Abstract
A total of 207 Staphylococcus aureus strains, including 105 well-characterized strains with decreased susceptibility to vancomycin (17 vancomycin-intermediate S. aureus [VISA] and 88 heteroresistant VISA [hVISA] strains) and 102 wild-type methicillin-resistant S. aureus (MRSA-WT) strains were tested by reference/standardized broth microdilution and disk diffusion methods, as well as by Etest (AB BIODISK, Solna, Sweden), against daptomycin and vancomycin. The lowest concentration of antimicrobial agent that killed ≥99.9% of the initial inoculum was defined as the minimum bactericidal concentration (MBC) endpoint, and time-kill curves were performed in selected strains to further evaluate bactericidal activity. All MRSA-WT and hVISA strains were inhibited by ≤1 μg/ml of daptomycin, while the VISA strains showed slightly higher daptomycin MICs (range, 0.5 to 4 μg/ml). All daptomycin MBC results were at the MIC or twofold higher. In contrast, 14.7% of MRSA-WT, 69.3% of hVISA, and all VISA strains showed a vancomycin MBC/MIC ratio of ≥32 or an MBC of ≥16 μg/ml (tolerant). The correlation coefficients between broth microdilution and disk diffusion method results were low for daptomycin (0.07) and vancomycin (0.11). Eight (3.8%) strains (all hVISA or VISA) were “nonsusceptible” to daptomycin by broth microdilution methods but susceptible by the disk diffusion method. For vancomycin, 35 (16.9%) strains were nonsusceptible by broth microdilution methods but susceptible by disk diffusion methods. In conclusion, daptomycin was highly bactericidal against S. aureus strains, and its bactericidal activity was not affected by decreased susceptibility to vancomycin. In contrast, many (one in seven) contemporary MRSA-WT, the majority of hVISA, and all VISA strains showed vancomycin MBC/MIC ratios consistent with tolerance, a predictor of poor clinical response. Disk diffusion tests generally failed to detect strains categorized as nonsusceptible to daptomycin or vancomycin by the reference broth microdilution method or Etest, and reassessment of breakpoints should be immediately attempted for MIC methods suggested as the test of choice.
The superiority of bactericidal over bacteriostatic antimicrobials is very controversial and may not translate to better clinical outcome for the majority of infections. However, there are a few studies suggesting that a bactericidal agent would be preferable in some clinical situations, such as endocarditis, meningitis, osteomyelitis, and systemic infections in immunocompromised patients (1, 13, 19). In endocarditis, for example, bacteria within cardiac valvular vegetations may reach very high concentrations (108 to 1010 organisms per gram of tissue), and at such densities, rates of metabolism and cell division appear to be reduced, resulting in decreased susceptibility to the bactericidal effects of cell wall-active agents. The bacteria are somewhat dormant, being surrounded by fibrin, platelets, and possibly calcified material. Bacteria that could be considered susceptible to various antimicrobials in most clinical situations are relatively resistant in endocarditis. Clinical cure can often be achieved, but prolonged administration of relatively high doses of a bactericidal, cell wall-active antibacterial agent is generally required for true sterilization of the vegetation to kill any dormant bacteria (1, 24). However, the clinical importance of bactericidal activity in the treatment of infectious endocarditis is very difficult to evaluate due to the high mortality rates of the disease (11, 15, 27).
Daptomycin is a cyclic lipopeptide which has been recently approved by the U.S. Food and Drug Administration for the treatment of complicated skin and skin structure infections and has been evaluated for the treatment of several other infections, including successful studies of bacteremia and infectious endocarditis (14, 21, 26). Daptomycin has a unique mechanism of action with no cross-resistance to glycopeptide (teicoplanin and vancomycin)-resistant strains. This lipopeptide antimicrobial acts on the cytoplasmic membrane in the presence of physiological levels of calcium ions, and its in vitro susceptibility testing requires appropriate supplementation of the test media with calcium (50 μg/liter in Mueller-Hinton broth) (12).
Vancomycin, the first glycopeptide antimicrobial agent, has been used clinically for nearly 5 decades, and susceptibility testing results document continued in vitro activity against a wide variety of gram-positive species. However, more recently, the emergence of vancomycin-intermediate (VISA) and -resistant Staphylococcus aureus has called into question the efficacy of this antimicrobial in the treatment of some serious staphylococcal infections (3, 8, 10, 18, 25, 28). Also, there have been several studies demonstrating that vancomycin bactericidal activity is significantly reduced during the bacterial stationary phase, under anaerobic conditions, and at an increased inoculum (1, 11, 20), even when vancomycin MICs are in the susceptible range.
We evaluated the bactericidal activities of daptomycin and vancomycin against a very large contemporary collection of VISA and heteroresistant VISA (hVISA) strains compared to a collection of wild-type methicillin-resistant S. aureus (MRSA-WT) strains. We also evaluated the correlation between the susceptibility testing results generated by reference broth microdilution (6), disk diffusion (7), and Etest (AB BIODISK, Solna, Sweden) for these two antimicrobials.
MATERIALS AND METHODS
Organism collection.
A collection of 207 S. aureus strains was selected for the study. The collection was composed of two groups of strains.
(i) hVISA/VISA group.
The hVISA/VISA group consisted of 105 isolates. The hVISA subset included 88 isolates with vancomycin MIC results of ≤4 μg/ml by the reference broth microdilution method that showed a subpopulation with a vancomycin MIC at >4 μg/ml when tested with a high inoculum (a heterogeneous population). This collection has been well characterized by Wootton et al. (29) and Howe et al. (17). The VISA subset included 17 isolates with vancomycin MIC results of 8 μg/ml and a homogeneous population. Ten strains were defined by Wooton et al. (29), and seven strains were provided by the Network on Antimicrobial Resistance in S. aureus (http://www.narsa.net).
(ii) MRSA-WT group.
The MRSA-WT group consisted of 102 oxacillin-resistant S. aureus strains with a homogeneous population of vancomycin MIC results at ≤2 μg/ml (wild type). These isolates were collected from more than 50 medical centers worldwide in 2003. No more than two strains per medical center were included.
Eighteen strains, selected from the MRSA-WT group based on the vancomycin minimum bactericidal concentration (MBC) results, were further evaluated by time-kill curve experiments. This collection included 15 MRSA-WT clinical strains that demonstrated elevated MBC/MIC ratios (tolerance) for vancomycin and 3 MRSA-WT clinical strains with a vancomycin MBC equal to the MIC at the same value. In addition, three S. aureus ATCC strains (29213, 43300, and 33591) were included in the time-kill curve experiments.
Susceptibility testing.
MICs were determined by broth microdilution for daptomycin, vancomycin, and oxacillin with appropriate medium variations (50 mg/liter of calcium) for testing daptomycin (6, 12). The log2 dilution ranges tested were as follows: daptomycin, 128 to 0.12 μg/ml; oxacillin, 4 to 1 μg/ml; and vancomycin 32 to 1 μg/ml.
MBCs for daptomycin and vancomycin were assessed by plating the entire volume of broth (100 μl) from the broth microdilution MIC well and from the log2 dilutions above the MIC for each organism onto agar growth media. Quantitative colony counts were performed on the starting inoculum at the time the MIC test was performed. The lowest concentration of antimicrobial agent that killed ≥99.9% of the starting inoculum was defined as the MBC endpoint (22, 23). Tolerance was defined as an MBC/MIC ratio of ≥32, and isolates with an MBC/MIC ratio of 16 associated with an MBC of ≥16 μg/ml, the proposed CLSI resistant breakpoint for vancomycin (6), were also considered tolerant (20).
Disk diffusion tests with 30-μg daptomycin and vancomycin disks were performed on all 207 isolates according to the method published by the CLSI for 100-mm Mueller-Hinton agar plates (7). All isolates were also susceptibility tested against daptomycin and vancomycin by Etest, according to the manufacturer's recommendations. In addition, all MRSA-WT strains with vancomycin MBC/MIC ratios consistent with tolerance (15 strains; MBC/MIC ratio ≥ 32 and/or MBC ≥ 16 μg/ml) were evaluated by the Etest methodology on brain-heart infusion agar against vancomycin and teicoplanin using two inocula (0.5 and 2 McFarland standards). This technique was used to detect hVISA strains, and the criteria used to characterize hVISA were as follows: MICs ≥ 8 μg/ml for both vancomycin and teicoplanin after 48 h of incubation at 37°C (29).
The quality control strains, S. aureus ATCC 25923 and Enterococcus faecalis ATCC 29212, were tested along with every set of tests.
Time-kill curve experiments.
Time-kill bactericidal activities were determined for daptomycin and vancomycin (22, 23). Vancomycin was tested at 2×, 4×, and 8× MIC, with colonies counted at time zero (T0), T4, T8, T24, and T48, while daptomycin was tested at 2×, 4×, and 8× MIC with colony counts performed at T0, T4, T8, and T24. Bactericidal activity was defined as ≥3 log10 reduction in the initial inoculum within 24 h of incubation (22).
RESULTS
Table 1 summarizes the MIC and MBC results for daptomycin and vancomycin. The strains were grouped according to the type of pattern of susceptibility to vancomycin as (i) MRSA-WT strains, (ii) hVISA strains, and (iii) VISA strains. All MRSA-WT and hVISA strains were inhibited by ≤1 μg/ml of daptomycin. A slight skewing toward a higher daptomycin MIC result was noted when the hVISA (MIC50, 0.5 μg/ml; MIC90, 1 μg/ml) and VISA (MIC50, 1 μg/ml; MIC90, 2 μg/ml) strains were compared to the MRSA-WT group (MIC50 and MIC90, 0.5 μg/ml). The highest daptomycin MBC result observed was 4 μg/ml (three isolates, all VISA), and 93.2% of the isolates showed a daptomycin MBC of ≤1 μg/ml (Table 1). Eight of 11 daptomycin MBC results of 2 μg/ml and all three MBC results of 4 μg/ml were observed among the VISA strains.
TABLE 1.
MIC and MBC results for daptomycin and vancomycin
| Antimicrobial agent/organism (total no. of isolates) | No. of isolates (cumulative %) at MIC/MBC (μg/ml) of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | >16 | |
| MIC | |||||||||
| Daptomycin | |||||||||
| MRSA-WT (102)a | 2 (2.0) | 41 (42.2) | 56b (97.1) | 3 (100.0) | 0 | 0 | 0 | 0 | 0 |
| hVISA (88) | 0 | 2 (2.3) | 52 (61.4) | 34 (100.0) | 0 | 0 | 0 | 0 | 0 |
| VISA (17) | 0 | 0 | 2 (11.8) | 7 (52.9) | 7 (94.1) | 1 (100.0) | 0 | 0 | 0 |
| Vancomycin | |||||||||
| MRSA-WT (102)a | −c | − | 9 (8.8) | 82 (89.2) | 11 (100.0) | 0 | 0 | 0 | 0 |
| hVISA (88) | − | − | 0 (0.0) | 9 (10.2) | 61 (79.5) | 18 (100.0) | 0 | 0 | 0 |
| VISA (17) | − | − | 0 (0.0) | 0 (0.0) | 0 (0.0) | 11 (64.7) | 6 (100.0) | 0 | 0 |
| MBC | |||||||||
| Daptomycin | |||||||||
| MRSA-WT (102)a | 0 | 29 (28.4) | 65 (92.2) | 7 (99.0) | 1 (100.0) | 0 | 0 | 0 | 0 |
| hVISA (88) | 0 | 2 (2.3) | 35 (40.9) | 49 (97.7) | 2 (100.0) | 0 | 0 | 0 | 0 |
| VISA (17) | 0 | 0 | 2 (11.8) | 4 (35.3) | 8 (82.4) | 3 (100.0) | 0 | 0 | 0 |
| Vancomycin | |||||||||
| MRSA-WT (102)a | − | − | 2 (2.0) | 38 (39.2) | 23 (61.8) | 9 (70.6) | 15 (85.3) | 4 (89.2) | 11 (100.0) |
| hVISA (88) | − | − | 0 | 3 (3.4) | 9 (14.9) | 5 (19.3) | 3 (22.7) | 7 (30.7) | 61 (100.0) |
| VISA (17) | − | − | 0 | 0 | 0 | 0 | 0 | 0 | 17 (100.0) |
Clinical MRSA isolate with vancomycin MIC of ≤2 μg/ml (homogeneous populations) collected from medical centers worldwide in 2003.
The underlined numbers are modal values.
—, dilution not tested.
Only 61.8% of the MRSA-WT isolates showed vancomycin MBC results of ≤2 μg/ml, the proposed CLSI (6) vancomycin-susceptible MIC breakpoint (Table 1). Furthermore, 14.9% of the hVISA and none of the VISA strains showed vancomycin MBC results of ≤2 μg/ml. The number of occurrences (percentage) of isolates with vancomycin MBC results of ≥16 μg/ml (the CLSI resistant breakpoint) (6) were 15 (14.7%), 68 (77.3%), and 17 (100.0%) among the MRSA-WT, hVISA, and VISA groups, respectively (Table 1).
All daptomycin MBC results were at the MIC or only twofold higher than the MIC, and the MBC/MIC ratio results were not significantly affected by susceptibility to vancomycin (Table 2). All three groups of S. aureus (MRSA-WT, hVISA, and VISA) showed very similar MBC/MIC ratio results for daptomycin. Conversely, 14.7% of MRSA-WT strains, 69.3% of hVISA strains, and all VISA strains showed vancomycin MBC/MIC ratios consistent with a definition of tolerance (MBC/MIC ≥ 32 and/or MBC ≥ 16 μg/ml).
TABLE 2.
Distribution of isolates according to MBC/MIC ratio for daptomycin and vancomycin
| MBC/MIC ratio | No. of isolates (%)
|
|||||
|---|---|---|---|---|---|---|
| Daptomycin
|
Vancomycin
|
|||||
| MRSA-WT | hVISA | VISA | MRSA-WT | hVISA | VISA | |
| 1 | 80 (78.4) | 69 (78.4) | 12 (70.6) | 42 (41.2) | 11 (12.5) | |
| 2 | 22 (21.6) | 19 (21.6) | 5 (29.4) | 19 (18.6) | 5 (5.7) | |
| 4 | 12 (11.8) | 4 (4.5) | ||||
| 8 | 14 (13.7) | 7 (8.0) | ||||
| ≥16 | 15 (14.7)a | 61 (69.3)a | 17 (100.0)a | |||
All isolates had vancomycin MBCs of >16 μg/ml.
All MRSA-WT strains with vancomycin MBC/MIC ratios consistent with tolerance (15 strains) showed an increase in the vancomycin and teicoplanin MIC results when tested by the Etest method using the high inoculum (data not shown). However; according to the criteria proposed by Wootton et al. (29), only one strain was considered hVISA (strain 16546A; MICs of ≥8 μg/ml for both vancomycin and teicoplanin after 48 h of incubation).
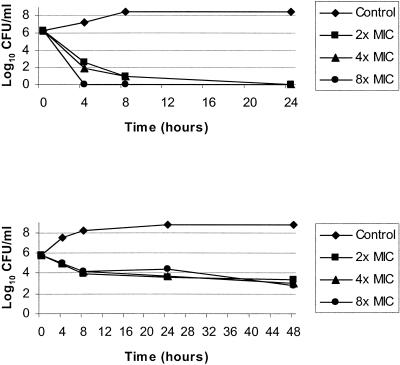
The time-kill curve results for vancomycin and daptomycin are summarized in Table 3. Since both antimicrobials demonstrated time-dependent killing, the concentration of the drug (2×, 4×, and 8× MIC) had little effect on the killing activity, and Table 3 presents average colony count values for 2×, 4×, and 8× MIC in log10 CFU/ml. Daptomycin generally showed potent and rapid bactericidal activity (Table 3 and Fig. 1, top). Bactericidal action by daptomycin was achieved after 4 h of incubation for 16 of 21 strains tested (76.2%), including 12 of the 15 clinical strains (80.0%) with high vancomycin MBC/MIC ratios. A ≥3 log10 reduction in the initial inoculum was observed with 20 strains (95%) after 8 h of incubation and with all strains after 24 h of incubation. In contrast, vancomycin exhibited only modest and slow killing (Table 3 and Fig. 1, bottom). The reduction in CFU/ml was generally 1 log10 unit after 8 h of incubation and 3 log10 units after 24 h of incubation. Among the 15 MRSA-WT strains that exhibited vancomycin MBC/MIC ratios consistent with tolerance, vancomycin showed bacteriostatic effects in only 9 strains (60.0%).
TABLE 3.
Time-kill curve results for vancomycin and daptomycin tested against bacteremic MRSA strains
| Isolate | Antimicrobial agent | Reduction in log10 CFU/ml ata:
|
Activity categoryb | |||
|---|---|---|---|---|---|---|
| T4 | T8 | T24b | T48 | |||
| 170A | Vancomycin | 0.9 | 1.7 | 2.0 | 2.8 | Static |
| Daptomycin | 4.8 | 5.6 | 6.3c | −d | Cidal | |
| 100A | Vancomycin | 0.5 | 1.1 | 1.9 | 2.1 | Static |
| Daptomycin | 6.1c | 6.1c | 6.1c | − | Cidale | |
| 13851A | Vancomycin | 0.7 | 1.1 | 2.9 | 4.3 | Statice |
| Daptomycin | 4.8 | 6.1c | 6.1c | − | Cidal | |
| 16546A | Vancomycin | 0.3 | 0.8 | 2.6 | 3.6 | Staticf |
| Daptomycin | 3.5 | 4.8 | 5.7c | − | Cidalg | |
| 14382A | Vancomycin | 0.1 | 0.4 | 1.7 | 3.0 | Static |
| Daptomycin | 2.7 | 4.4 | 5.6c | 5.6c | Cidal | |
| 2345A | Vancomycin | 0.5 | 1.0 | 2.4 | 3.4 | Statice |
| Daptomycin | 4.0 | 6.0c | 6.0c | 6.0c | Cidal | |
| 2308A | Vancomycin | 0.8 | 1.3 | 3.0 | 4.1 | Cidal |
| Daptomycin | 4.2 | 6.0c | 6.0c | − | Cidal | |
| 1416A | Vancomycin | 0.1 | 0.8 | 2.8 | 3.9 | Statice |
| Daptomycin | 3.4 | 4.8 | 5.9c | − | Cidal | |
| 2336A | Vancomycin | 0.4 | 0.9 | 3.2 | 5.6c | Cidal |
| Daptomycin | 3.2 | 5.8c | 5.8c | − | Cidal | |
| 16542A | Vancomycin | 0.3 | 1.0 | 3.8 | 5.5c | Cidal |
| Daptomycin | 3.2 | 4.1 | 5.5c | − | Cidal | |
| 16160A | Vancomycin | 0.5 | 1.7 | 3.6 | 4.2 | Cidal |
| Daptomycin | 2.2 | 2.6 | 3.8 | 6.0c | Cidal | |
| 5642A | Vancomycin | 0.5 | 0.7 | 2.4 | 3.7 | Statice |
| Daptomycin | 2.6 | 4.0 | 4.3 | − | Cidalg | |
| 561A | Vancomycin | 0.3 | 0.8 | 2.0 | 3.6 | Statice |
| Daptomycin | 3.6 | 5.7c | 5.7c | − | Cidal | |
| 16325A | Vancomycin | (0.1)h | 0.3 | 2.3 | 3.2 | Statice |
| Daptomycin | 3.8 | 6.0c | 6.0c | − | Cidal | |
| 456A | Vancomycin | 0.5 | 1.3 | 3.6 | 3.9 | Cidal |
| Daptomycin | 4.5 | 5.6c | 5.6c | − | Cidal | |
| 424A | Vancomycin | 0.4 | 1.0 | 3.5 | 3.5 | Cidal |
| Daptomycin | 3.1 | 5.7c | 4.8 | − | Cidal | |
| 3811A | Vancomycin | 1.0 | 1.6 | 2.8 | 3.9 | Statice |
| Daptomycin | 2.8 | 3.7 | 4.9 | − | Cidal | |
| 14854A | Vancomycin | 0.8 | 1.2 | 3.0 | 4.2 | Cidal |
| Daptomycin | 4.4 | 5.2 | 6.0c | − | Cidal | |
| ATCC 29213 | Vancomycin | 1.2 | 1.4 | 2.7 | 4.6 | Statice |
| Daptomycin | 3.2 | 5.9c | 5.9c | 5.9c | Cidal | |
| ATCC 43300 | Vancomycin | 0.7 | 1.0 | 2.8 | 3.7 | Staticf |
| Daptomycin | 2.3 | 2.9 | 4.0 | − | Cidalg | |
| ATCC 33591 | Vancomycin | 0.5 | 1.3 | 3.8 | 4.5 | Cidal |
| Daptomycin | 4.0 | 4.8 | 5.7c | − | Cidal | |
Compared to the initial inoculum. The values represent averages of 2×, 4×, and 8× reductions in log10 CFU/ml.
Bactericidal (Cidal) activity was defined as ≥3 log10 reduction in the initial inoculum within 24 h of incubation.
No bacterial growth was detected.
−, incubated for 24 h only.
Bactericidal activity was achieved only at 48 h of incubation.
Bactericidal activity was achieved at 24 h when tested at 4× and/or 8× MIC.
Bacteriostatic activity when tested at 2× MIC.
Value in parentheses represents the increase in the log10 CFU/ml.
FIG. 1.
Time-kill curve results for strain 170A tested against daptomycin (top) and vancomycin (bottom).
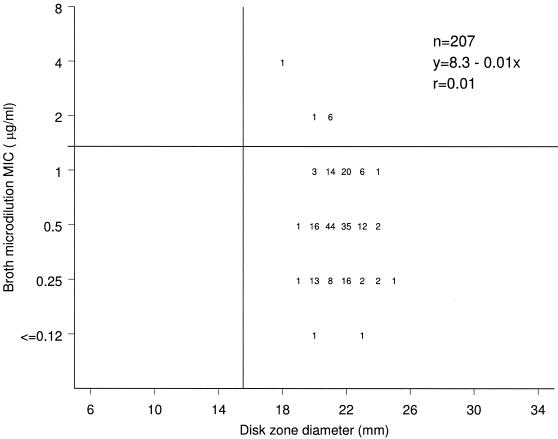
A scattergram illustrating the correlation between the broth microdilution MIC and the disk diffusion zone diameter results for daptomycin is shown in Fig. 2. Eight strains were considered nonsusceptible to daptomycin (MIC ≥ 2 μg/ml) when tested by the broth microdilution method but susceptible when tested by the disk diffusion method using the current CLSI (7) interpretation criteria. All eight strains were in the hVISA/VISA groups. The correlation coefficient was only 0.01.
FIG. 2.
Scattergram showing the correlation between the daptomycin broth microdilution MIC and disk zone diameter results.
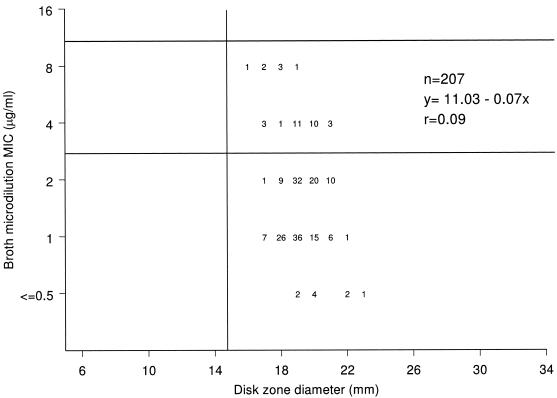
A scattergram showing the correlation between the broth microdilution MIC and the disk diffusion zone diameter results for vancomycin is shown in Fig. 3. Thirty-five strains (17 VISA and 18 hVISA) had intermediate vancomycin MICs (4 to 8 μg/ml) as categorized by the proposed new CLSI breakpoints (6). However, these isolates had inhibition zone diameters of ≥16 mm and were considered susceptible according to CLSI breakpoints for the disk diffusion test (≥15 mm) (6, 7). The inhibitory-zone diameter varied from 16 to 19 mm among the seven VISA strains with broth microdilution MICs of 8 μg/ml. The correlation coefficientwas also very low (0.09).
FIG. 3.
Scattergram showing the correlation between the vancomycin broth microdilution MIC and disk zone diameter results.
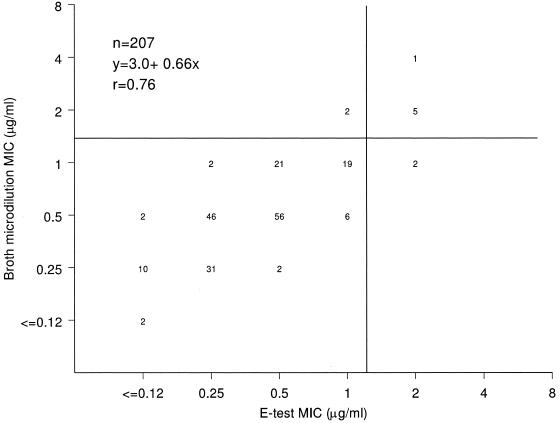
The Etest results correlated well with broth microdilution MIC results for daptomycin (Fig. 4), with a slight tendency toward lower Etest MICs. There was nearly complete categorical agreement between the two methods when MRSA-WT strains were tested, and the rates of major and very major errors for the Etest method were 1.9% (2 of 102) when hVISA/VISA strains were tested (1.0% overall). However, if the number of resistant strains had been used as the denominator, the very major error rate for the Etest would have been 25% (2 of 8).
FIG. 4.
Scattergram showing the correlation between the daptomycin broth microdilution and Etest MIC results.
DISCUSSION
For the treatment of high-bacterial-density infections, such as bacterial endocarditis and serious infections in immunocompromised patients, bactericidal activity is preferred (9, 11, 19, 26). The results of the present study endorse previous experiences that showed a more rapid and potent bactericidal activity for daptomycin than for vancomycin (2, 4, 5, 13). In addition, we demonstrated that daptomycin remained highly active (bactericidal) against S. aureus strains with reduced susceptibility to vancomycin (VISA and hVISA strains).
Our results also indicate that the disk diffusion method fails to detect resistance to either vancomycin or daptomycin as currently defined by the CLSI documents (6, 7). The fact that the disk diffusion method does not differentiate strains with reduced susceptibility to vancomycin (MIC, 4 to 8 μg/ml) from susceptible strains (MIC ≤ 2 μg/ml) is well known (6, 7, 16). However, the CLSI still provides vancomycin disk diffusion breakpoints for staphylococci (6, 7). Regarding daptomycin, the disk has been removed from the market and the disk diffusion breakpoints were correctly withdrawn from CLSI documents until the method can be adjusted for appropriate detection of daptomycin-nonsusceptible strains (7). Etest results showed excellent correlation with broth microdilution MIC results for testing daptomycin and represent a reasonable option for susceptibility testing with this antimicrobial agent by an agar diffusion technique. However, the ability of the Etest to detect isolates with decreased susceptibility to daptomycin could not be evaluated properly, since only eight daptomycin-nonsusceptible strains could be included in the study.
In summary, daptomycin was highly bactericidal against essentially all S. aureus strains, and its bactericidal activity was not affected by decreased susceptibility to vancomycin. In contrast, the majority of hVISA strains (69.3%), all VISA strains, and a significant percentage of MRSA-WT strains (14.7%) showed vancomycin MBC/MIC ratios consistent with drug tolerance. Thus, the results of this study, along with previous reports, suggest that daptomycin may be more appropriate than vancomycin for the treatment of infections that require a bactericidal agent. These results also encourage the wider use of MBC testing when vancomycin is selected for therapy for serious MRSA infections.
Acknowledgments
We thank Jeffrey T. Kirby for excellent technical support. We also acknowledge the National Institutes of Health/National Institute of Allergy and Infectious Diseases and its network of Antimicrobial Resistance Staphylococcus aureus programs for kindly providing isolates for this study.
This study was funded by Cubist Pharmaceuticals.
REFERENCES
- 1.Asseray, N., C. Jacqueline, V. Le Mabecque, E. Batard, D. Bugnon, G. Potel, and J. Caillon. 2005. Activity of glycopeptides against Staphylococcus aureus infection in a rabbit endocarditis model: MICs do not predict in vivo efficacy. Antimicrob. Agents Chemother. 49:857-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantoni, L., M. P. Glauser, and J. Bille. 1990. Comparative efficacy of daptomycin, vancomycin, and cloxacillin for the treatment of Staphylococcus aureus endocarditis in rats and role of test conditions in this determination. Antimicrob. Agents Chemother. 34:2348-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 4.Cha, R., W. J. Brown, and M. J. Rybak. 2003. Bactericidal activities of daptomycin, quinupristin/dalfopristin and linezolid against vancomycin-resistant Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 47:3960-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha, R., and M. J. Rybak. 2003. Daptomycin against multidrug-resistant staphylococcus and enterococcus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Diagn. Microbiol. Infect. Dis. 47:539-546. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Document M7-A7. CLSI, Wayne, Pa.
- 7.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests. Approved standard, 9th ed. Document M2-A9. CLSI, Wayne, Pa.
- 8.Cosgrove, S. E., K. C. Carroll, and T. M. Perl. 2004. Staphylococcus aureus with reduced susceptibility to vancomycin. Clin. Infect. Dis. 39:539-545. [DOI] [PubMed] [Google Scholar]
- 9.Denny, A. E., L. R. Peterson, D. N. Gerding, and W. H. Hall. 1979. Serious staphylococcal infections with strains tolerant to bactericidal antibiotics. Arch. Intern. Med. 139:1026-1031. [PubMed] [Google Scholar]
- 10.Domenech, A., S. Ribes, C. Cabellow, M. A. Dominguez, A. Montero, J. Linares, J. Ariza, and F. Gudiol. 2004. A mouse peritonitis model for the study of glycopeptide efficacy in GISA infections. Microb. Drug Resist. 10:346-353. [DOI] [PubMed] [Google Scholar]
- 11.Drake, T. A., and M. A. Sande. 1985. Studies of the chemotherapy of endocarditis: correlation of in vitro, animal model, and clinical studies. Rev. Infect. Dis. 5(Suppl. 2):S345-S353. [Google Scholar]
- 12.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2000. Daptomycin susceptibility tests: interpretive criteria, quality control, and effect of calcium on in vitro tests. Diagn. Microbiol. Infect. Dis. 38:51-58. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs, P. C., A. L. Barry, and S. D. Brown. 2002. In vitro bactericidal activity of daptomycin against staphylococci. J. Antimicrob. Chemother. 49:467-470. [DOI] [PubMed] [Google Scholar]
- 14.Garcia, C., F. Marco, Y. Armero, E. Amat, D. Soy, A. Del Rio, A. Moreno, M. Almela, C. Manzardo, X. Claramonte, C. Mestres, N. Perez, J. Gatell, M. Jimenez de Anta, J. Miro, and the Hospital Endocarditis Study Group. 2005. Efficacy of daptomycin in the treatment of experimental endocarditis due to methicillin-resistant (MRSA) and reduced susceptibility to glycopeptides (GISA) Staphlococcus aureus, abstr. B-2002. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, D.C.
- 15.Gopal, V., A. Bisno, and J. Silverblatt. 1976. Failure of treatment in Staphylococcus aureus endocarditis. In vivo and in vitro observations. JAMA 236:1604-1606. [PubMed] [Google Scholar]
- 16.Hageman, J. C., S. K. Fridkin, J. M. Mohammed, C. D. Steward, R. P. Gayne, F. C. Tenover, and the National Nosocomial Infections Surveillance System Hospitals. 2003. Antimicrobial proficiency testing of National Nosocomial Infections Surveillance System hospital laboratories. Infect. Control Hosp. Epidemiol. 24:356-361. [DOI] [PubMed] [Google Scholar]
- 17.Howe, R. A., A. Monk, M. Wootton, T. R. Walsh, and M. C. Enright. 2004. Vancomycin susceptibility within methicillin-resistant Staphylococcus aureus lineages. Emerg. Infect. Dis. 10:855-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, R. N. 2006. Microbiology of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin. Infect. Dis. 42(Suppl. 1):S13-S24. [DOI] [PubMed] [Google Scholar]
- 19.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid and daptomycin, alone and in combination with gentamicin in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May, J., K. Shannon, A. King, and G. French. 1998. Glycopeptide tolerance in Staphylococcus aureus. J. Antimicrob. Chemother. 42:189-197. [DOI] [PubMed] [Google Scholar]
- 21.Mohan, S. S., B. P. McDermott, and B. A. Cunha. 2005. Methicillin-resistant Staphylococcus aureus prosthetic aortic valve endocarditis with paravalvular abscess treated with daptomycin. Heart Lung 34:69-71. [DOI] [PubMed] [Google Scholar]
- 22.Moody, J., and C. Knapp. 2004. Tests to assess bactericidal activity, p. 5.10.1.1-5.10.3.6. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook. ASM Press, Washington, D.C.
- 23.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antibacterial agents; approved guideline. NCCLS document M26-A. NCCLS, Wayne, Pa.
- 24.Pankey, G. A., and L. D. Sabath. 2004. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 38:864-870. [DOI] [PubMed] [Google Scholar]
- 25.Quirk, M. 2002. First VRSA isolate identified in USA. Lancet Infect. Dis. 2:510.12206952 [Google Scholar]
- 26.Sakouloas, G., G. M. Eliopoulos, J. Alder, and C. Thauvin-Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Small, P., and F. Chambers. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenover, F. C., J. W. Biddle, and M. V. Lancaster. 2001. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerg. Infect. Dis. 7:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wootton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399-403. [DOI] [PubMed] [Google Scholar]