 3.04 μM) and 18a (IC50, 61.67 ± 27.00 μM) also impaired the growth of the J774.1 macrophage cell line. In contrast, compounds 13b and 13e (structures illustrated in Fig. 1B) were not toxic against the macrophage cell line, even at concentrations of 100 μM, suggesting a selective antiparasitic effect. The IC50 of amphotericin B, which was used as a reference compound, was 5.07 ± 0.05 μM for L. major promastigotes, without affecting the growth of J774.1 macrophages. Together, these results demonstrate that the aziridine-2,3-dicarboxylates 13b and 13e are effective against Leishmania promastigotes but do not impair the growth of the J774.1 macrophage cell line.
Cytotoxic activity of aziridine-2,3-dicarboxylates against macrophages, BMDCs, and NIH 3T3 fibroblasts.
Since the results described above indicate that two of the aziridine-2,3-dicarboxylates are toxic against Leishmania parasites at concentrations that do not affect the growth of J774.1 macrophages, we analyzed the influence of compounds 13b and 13e on the growth of NIH 3T3 fibroblasts as well as on the survival of freshly isolated macrophages and in vitro-generated BMDCs from BALB/c mice. The different cell types were treated with increasing concentrations of these compounds. No deleterious effects were observed against NIH 3T3 fibroblasts and peritoneal macrophages even at 100 μM, and concentrations higher than 80 μM were needed to reach the IC50 against BMDCs (data not shown), confirming the low toxicity of compounds 13b and 13e against host cells and their selective antiparasitic effect.
Effect of aziridine-2,3-dicarboxylates on the infection rate of macrophages.
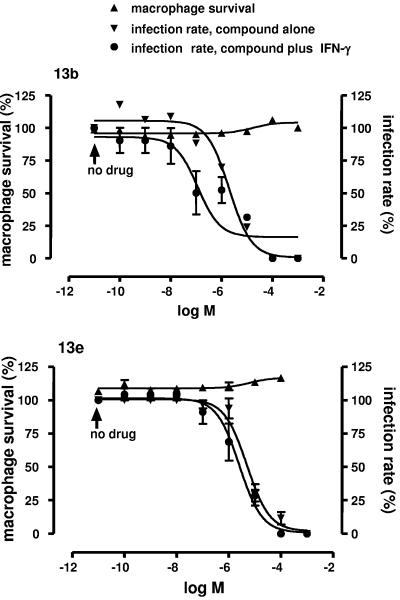
To further analyze the antileishmanial activity of compounds 13b and 13e, freshly isolated peritoneal macrophages were infected with L. major for 24 h and then treated with increasing concentrations of the compounds for 48 h. Figure 2 presents a summary of the data for macrophage survival and decrease in infection rate. Macrophages that were not treated with compounds had infection rates of 20 to 30%, consistent within each experiment. The infection rates of untreated macrophages were normalized to 100% for further analysis of the results. Macrophage survival was not affected by the compounds 13b and 13e (Fig. 2). Treatment of infected macrophages with compounds 13b and 13e resulted in a dose-dependent decrease in infection rate. A 50% decrease in infection rate (EC50) was obtained at concentrations of 2.71 ± 0.09 μM and 4.98 ± 0.08 μM, respectively, for compounds 13b and 13e (Fig. 2). Treatment of infected macrophages with IFN-γ decreased the infection rate by 14 to 25%, and simultaneous treatment of these cells with the compounds plus IFN-γ (Fig. 2) decreased the EC50 to 0.158 ± 0.026 μM (P < 0.005) for compound 13b but did not modify the EC50 for compound 13e. The EC50 for amphotericin B, which was used as a reference compound, was 0.075 ± 0.019 μM. These results indicate that a significant decrease in macrophage infection rate is obtained at concentrations of compounds 13b and 13e that are 8- to 13-fold lower than those needed to inhibit the growth of L. major promastigotes and that there is a synergistic effect of compound 13b and IFN-γ. Notably, the toxicity against cell lines and freshly isolated cells from BALB/c mice is at least 16-fold lower than that against intracellular amastigotes.
FIG. 2.
Dose-response curves showing the effects of compounds 13b (upper panel) and 13e (lower panel) on macrophage survival and macrophage infection rate in the absence or presence of IFN-γ. Macrophages were infected with stationary-phase L. major promastigotes for 24 h. After removal of extracellular parasites, the cells were incubated further for 48 h in the absence of drugs, or in the presence of increasing concentrations of compounds with or without IFN-γ (100 U ml−1). The arrows point out normalized survival and infection rates of macrophages incubated without drugs, i.e., in the absence or presence of IFN-γ alone.
Modulation of macrophage cytokine secretion by aziridine-2,3-dicarboxylates.
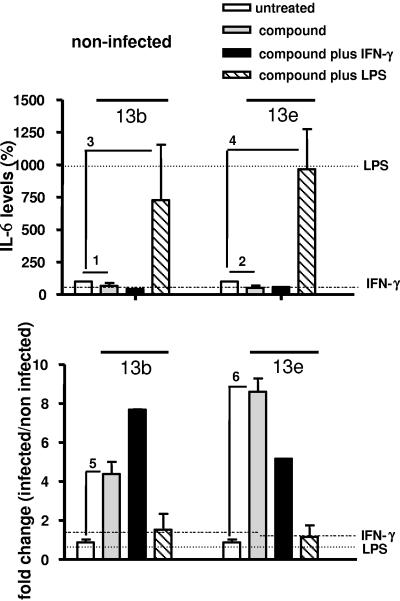
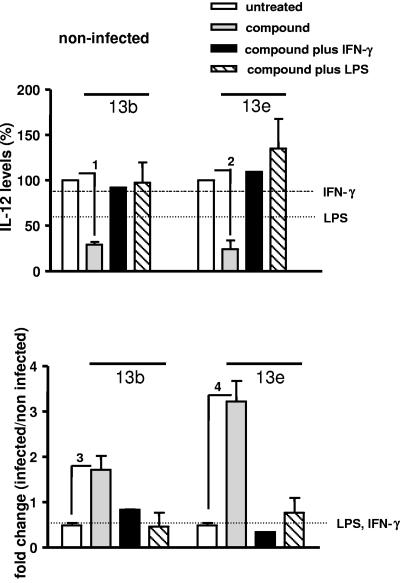
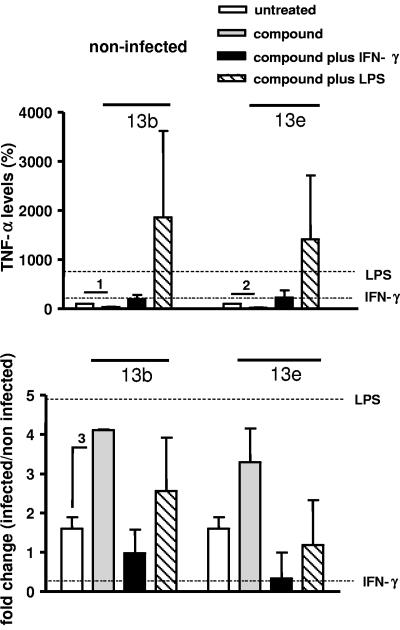
Next, we analyzed the effect of aziridine-2,3-dicarboxylates on the production of cytokines by peritoneal macrophages. To this end, we determined the levels of the proinflammatory cytokines IL-6 and TNF-α as well as the amount of IL-12, which is pivotal in inducing a Th1 response, in culture supernatants of noninfected or infected cells treated either with compounds 13b and 13e alone or simultaneously with two types of macrophage stimulators, the cytokine IFN-γ or bacterial LPS. Control supernatants were obtained from cells cultured with IFN-γ or LPS alone. Noninfected untreated cells secreted 5.058 ± 1.131 ng ml−1 of IL-6, 0.417 ± 0.014 ng ml−1 of IL-12, and 0.072 ± 0.013 ng ml−1 of TNF-α. Treatment of noninfected cells with IFN-γ alone did not affect cytokine secretion, while treatment with LPS alone increased IL-6 and TNF-α levels and decreased the IL-12 level (Fig. 3, 4, and 5, upper panels). After treatment with compounds 13b and 13e, IL-6, IL-12, and TNF-α levels decreased in noninfected cells (Fig. 3, 4, and 5, upper panels). Compared to untreated cells, the cytokine levels did not change in noninfected cells incubated with compounds plus IFN-γ. In the presence of compounds plus LPS, IL-6 levels increased considerably, while the enhancement of TNF-α production was statistically not significant. Moreover, the increased IL-6 and TNF-α levels in response to compounds plus LPS were not significantly different from those observed after exposure to LPS alone.
FIG. 3.
IL-6 secretion by macrophages. Macrophages were infected with stationary-phase L. major promastigotes for 24 h and treated with the compounds in the absence or presence of IFN-γ or LPS. (Upper panel) Percentages of IL-6 levels in the supernatants of noninfected macrophages cultured in the presence of compound 13b (40 μM) or compound 13e (40 μM), in the absence or presence of IFN-γ (100 U ml−1) or LPS (15 μg ml−1). (Lower panel) Change (n-fold) (infected/noninfected) of IL-6 levels in the supernatants of infected macrophages cultured in the presence of compound 13b or compound 13e, in the absence or presence of IFN-γ or LPS. The horizontal lines indicate changes in cytokine production induced by IFN-γ or LPS alone. A ratio of 1 means no change. Indicated comparisons 1, 5, and 6, P < 0.001; indicated comparisons 2, 3, and 4, P < 0.05.
FIG. 4.
IL-12 secretion by macrophages. Macrophages were infected with stationary-phase L. major promastigotes for 24 h and treated with the compounds in the absence or presence of IFN-γ or LPS. (Upper panel) Percentages of IL-12 levels in the supernatants of noninfected macrophages cultured in the presence of compound 13b (40 μM) or compound 13e (40 μM), in the absence or presence of IFN-γ (100 U ml−1) or LPS (15 μg ml−1). (Lower panel) Change (n-fold) (infected/noninfected) of IL-12 levels in the supernatants of infected macrophages cultured in the presence of compound 13b or compound 13e, in the absence or presence of IFN-γ or LPS. The horizontal lines indicate changes in cytokine production induced by IFN-γ or LPS alone. A ratio of 1 means no change. Indicated comparisons 1, 3, and 4, P < 0.001; indicated comparison 2, P < 0.005.
FIG. 5.
TNF-α secretion by macrophages. Macrophages were infected with stationary-phase L. major promastigotes for 24 h and treated with the compounds in the absence or presence of IFN-γ or LPS. (Upper panel) Percentages of TNF-α levels in the supernatants of noninfected macrophages cultured in the presence of compound 13b (40 μM) or compound 13e (40 μM), in the absence or presence of IFN-γ (100 U ml−1) or LPS (15 μg ml−1). (Lower panel) Change (n-fold) (infected/noninfected) of TNF-α levels in the supernatants of infected macrophages cultured in the presence of compound 13b or compound 13e, in the absence or presence of IFN-γ or LPS. The horizontal lines indicate changes in cytokine production induced by IFN-γ or LPS alone. A ratio of 1 means no change. Indicated comparisons 1 and 2, P < 0.005; indicated comparison 3, P < 0.001.
Infection with Leishmania did not affect the IL-6 level but induced a 1.6-fold increase of the TNF-α level and a 2-fold decrease of the IL-12 level (white bars in Fig. 3, 4, and 5, lower panels). Importantly, in infected cells treated with compound 13b, the levels of IL-6, IL-12, and TNF-α increased significantly (P < 0.001) (Fig. 3, 4, and 5, lower panels), and in infected cells treated with compound 13e, IL-6 (P < 0.001) and IL-12 (P < 0.005) increased significantly (Fig. 3 and 4, lower panels). Increased IL-6 levels were also obtained in the presence of compounds plus IFN-γ, but not after exposure to IFN-γ alone or after treatment with LPS (Fig. 3, lower panel). For the other cytokines, these modulations were not evident in cells treated simultaneously with the compounds and LPS or IFN-γ. Together, these findings suggest that aziridine-2,3-dicarboxylates modulate cytokine secretion by macrophages and that the effect of the compounds is influenced by macrophage activators.
Increased NO secretion by macrophages treated with aziridine-2,3-dicarboxylates.
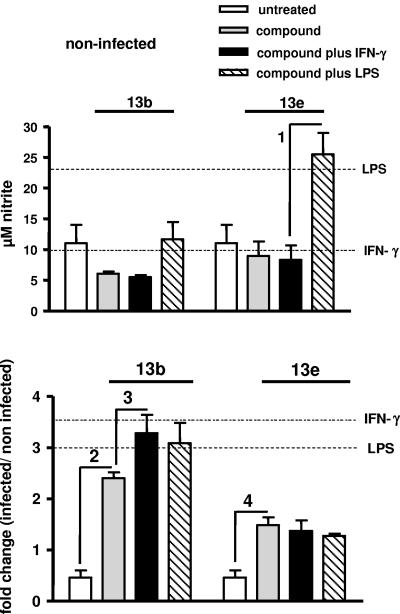
To analyze whether the decrease in macrophage infection rate observed in the presence of aziridine-2,3-dicarboxylates correlates with the capacity of macrophages to secrete NO, we evaluated the nitrite production in noninfected and infected cells treated with compounds 13b and 13e. The NO release was measured after 48 h of incubation of infected macrophages with the compounds (Fig. 6). Noninfected cells treated with compounds 13b and 13e secreted NO levels that were similar to those of noninfected untreated cells (Fig. 6, upper panel). These levels did not change in cells incubated with IFN-γ alone or with compounds plus IFN-γ (Fig. 6, upper panel) but increased in cells treated with LPS alone or with compound 13e plus LPS (P < 0.005).
FIG. 6.
NO production by macrophages. Macrophages were infected with stationary-phase L. major promastigotes for 24 h. Cells were then washed and incubated for 48 h further with the compounds in the absence or presence of IFN-γ or LPS. Thereafter, the culture supernatants were collected for determination of the nitrite concentrations. (Upper panel) Micromolar concentrations of NO2− in the supernatants of noninfected macrophages cultured in the presence of compound 13b (40 μM) or compound 13e (40 μM), in the absence or presence of IFN-γ (100 U ml−1) or LPS (15 μg ml−1). (Lower panel) Change (n-fold) (infected/noninfected) of NO levels in supernatants of infected macrophages cultured in the presence of compound 13b or compound 13e, in the absence or presence of IFN-γ or LPS. The horizontal lines indicate changes in NO production induced by IFN-γ or LPS alone. A ratio of 1 means no change. Indicated comparisons 1 and 2, P < 0.005; indicated comparisons 3 and 4, P < 0.05.
Infection with Leishmania promastigotes decreased NO production (Fig. 6, lower panel). These levels increased 3- or 3.5-fold in cells treated with LPS or IFN-γ alone, 2.5-fold in cells incubated with compound 13b alone (P < 0.005), and 3.1- to 3.3-fold in cells incubated simultaneously with compound 13b and LPS or IFN-γ (P < 0.05), respectively. NO production increased 1.5-fold in cells incubated with compound 13e (P < 0.05). Levels of NO did not increase in cells incubated with amphotericin B used as the control compound (data not shown). These results suggest that the decrease in infection rate observed with macrophages treated with aziridine-2,3-dicarboxylates correlates with a higher production of NO that is further increased in cells treated simultaneously with compound 13b and IFN-γ.
DISCUSSION
CPs are enzymes of great significance as virulence factors, and their relevance as drug targets and modulators of immune responses has been extensively studied (2, 28, 34). Since CPs are crucial for parasite metabolism, differentiation, and survival, an important strategy against leishmaniasis could be their selective inhibition (28). Herein, we demonstrate that the aziridine-2,3-dicarboxylates 13b and 13e, which are inhibitors of the cathepsin L subfamily of the papain clan of CPs (CAC1), e.g., of the cathepsin L-like proteases rhodesain (Ki, 1.1 to 1.2 μM) from Trypanosoma brucei rhodesiense (43) and falcipain 2 (Ki, 28 to 44 μM) from Plasmodium falciparum (17), are promising leishmanicidal pharmacophores.
The compounds 13b and 13e impaired the growth of L. major promastigotes with IC50 values around 40 μM and decreased the infection rate of macrophages with EC50 values of 3 to 5 μM. The IC50 of amphotericin B against extracellular parasites was 5.07 ± 0.05 μM, and its EC50 for decreasing macrophage infection rate was 0.075 + 0.019 μM, similar to what has been previously described (14). Compared to amphotericin B, compounds 13b and 13e were thus less potent against extracellular and intracellular parasites. However, at concentrations near their IC50 against extracellular parasites, these compounds, but not amphotericin B (36), modulated the secretion of cytokines relevant for the immune response against leishmaniasis and, most noteworthy, were able to stimulate the production of NO by infected macrophages. It is of interest that the EC50 value obtained for compound 13b, but not that obtained for compound 13e, decreased 17-fold when infected cells were simultaneously treated with IFN-γ. This different behavior of compounds 13b and 13e in the presence of IFN-γ may be suggestive of different mechanisms of action or distinct parasite targets of the two compounds. Importantly, at the concentrations tested in the present study, compounds 13b and 13e were not toxic against J774.1 macrophage and NIH 3T3 fibroblast lines or BMDCs and freshly isolated macrophages from BALB/c mice. Together, these results suggest that the activities of compounds 13b and 13e are mainly directed against the parasite.
The aziridine-2,3-dicarboxylates 13b and 13e decreased the rate of macrophage infection with L. major at concentrations that were 8- to 13-fold lower than those needed to inhibit the growth of promastigotes and more than 20-fold lower than those needed to affect the growth or survival of cell lines and cells freshly isolated from BALB/c mice. Contrary to what has been described for vinyl sulfone-based peptides (13), the inhibitors tested herein were selective for parasite over host cells and probably for parasite over host cell CPs; even at 100 μM, they did not affect host cell proliferation and survival but inhibited parasite growth and reduced the infection rate of macrophages.
The fact that CP expression is higher in amastigotes than in promastigotes of Leishmania sp. parasites (4, 28, 37) is in line with the finding that lower inhibitor concentrations decreased the infection rate of macrophages but did not kill promastigotes (Table 1; Fig. 2). Alternatively, this effect may be caused by a selective uptake mechanism at the macrophage plasma membrane with enhanced concentrations reaching the parasitophorous vacuole within the host cell. In order to address these open questions, it will be important to identify the cellular targets of aziridine-3,4-dicarboxylates and to analyze the transport kinetics of the compounds.
Th1 and Th2 cytokine profiles are induced by parasite CPs during murine cutaneous leishmaniasis (2); furthermore, Leishmania infection causes changes in cytokine production by macrophages (5, 8, 20, 44). Secretion of proinflammatory cytokines and chemokines after exposure to amphotericin B has been demonstrated to occur at concentrations higher than those used in our study (36). Notably, compounds 13b and 13e at concentrations near their IC50 against extracellular parasites modulate cytokine secretion by infected macrophages. In fact, compound 13b increases IL-6, IL-12, and TNF-α levels and compound 13e increases IL-6 and IL-12 levels in infected macrophages, while IL-1β levels were not modulated by the compounds (data not shown). Infection with Leishmania inhibits IL-12 secretion by macrophages (8), a cytokine that is critical for the induction of a protective Th1-cell immune response (14, 41).
Importantly, our finding that aziridine-2,3-dicarboxylates strongly upregulate IL-12 production by infected macrophages suggests that the compounds, besides facilitating parasite control by a direct leishmanicidal effect, may favor the development of a Th1 immune response that is essential for the host's ability to control Leishmania infection (41). This hypothesis needs further evaluation by in vivo experiments to link the in vitro results with the immune responses of animals to Leishmania infection.
The importance of reactive nitrogen intermediates produced by the inducible NO synthase (iNOS) in murine leishmaniasis is well established (6). NO synthesis is enhanced significantly in infected macrophages stimulated with IFN-γ (33), and the NO is involved in the intracellular killing of Leishmania (6). Herein we demonstrate that the decrease in the infection rate of macrophages treated with compounds 13b and 13e correlated with an increased NO production; treatment of infected macrophages with compound 13b upregulates NO production 2.5-fold (3.3-fold if IFN-γ is included in the treatment). Leishmania survival and persistence in the host correlate with the suppression of iNOS and parasite entry into iNOS-negative cells (6, 7), and NO donors inactivate parasite CPs (3). In BALB/c mice infected with Leishmania donovani and treated with the natural CP inhibitor cystatin and IFN-γ, elimination of spleen parasite burden correlated with enhanced amounts of NO (10). Since higher NO levels were seen when infected macrophages were treated with compound 13b, an activation of iNOS may be part of its effect.
Together, our results indicate that the aziridine-2,3-dicarboxylates 13b and 13e act directly on Leishmania, without affecting the viability of the host cell. Additionally, they show that treatment of infected cells with compounds 13b and 13e modulates the cytokine secretion by host macrophages. These results suggest that compounds 13b and 13e are promising candidates for further investigation as lead compounds against Leishmania parasites.
Acknowledgments
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG Collaborative Research Center 630).
We are grateful to Calin Apetrei and Simon Berzel for help with cell isolation, Christina de Witt and Christine Hambrecht for technical assistance, and Ulrich Körner, Marcela Fajardo-Moser, and Katharina Remer for fruitful discussions.
REFERENCES
- 1.Ahmed, S. A., R. M. Gogal, and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 2.Alves, C. R., T. C. Benevolo-De-Andrade, J. L. Alves, and C. Pirmez. 2004. Th1 and Th2 immunological profile induced by cysteine proteinase in murine leishmaniasis. Parasite Immunol. 26:127-135. [DOI] [PubMed] [Google Scholar]
- 3.Ascenzi, P., A. Bocedi, M. Gentile, P. Visca, and L. Gradoni. 2004. Inactivation of parasite cysteine proteinases by the NO-donor 4-(phenylsulfonyl)-3-((2-(dimethylamino)ethyl)thio)-furoxan oxalate. Biochim. Biophys. Acta 1703:69-77. [DOI] [PubMed] [Google Scholar]
- 4.Bates, P. A., C. D. Robertson, and G. H. Coombs. 1994. Expression of cysteine proteinases by metacyclic promastigotes of Leishmania mexicana. J. Eukaryot. Microbiol. 41:199-203. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y., B. Butcher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389-1400. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan, C., M. Röllinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 7.Brandonisio, O., M. A. Panaro, M. Sisto, A. Acquafredda, L. Fumaro, and D. Leogrande. 2000. Interactions between Leishmania parasites and host cells. Parassitologia 42:183-190. [PubMed] [Google Scholar]
- 8.Carrera, L., R. T. Gazzinelli, R. Badolato, S. Hieny, W. Muller, R. Kuhn, and D. L. Sacks. 1996. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J. Exp. Med. 183:515-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombs, G. H., and J. Baxter. 1984. Inhibition of Leishmania amastigote growth by antipain and leupeptin. Ann. Trop. Med. Parasitol. 78:21-24. [DOI] [PubMed] [Google Scholar]
- 10.Das, L., N. Datta, S. Bandyopadhyay, and P. K. Das. 2001. Successful therapy of lethal murine visceral leishmaniasis with cystatin involves up-regulation of nitric oxide and a favorable T cell response. J. Immunol. 166:4020-4028. [DOI] [PubMed] [Google Scholar]
- 11.Davis, A. J., H. W. Murray, and E. Handman. 2004. Drugs against leishmaniasis: a synergy of technology and partnerships. Trends Parasitol. 20:73-76. [DOI] [PubMed] [Google Scholar]
- 12.Denise, H., K. McNeil, D. R. Brooks, J. Alexander, G. H. Coombs, and J. C. Mottram. 2003. Expression of multiple CPB genes encoding cysteine proteases is required for Leishmania mexicana virulence in vivo. Infect. Immun. 71:3190-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai, P. V., A. Patny, Y. Sabnis, B. Tekwani, J. Gut, P. Rosenthal, A. Srivastava, and M. Avery. 2004. Identification of novel parasitic cysteine protease inhibitors using virtual screening. 1. The ChemBridge database. J. Med. Chem. 47:6609-6615. [DOI] [PubMed] [Google Scholar]
- 14.Escobar, P., S. Matu, C. Marques, and S. L. Croft. 2002. Sensitivities of Leishmania species to hexadecylphosphocoline (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Trop. 81:151-157. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes, A. P., F. A. Carvalho, C. A. Tavares, H. C. Santiago, G. A. Castro, W. L. Tafuri, L. A. Ferreira, and R. T. Gazzinelli. 2001. Combined interleukin-12 and topical chemotherapy for established leishmaniasis drastically reduces tissue parasitism and relapses in susceptible mice. J. Infect. Dis. 183:1646-1652. [DOI] [PubMed] [Google Scholar]
- 16.Gantt, K. R., T. L. Goldman, M. L. McCormick, M. A. Miller, S. M. Jeronimo, E. T. Nascimento, B. E. Britigan, and M. E. Wilson. 2001. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J. Immunol. 167:893-901. [DOI] [PubMed] [Google Scholar]
- 17.Gelhaus, C., R. Vicik, R. Hilgenfeld, C. L. Schmidt, M. Leippe, and T. Schirmeister. 2004. Synthesis and antiplasmodial activity of a cysteine protease-inhibiting biotinylated aziridine-2,3-dicarboxylate. Biol. Chem. 385:435-438. [DOI] [PubMed] [Google Scholar]
- 18.Gradoni, L., and P. Ascenzi. 2004. Nitric oxide and anti-protozoan chemotherapy. Parassitologia 46:101-103. [PubMed] [Google Scholar]
- 19.Granger, D. L., R. L. Taintor, K. S. Boockvar, and J. B. Hibbs. 1996. Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods Enzymol. 268:142-151. [DOI] [PubMed] [Google Scholar]
- 20.Ho, J. L., R. Badaro, A. Schwartz, C. A. Dinarello, J. A. Gelfand, J. Sobel, A. Barral, M. B. Netto, E. M. Carvalho, S. G. Reed, and W. D. Johnson. 1992. Diminished in vitro production of interleukin-1 and tumor necrosis factor-alpha during acute visceral leishmaniasis and recovery after therapy. J. Infect. Dis. 165:1094-1102. [DOI] [PubMed] [Google Scholar]
- 21.Huber, W., and J. C. Koella. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55:257-261. [DOI] [PubMed] [Google Scholar]
- 22.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Röβner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Meth. 223:77-92. [DOI] [PubMed] [Google Scholar]
- 23.Maekawa, Y., K. Himeno, H. Ishikawa, H. Hisaeda, T. Sakai, T. Dainichi, T. Asao, R. A. Good, and N. Katunuma. 1998. Switch of CD4+ T cell differentiation from Th2 to Th1 by treatment with cathepsin B inhibitor in experimental leishmaniasis. J. Immunol. 161:2120-2127. [PubMed] [Google Scholar]
- 24.Mahmoudzadeh-Nikam, H., and J. H. McKerrow. 2004. Leishmania tropica: cysteine proteases are essential for growth and pathogenicity. Exp. Parasitol. 106:158-163. [DOI] [PubMed] [Google Scholar]
- 25.McKerrow, J. H., J. C. Engel, and C. R. Caffrey. 1999. Cysteine protease inhibitors as chemotherapy for parasitic infections. Bioorg. Med. Chem. 7:639-644. [DOI] [PubMed] [Google Scholar]
- 26.Mikus, J., and D. Steverding. 2000. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue. Parasitol. Int. 48:265-269. [DOI] [PubMed] [Google Scholar]
- 27.Mottram, J. C., D. R. Brooks, and G. H. Coombs. 1998. Roles of cysteine proteinases of trypanosomes and Leishmania in host-parasite interactions. Curr. Opin. Microbiol. 1:455-466. [DOI] [PubMed] [Google Scholar]
- 28.Mottram, J. C., G. H. Coombs, and J. Alexander. 2004. Cysteine peptidases as virulence factors of Leishmania. Curr. Opin. Microbiol. 7:375-381. [DOI] [PubMed] [Google Scholar]
- 29.Mundodi, V., A. S. Kucknoor, and L. Gedamu. 2005. Role of Leishmania (Leishmania) chagasi amastigote cysteine protease in intracellular parasite survival: studies by gene disruption and antisense mRNA inhibition. BMC Mol. Biol. 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onishi, K., Y. Li, K. Ishii, H. Hisaeda, L. Tang, X. Duan, T. Dainichi, Y. Maekawa, N. Katunuma, and K. Himeno. 2004. Cathepsin L is crucial for a Th1-type immune response during Leishmania major infection. Microbes Infect. 6:468-474. [DOI] [PubMed] [Google Scholar]
- 31.Ouellette, M., J. Drummelsmith, and B. Papadopoulou. 2004. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist. Updates 7:257-266. [DOI] [PubMed] [Google Scholar]
- 32.Ponte-Sucre, A., Y. Campos, M. Fernández, H. Moll, and A. Mendoza-León. 1998. Leishmania sp.: growth and survival are impaired by ion channel blockers. Exp. Parasitol. 88:11-19. [DOI] [PubMed] [Google Scholar]
- 33.Ponte-Sucre, A., A. Mendoza-León, and H. Moll. 2001. Experimental leishmaniasis: synergistic effect of ion channel blockers and interferon-γ on the clearance of Leishmania major parasites. Parasitol. Res. 87:27-31. [DOI] [PubMed] [Google Scholar]
- 34.Rafati, S., S. Couty-Jouve, M. H. Alimohammadian, and J. A. Louis. 1997. Biochemical analysis and immunogenicity of Leishmania major amastigote fractions in cutaneous leishmaniasis. Clin. Exp. Immunol. 110:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramírez-Pineda, J. R., A. Fröhlich, C. Berberich, and H. Moll. 2004. Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J. Immunol. 172:6281-6289. [DOI] [PubMed] [Google Scholar]
- 36.Razonable, R. R., M. Henault, L. N. Lee, C. Laethem, P. A. Johnston, H. L. Watson, and C. V. Paya. 2005. Secretion of proinflammatory cytokines and chemokines during amphoptericin B exposure is mediated by coactivation of Toll-like receptors 1 and 2. Antimicrob. Agents Chemother. 49:1617-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakanari, J. A., S. A. Nadler, V. J. Chan, J. C. Engel, C. Leptak, and J. Bouvier. 1997. Leishmania major: comparison of the cathepsin L- and B- like cysteine protease genes with those of other trypanosomatids. Exp. Parasitol. 85:63-76. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Delgado, R. A., A. Anzellotti, and L. Suárez. 2004. Metal complexes as chemotherapeutic agents against tropical diseases: malaria, trypanosomiasis and leishmaniasis. Met. Ions Biol. Syst. 41:379-419. [PubMed] [Google Scholar]
- 39.Schirmeister, T. 1999. New peptidic cysteine protease inhibitors derived from the electrophilic α-amino acid aziridine-2,3-dicarboxylic acid. J. Med. Chem. 42:560-572. [DOI] [PubMed] [Google Scholar]
- 40.Schirmeister, T., and M. Peric. 2000. Aziridinyl peptides as inhibitors of cysteine proteases: effect of a free carboxylic acid function on inhibition. Bioorg. Med. Chem. 8:1281-1291. [DOI] [PubMed] [Google Scholar]
- 41.Scott, P. 2003. Development and regulation of cell-mediated immunity in experimental leishmaniasis. Immunol. Res. 27:489-498. [DOI] [PubMed] [Google Scholar]
- 42.Vianna, G. O. 1912. Tratamento da leishmaniose tegumentar por injeções intravenosas de tártaro emético. Arch. Brasil. Med. 3:1171. [Google Scholar]
- 43.Vicik, R., V. Hoerr, M. Glaser, M. Schultheis, E. Hansell, J. H. McKerrow, U. Holzgrabe, C. R. Caffrey, A. Ponte-Sucre, H. Moll, A. Stich, and T. Schirmeister. 2006. Aziridine-2,3-dicarboxylate inhibitors targeting the major cysteine protease of Trypanosoma brucei as lead trypanocidal agents. Bioorg. Med. Chem. Lett. 16:2753-2757. [DOI] [PubMed] [Google Scholar]
- 44.Weinheber, N., M. Wolfram, D. Harbecke, and T. Aebischer. 1998. Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. Eur. J. Immunol. 28:2467-2477. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, T., Y. Maekawa, T. Sakai, Y. Nakano, K. Ishii, H. Hisaeda, T. Dainichi, T. Asao, N. Katunuma, and K. Himeno. 2001. Treatment with cathepsin L inhibitor potentiates Th2-type immune response in Leishmania major-infected BALB/c mice. Int. Immunol. 13:975-982. [DOI] [PubMed] [Google Scholar]