Abstract
Four isogenic derivatives with stably increased glycopeptide MICs (all become resistant to teicoplanin) were obtained from four glycopeptide-susceptible clinical isolates of Staphylococcus haemolyticus. All strains were extensively analyzed and compared for a number of distinctive features. In particular, the results provided insights into the puzzling issue of antistaphylococcal interactions between glycopeptides and β-lactams, especially the paradox of double zones around β-lactam disks and the relationships between autolysis rate and type of interaction.
Synergistic interactions between glycopeptides and β-lactams against methicillin-resistant staphylococci have been documented in several studies (3, 10, 13, 16, 17). As most extensively demonstrated by Climo and coworkers (10), combinations of vancomycin and β-lactams appear to be synergistic, especially against staphylococcal strains with reduced susceptibility to vancomycin. Consistent with these findings, Sieradzki and Tomasz (31) noted that a highly vancomycin-resistant laboratory mutant of Staphylococcus aureus became extremely susceptible to β-lactams compared to its vancomycin-susceptible parent. In contrast, other studies reported possibly concentration-dependent antagonistic effects against S. aureus strains with various degrees and phenotypes of glycopeptide resistance (1, 22, 24). False synergism due to inappropriate testing methods has also been described (21). This study was aimed at gaining new insights into this puzzling issue by analyzing and comparing four isogenic pairs of teicoplanin-susceptible and -resistant strains of Staphylococcus haemolyticus.
S. haemolyticus and glycopeptide resistance.
S. haemolyticus is second in frequency only to Staphylococcus epidermidis among clinical isolates of coagulase-negative staphylococci (CNS) (2). Since early studies, S. haemolyticus is regarded as an important nosocomial pathogen with a tendency to develop multiple resistances (20). Indeed, it was the first gram-positive pathogen to acquire glycopeptide resistance (6), earlier than other staphylococcal species and enterococci, and has been suggested to be unique among CNS in being predisposed to develop glycopeptide resistance (30), which in this species may be multifactorial (7). Population analysis indicated that heterogeneous expression of teicoplanin resistance is prevalent among S. haemolyticus clinical strains and may be associated with heterogeneous resistance to vancomycin (5). Moreover, in cultures of this more often than of other staphylococcal species, glycopeptides have been seen, under laboratory conditions, to select for clones with increased glycopeptide (especially teicoplanin) MICs (6). It is worth noting that, among clinical isolates, exposure to glycopeptides appears to be a prerequisite to the development of resistance (10); in fact, glycopeptide-resistant S. haemolyticus strains have typically been recovered from patients subjected to prolonged courses of glycopeptides (6).
Isogenic pairs of strains.
Four glycopeptide-susceptible clinical isolates of S. haemolyticus, identified by the API test system (bioMérieux, Marcy-l'Etoile, France) and confirmed by additional laboratory tests (2), were used to obtain derivatives with increased glycopeptide MICs. Of the four isolates, two (Sh1 and Sh2) were methicillin susceptible (MS) and two (Sh3 and Sh4) methicillin resistant (MR); all were β-lactamase producers. After exposure to 10 μg/ml teicoplanin, according to a procedure successfully used in previous studies in our laboratory (4), stable clones (Sh1R, Sh2R, Sh3R, and Sh4R) were obtained from each parent.
Strain characterization and comparison.
Broth microdilution MICs (11) of teicoplanin (Sanofi-Aventis Italia, Milan, Italy), vancomycin (Eli Lilly Italia, Sesto Fiorentino, Italy), and three β-lactams (oxacillin, penicillin, and ampicillin; all from Sigma-Aldrich, Milan, Italy) for the four parents and the four derivatives as well as other characteristics of these strains are shown in Table 1. While an increased vancomycin MIC was observed in a single clone (Sh2R; a twofold increase still consistent with susceptibility), all four derivatives turned resistant to teicoplanin, with MICs increasing 4 to 16 times. Population analysis profiles (PAPs), determined as described elsewhere (5), indicated that the four parents all had a heterogeneous phenotype for teicoplanin and that only one (Sh3) had a heterogeneous phenotype for vancomycin. Of the derivatives, one (Sh1R) exhibited a heterogeneous profile for teicoplanin and none for vancomycin. For both parents and derivatives, vancomycin PAP MICs, defined as the lowest antibiotic concentration inhibiting 99.9% of growth (3-log10 decrease in the number of CFU) (32), were two to four times higher than conventional vancomycin MICs; a more heterogeneous range of ratios was obtained with PAP MICs and conventional MICs of teicoplanin. As regards β-lactams, no changes were recorded for oxacillin MICs, and the mecA gene was detected by PCR (27) in the MR derivatives as well as in their respective parents. Penicillin MICs, unchanged in the two MR derivatives, fell by 64 and 4 times, respectively, in the two MS derivatives Sh1R and Sh2R, whereas ampicillin MICs varied only in one MS derivative (Sh1R; a 16-fold decrease). blaZ, the structural gene of staphylococcal β-lactamase, was detected by PCR (27) in all parents and derivatives, with β-lactamase production being confirmed by the nitrocefin and iodometric assays (26). All derivatives demonstrated ultrastructural differences with respect to their parents that consisted mainly in cell wall thickening and roughened surfaces observed by transmission and scanning electron microscopy, respectively (data not shown). Increased rates of autolysis, monitored by a turbidimetric assay as described previously (14), were observed in all derivatives compared with those observed in their parents, but these values dropped to parental rates or even lower when subinhibitory teicoplanin was present in the growth medium and/or the autolysis buffer. Though variable among parent strains, resistance to lysostaphin, estimated from the rates of survival to its action (12), was consistently enhanced in all derivatives and further increased when the derivatives were grown in the presence of subinhibitory teicoplanin.
TABLE 1.
Glycopeptide and β-lactam susceptibilities and related characteristics of four isogenic pairs of teicoplanin-susceptible and -resistant strains of S. haemolyticusa
| Strain | MIC (μg/ml) for: |
Population analysis profile/PAP MIC (μg/ml) forb: |
Detection of mecA gene | Detection of blaZ gene | Detection of β-lactamase production | % Decrease in OD620c |
% Survival to lysostaphind |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAN | TEC | OXA | PEN | AMP | VAN | TEC | Without TEC | With TEC | Without TEC | With TEC | ||||
| Sh1 | 1 | 4 | 1 | 8 | 8 | homo/4 | hetero/4 | − | + | + | 8 | 0.3 | ||
| Sh1R | 1 | 32 | 1 | 0.125 | 0.5 | homo/2 | hetero/16 | − | + | + | 49 | 10 | 1.9 | 21.7 |
| Sh2 | 2 | 8 | 0.5 | 2 | 2 | homo/4 | hetero/4 | − | + | + | 36 | 1.0 | ||
| Sh2R | 4 | 64 | 0.5 | 0.5 | 2 | homo/8 | homo/64 | − | + | + | 70 | 17 | 8.8 | 69.2 |
| Sh3 | 4 | 8 | 512 | 64 | 64 | hetero/8 | hetero/64 | + | + | + | 29 | 0.3 | ||
| Sh3R | 4 | 32 | 512 | 64 | 64 | homo/16 | homo/128 | + | + | + | 82 | 42 | 49.5 | 98.9 |
| Sh4 | 1 | 2 | 256 | 64 | 64 | homo/2 | hetero/8 | + | + | + | 22 | 0.03 | ||
| Sh4R | 1 | 32 | 256 | 64 | 64 | homo/2 | homo/32 | + | + | + | 55 | 33 | 4.8 | 92.5 |
VAN, vancomycin; TEC, teicoplanin; OXA, oxacillin; PEN, penicillin; AMP, ampicillin. +, presence; −, absence.
homo, homogeneous profile; hetero, heterogeneous profile.
Percent decrease in optical density at 620 nm in the absence of teicoplanin or, with the derivatives, in the presence of 10 μg/ml teicoplanin in the growth medium and the autolysis buffer.
Percent survival after 45 min of incubation in 250 μg/ml lysostaphin of bacterial cells grown in antibiotic-free medium or, with the derivatives, in medium containing 10 μg/ml teicoplanin.
Checkerboard assays.
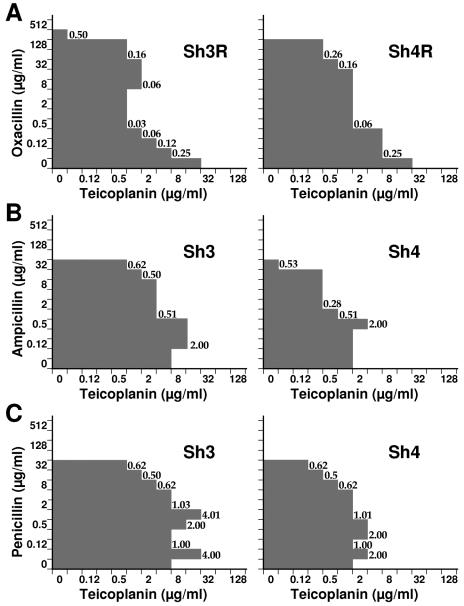
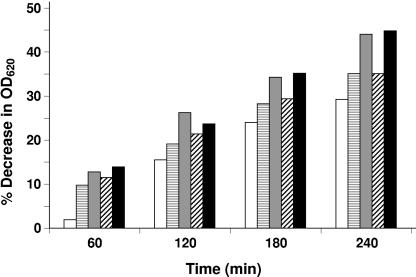
Interactions between glycopeptides and β-lactams were investigated by standard checkerboard experiments performed in microtiter trays with Mueller-Hinton II broth (Becton Dickinson Italia, Milan, Italy) containing 2% NaCl, with synergism being determined from the fractional inhibitory concentration (FIC) index (19). FIC indices were interpreted as indicating synergism if values were ≤0.5, additivity if they were >0.5 to 1, indifference if they were >1 to 4, and antagonism if they were >4. Glycopeptide-plus-β-lactam combinations demonstrating synergism (FIC indices of ≤0.5) were recorded against all parents and derivatives. Synergism was most evident against MR derivatives, especially when the glycopeptide in the combination was teicoplanin (Fig. 1A). However, particularly with teicoplanin-containing combinations against MR parental strains (Fig. 1B and C), additivity (with oxacillin or penicillin) and indifference (with penicillin or ampicillin) were observed at some drug concentrations; even antagonism was seen against strain Sh3 at penicillin concentrations of 0.06 and 1 μg/ml. Interestingly, the rate of autolysis depended on penicillin concentration, with a lower rate being displayed by strain Sh3, in the presence of 1 μg/ml teicoplanin, at the same penicillin concentrations causing antagonism (Fig. 2).
FIG. 1.
Combination effects, resulting from broth microdilution checkerboard tests, of teicoplanin plus oxacillin against test strains Sh3R and Sh4R (A), teicoplanin plus ampicillin against test strains Sh3 and Sh4 (B), and teicoplanin plus penicillin against test strains Sh3 and Sh4 (C). FIC indices are given at appropriate points.
FIG. 2.
Autolysis rates of strain Sh3 at different times (expressed as percent decrease in optical density at 620 nm [OD620]) in the presence of 1 μg/ml teicoplanin and different penicillin concentrations: ▤, 0.06 μg/ml; ░⃞, 0.25 μg/ml; ▨, 1 μg/ml; and ▪, 8 μg/ml. □, control (antibiotic-free medium).
Diffusion tests.
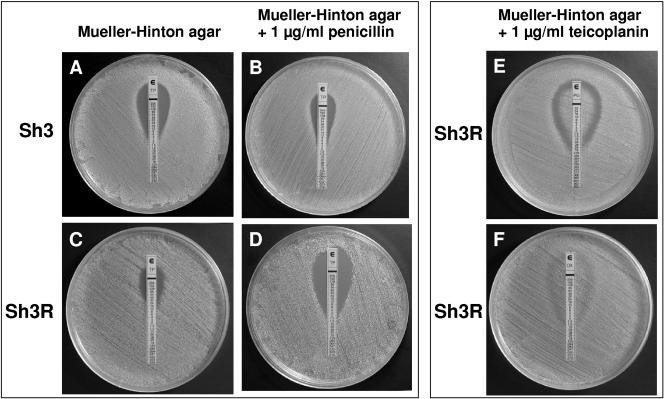
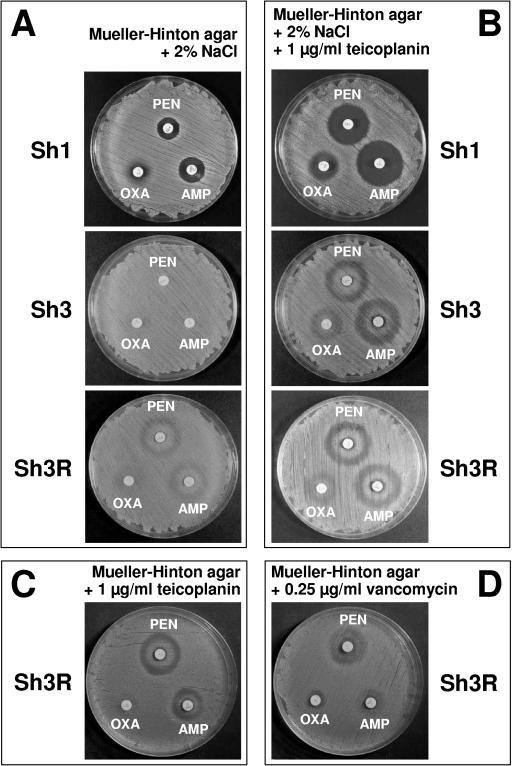
Concentration-dependent teicoplanin-β-lactam interactions were also observed using Etest strips (AB Biodisk, Solna, Sweden). Synergism (strain Sh3R) and antagonism (strain Sh3) were demonstrated by comparing teicoplanin strips on Mueller-Hinton agar unsupplemented (Fig. 3A and C) or supplemented with 1 μg/ml penicillin (Fig. 3B and D). Using penicillin and oxacillin strips, incomplete inhibition, with a double zone (growth immediately around the strip with a zone of inhibition farther out) around the former (Fig. 3E) and a barely appreciable double zone around the latter (Fig. 3F), was observed for all MR test strains (both parents and derivatives). Further diffusion tests were performed using commercial disks (Oxoid Ltd., Basingstoke, United Kingdom) of oxacillin (1 μg), penicillin (10 μg), and ampicillin (10 μg) on Mueller-Hinton agar, variably supplemented with teicoplanin or vancomycin and 2% NaCl. All MS test strains showed increased zones of inhibition compared to those observed in equivalent medium without teicoplanin. The difference was especially apparent with strain Sh1 around penicillin and ampicillin disks (Fig. 4A and B). All MR test strains showed characteristic double zones around penicillin and ampicillin disks and an incomplete zone of inhibition (with colonies growing within the zone) around oxacillin (strain Sh3 is shown in Fig. 4B). Such unusual zones were not observed in teicoplanin-free medium (strain Sh3 is shown in Fig. 4A), except for derivative Sh3R; indeed, this was the sole strain showing double zones also in the absence of teicoplanin, provided that the medium contained 2% NaCl (Fig. 4A). Slightly smaller double zones around penicillin and ampicillin disks were still observed with MR strains in NaCl-free medium supplemented with 1 μg/ml teicoplanin (Fig. 4C) or 0.25 μg/ml vancomycin, albeit in this case less evidently and only with strain Sh3R (Fig. 4D).
FIG. 3.
Diffusion tests using Etest strips. Zones of inhibition around teicoplanin strips (A to D) on Mueller-Hinton agar unsupplemented or supplemented with 1 μg/ml penicillin and inoculated with strain Sh3 or strain Sh3R. Incomplete growth inhibition around penicillin (E) and oxacillin (F) strips, with a clear double zone around the former and a barely distinguishable one around the latter, on Mueller-Hinton agar supplemented with 1 μg/ml teicoplanin and inoculated with strain Sh3R.
FIG. 4.
Diffusion tests using commercial disks. Zones of inhibition around oxacillin, penicillin, and ampicillin disks for selected test strains, using Mueller-Hinton agar, supplemented with 2% NaCl (A), 2% NaCl and 1 μg/ml teicoplanin (B), 1 μg/ml teicoplanin (C), or 0.25 μg/ml vancomycin (D), as the test medium.
Final comments.
Contradictory observations about antistaphylococcal interactions between glycopeptides and β-lactams are not easy to explain and may partly reflect an incomplete knowledge of the mechanisms responsible for glycopeptide resistance, the expression of methicillin resistance, and the complex regulation of peptidoglycan synthesis in staphylococci. The implications are also clinical: at variance with others, who held that acquisition of glycopeptide resistance would eliminate the potential for achieving a synergistic effect by combining a glycopeptide with another antibiotic (25), Climo and coworkers (10) demonstrated that synergism between vancomycin and β-lactams applies especially to staphylococcal strains with reduced vancomycin susceptibility and concluded that combination therapy may be a reasonable alternative in treating infections caused by staphylococci with reduced susceptibility to glycopeptides. However, a recent study was unable to demonstrate in an in vivo experimental model the synergism described in vitro (18).
This study of four isogenic pairs of teicoplanin-susceptible and -resistant strains of S. haemolyticus showed synergism to be the predominant interaction, especially against MR derivatives when the glycopeptide used in the combination was teicoplanin. However, different interactions were observed at particular teicoplanin-β-lactam concentrations in both checkerboard and diffusion assays. The paradox whereby MR strains tested in teicoplanin-containing agar form alternate zones of growth and nongrowth at different distances from the disk (i.e., at different β-lactam concentrations) could reflect synergistic or antagonistic interaction depending on drug concentrations. This was even better documented in Etest assays, where the inhibition area of the double zone was likely to be the expression of synergism between teicoplanin and β-lactams over a limited concentration range. The double-zone phenomenon around a β-lactam disk has previously been noted, with CNS, around imipenem disks and explained as an effect of inducible drug resistance (8) or as a unique feature of MR strains of S. haemolyticus containing vancomycin-resistant subpopulations (29). However, other factors may have a critical role, including methicillin resistance, the presence of NaCl in the medium (enhancing the expression of staphylococcal resistance to both methicillin [9] and vancomycin [33]), and high-level glycopeptide resistance (resulting from population analysis and not conventional MICs). On the other hand, in S. haemolyticus (6, 7, 32), as well as in S. aureus (12, 17, 23, 31), high-level glycopeptide resistance may be associated with cell wall alterations that render the strain more sensitive to β-lactams and autolysins, whose action may further vary with the presence of glycopeptides.
Synergism between glycopeptides and β-lactams against MR staphylococci has been suggested to be related to the substrate specificity of PBP2a (10) for monomeric disaccharide pentapeptides (7, 15). Autolysis, whose increase reflects cell wall impairment rather than increased autolysin production (28), may play a major role in glycopeptide-β-lactam interactions against MR S. haemolyticus strains; at least in part, synergism or antagonism might result from higher or lower autolysis rates induced by specific β-lactam concentrations.
Acknowledgments
This work was supported in part by a grant from the Italian Ministry of Education, University and Research.
REFERENCES
- 1.Aritaka, N., H. Hanaki, L. Cui, and K. Hiramatsu. 2001. Combination effect of vancomycin and β-lactams against a Staphylococcus aureus strain, Mu3, with heterogeneous resistance to vancomycin. Antimicrob. Agents Chemother. 45:1292-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannerman, T. L. 2003. Staphylococcus, Micrococcus, and other catalase-positive cocci that grow aerobically, p. 384-404. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 3.Barr, J. G., E. T. Smyth, and G. M. Hogg. 1990. In vitro antimicrobial activity of imipenem in combination with vancomycin or teicoplanin against Staphylococcus aureus and Staphylococcus epidermidis. Eur. J. Clin. Microbiol. Infect. Dis. 9:804-809. [DOI] [PubMed] [Google Scholar]
- 4.Biavasco, F., E. Giovanetti, M. P. Montanari, R. Lupidi, and P. E. Varaldo. 1991. Development of in-vitro resistance to glycopeptide antibiotics: assessment in staphylococci of different species. J. Antimicrob. Chemother. 27:71-79. [DOI] [PubMed] [Google Scholar]
- 5.Biavasco, F., C. Vignaroli, R. Lazzarini, and P. E. Varaldo. 2000. Glycopeptide susceptibility profiles of Staphylococcus haemolyticus bloodstream isolates. Antimicrob. Agents Chemother. 44:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biavasco, F., C. Vignaroli, and P. E. Varaldo. 2000. Glycopeptide resistance in coagulase-negative staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 19:403-417. [DOI] [PubMed] [Google Scholar]
- 7.Billot-Klein, D., L. Gutmann, D. Bryant, D. Bell, J. van Heijenoort, J. Grewal, and D. M. Shlaes. 1996. Peptidoglycan synthesis and structure in Staphylococcus haemolyticus expressing increasing levels of resistance to glycopeptide antibiotics. J. Bacteriol. 178:4696-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthal, R. M., R. Raeder, C. D. Takemoto, and E. H. Freimer. 1983. Occurrence and expression of imipemide (N-formimidoyl thienamycin) resistance in clinical isolates of coagulase-negative staphylococci. Antimicrob. Agents Chemother. 24:61-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers, H. F., and C. J. Hackbarth. 1987. Effect of NaCl and nafcillin on penicillin-binding protein 2a and heterogeneous expression of methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 31:1982-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Climo, M. W., R. L. Patron, and G. L. Archer. 1999. Combinations of vancomycin and β-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 43:1747-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement M100-S14. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 12.Daum, R. S., S. Gupta, R. Sabbagh, and W. M. Milewski. 1992. Characterization of Staphylococcus aureus isolates with decreased susceptibility to vancomycin and teicoplanin: isolation and purification of a constitutively produced protein associated with decreased susceptibility. J. Infect. Dis. 166:1066-1072. [DOI] [PubMed] [Google Scholar]
- 13.Debbia, E., P. E. Varaldo, and G. C. Schito. 1986. In vitro activity of imipenem against enterococci and staphylococci and evidence for high rates of synergism with teicoplanin, fosfomycin, and rifampin. Antimicrob. Agents Chemother. 30:813-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Jonge, B. L. M., H. de Lencastre, and A. Tomasz. 1991. Suppression of autolysis and cell wall turnover in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J. Bacteriol. 173:1105-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Jonge, B. L. M., and A. Tomasz. 1993. Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus haemolyticus grown in the presence of methicillin: functional role for penicillin-binding protein 2A in cell wall synthesis. Antimicrob. Agents Chemother. 37:342-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domaracki, B. E., A. Evans, K. E. Preston, H. Fraimow, and R. A. Venezia. 1998. Increased oxacillin activity associated with glycopeptides in coagulase-negative staphylococci. Eur. J. Clin. Microbiol. Infect. Dis. 17:143-150. [DOI] [PubMed] [Google Scholar]
- 17.Domaracki, B. E., A. M. Evans, and R. A. Venezia. 2000. Vancomycin and oxacillin synergy for methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 44:1394-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domenech, A., S. Ribes, C. Cabellos, F. Taberner, F. Tubau, M. A. Domínguez, A. Montero, J. Liñares, J. Ariza, and F. Gudiol. 2005. Experimental study on the efficacy of combinations of glycopeptides and β-lactams against Staphylococcus aureus with reduced susceptibility to glycopeptides. J. Antimicrob. Chemother. 56:709-716. [DOI] [PubMed] [Google Scholar]
- 19.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, Md.
- 20.Froggatt, J. W., J. L. Johnston, D. W. Galetto, and G. L. Archer. 1989. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob. Agents Chemother. 33:460-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein, F. W., R. Atoui, A. Ben Ali, J. C. Nguyen, A. Ly, and M. D. Kitzis. 2004. False synergy between vancomycin and β-lactams against glycopeptide-intermediate Staphylococcus aureus (GISA) caused by inappropriate testing methods. Clin. Microbiol. Infect. 10:342-345. [DOI] [PubMed] [Google Scholar]
- 22.Haraga, I., S. Nomura, and A. Nagayama. 1999. The effects of vancomycin and β-lactam antibiotics against vancomycin low-level or intermediately resistant Staphylococcus aureus. N. Engl. J. Med. 341:1624-1625. [DOI] [PubMed] [Google Scholar]
- 23.Hiramatsu, K. 1998. Vancomycin resistance in staphylococci. Drug Resist. Updates 1:135-150. [DOI] [PubMed] [Google Scholar]
- 24.Howe, R. A., M. Wootton, P. M. Bennett, A. P. MacGowan, and T. R. Walsh. 1999. Interactions between methicillin and vancomycin in methicillin-resistant Staphylococcus aureus strains displaying different phenotypes of vancomycin susceptibility. J. Clin. Microbiol. 37:3068-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linden, P. K. 1998. Clinical implications of nosocomial gram-positive bacteremia and superimposed antimicrobial resistance. Am. J. Med. 104(Suppl. 5A):24-33. [DOI] [PubMed] [Google Scholar]
- 26.Livermore, D. M., and J. D. Williams. 1996. β-Lactams: mode of action and mechanisms of bacterial resistance, p. 502-578. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, Md.
- 27.Martineau, F., F. J. Picard, L. Grenier, P. H. Roy, M. Ouellette, M. G. Bergeron, and the ESPRIT Trial. 2000. Multiplex PCR assays for the detection of clinically relevant antibiotic resistance genes in staphylococci isolated from patients infected after cardiac surgery. J. Antimicrob. Chemother. 46:527-533. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda, K., K. Nakamura, Y. Adachi, M. Inoue, and M. Kawakami. 1995. Autolysis of methicillin-resistant Staphylococcus aureus is involved in synergism between imipenem and cefotiam. Antimicrob. Agents Chemother. 39:2631-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwalbe, R. S., W. J. Ritz, P. R. Verma, E. A. Barranco, and P. H. Gilligan. 1990. Selection for vancomycin resistance in clinical isolates of Staphylococcus haemolyticus. J. Infect. Dis. 161:45-51. [DOI] [PubMed] [Google Scholar]
- 30.Schwalbe, R. S., J. T. Stapleton, and P. H. Gilligan. 1987. Vancomycin-resistant staphylococcus. N. Engl. J. Med. 317:766-768. [DOI] [PubMed] [Google Scholar]
- 31.Sieradzki, K., and A. Tomasz. 1997. Inhibition of cell wall turnover and autolysis by vancomycin in a highly vancomycin-resistant mutant of Staphylococcus aureus. J. Bacteriol. 179:2557-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieradzki, K., P. Villari, and A. Tomasz. 1998. Decreased susceptibilities to teicoplanin and vancomycin among coagulase-negative methicillin-resistant clinical isolates of staphylococci. Antimicrob. Agents Chemother. 42:100-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong, S. S. Y., P. L. Ho, P. C. Y. Woo, and K. Y. Yuen. 1999. Bacteremia caused by staphylococci with inducible vancomycin heteroresistance. Clin. Infect. Dis. 29:760-767. [DOI] [PubMed] [Google Scholar]